Abstract
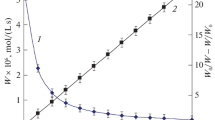
Formation of protonated 3,4,6-triisopropylsemiquinone radicals in solution of a mixture of 3,4,6- triisopropyl-о-benzoquinone and 3,4,6-triisopropylcatechol was shown using ESR spectroscopy. Heating the mixture to 110°С leads to the formation of the condensation products, additional amount of 3,4,6-triisopropylcatechol, and evolution of propylene. Mathematical simulation of the kinetics of the reaction was performed.
Similar content being viewed by others
References
Pointillart, F., Klementieva, S., Kuropatov, V., Le Gal, Y., Golhen, S., Cador, O., Cherkasov, V., and Ouahab, L., Chem. Commun., 2012, vol. 48, p. 714. doi 10.1039/c1cc16314k
Minkin, V.I., Starikova, A.A., and Starikov, A.G., Dalton Trans., 2015, vol. 44, p. 1982. doi 101039/4dt03053b
Haoyuan Li, Chavez, A.D., Huifang Li, Hong Li, Dichtel, W.R., and Bredas, J.-L., J. Am. Chem. Soc., 2017, vol. 139, p. 16310. doi 10.1021/jacs.7b09169
Thompson, C.M., Occhialini, G., Mc Candless, G.T., Alahakoon, S.B., Cameron, V., Nielsen, S.O., and Smaldone, R.A., J. Am. Chem. Soc., 2017, vol. 139, p. 10506. doi 10.1021/jacs.7b05555
Zemtsova, O.V. and Zheleznov, K.N., Russ. Chem. Bull., 2004, vol. 53, no. 8, p. 1743. doi 10.1007/s11172-005-0028-7
Zagorskii, A.L., Kalnin’sh, K.K., and Toropov, D.K., Russ. J. Appl. Chem., 2005, vol. 78, no. 8, p. 656. doi 10.1007/s11167-005-0363-3
Liogon’kii, B.I., Ragimov, A.V., Bektashi, F.T., and Nagiev, A.Yu., Dokl. Akad. Nauk SSSR, 1982, vol. 266, no. 4, p. 903.
Love, B.E., Duffy, B.C., and Simmons, A.L., Tetrahedron Lett., 2014, vol. 55, no. 12, p. 1994. doi 10.1016/j.tetlet.2014.02.17
Abakumov, G.A., Nevodchikov, V.L., Druzhkkov, N.O., Zakharov, L.N., Abakumova, L.G., Kurskii, Yu.A., and Cherkasov, V.K., Russ. Chem. Bull., 1997, vol. 46, no. 4, p. 771. doi 10.1007/BF02495211
Pokhodenko, V.D., Fenoksil’nye radikaly (Phenoxy Radicals), Kyiv: Naukova Dumka, 1969.
Cook, C.D. and Norcross, B.E., J. Am. Chem. Soc., 1956, vol. 78, no. 15, p. 3797. doi 10.1021/ja01596a064
Bodrikov, I.V., Grinval’d, I.I., Subbotin, A.Yu., Druzhkov, N.O., and Kocherova, T.N., Doklady Chem., 2013, vol. 448, no. 1, p. 19. doi 10.7868/S0869565213030134
Gurvich, L.V., Karachentsev, G.V., Kondrat’ev, Yu.A., Lebedev, Yu.A., Medvedev V.A., Potapov, V.K., and Khodeev, Yu.S., Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu (The Energy of Rupture of Chemical Bonds. Potentials of Ionization and Electron Affinity), Moscow: Nauka, 1974.
Zavelovich, E.B. and Prokof’ev, A.I., Chem. Phys. Lett., 1974, vol. 29, no. 2, p. 212. doi 10.1016/0009-2614(74) 85015-3
Abakumov, G.A., Vavilina, N.N., Kurskii, Yu.A., Nevodchikov, V.I., Cherkasov, V.K., and Shavyrin, A.S., Russ. Chem. Bull., 2003, vol. 52, no. 8, p. 1847. doi 10.1023/A:1026029410312
Cherkasov, V.K., Abakumov, G.A., Shavyrin, A.S., Kuz’michev, V.V., Baranov, E.V., Smolyaninov, I.V., and Kuropatov, V.A., Asian. J. Org. Chem., 2015, vol. 4, no. 5, p. 446. doi 10.1002/ajoc.201500005
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.А. Kurskii, А.S. Shavyrin, Т.I. Kulikova, V.А. Kuropatov, G.А. Abakumov, 2018, published in Zhurnal Obshchei Khimii, 2018, Vol. 88, No. 8, pp. 1262–1267.
Rights and permissions
About this article
Cite this article
Kurskii, Y.A., Shavyrin, A.S., Kulikova, T.I. et al. Reaction of 3,4,6-Triisopropyl-о-benzoquinone with 3,4,6-Triisopropylcatechol. Russ J Gen Chem 88, 1584–1589 (2018). https://doi.org/10.1134/S1070363218080054
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218080054