Abstract
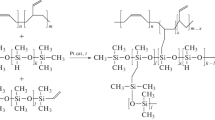
Polydimethylsildimethylene-dimethylsiloxane (PSDMS) and polydimethylsiltrimethylenedimethylsiloxane (PSTMS) have been first studied as pervaporation membrane materials for the recovery of butanol from aqueous media. New synthesis procedures that make it possible to obtain the monomers 2,2,5,5-tetramethyl-1-oxa-2,5-disilacyclopentane (1) and 2,2,6,6-tetramethyl-1-oxa-2,6-disilacyclohexane (2) in high yields and with high purity required for subsequent polymerization have been developed. The optimum concentration of the crosslinking agent (tetraethoxysilane (TEOS)) of 5% has been found, which provides the maximum degree of crosslinking without sacrificing high values of separation factor and permeate flux. It has been shown that the permselectivity of PSDMS or PSTMS for butanol–water is higher by a factor of 1.5 or- almost 2, respectively, than the selectivity of the industrial membrane polymer, PDMS, at comparable values of the butanol permeability coefficient.
Similar content being viewed by others
References
TR TS (Technical Regulations of the Customs Union) 013-2011: About Requirements to Motor and Aviation Gasoline, Diesel and Marine Fuel, Jet Fuel, and Fuel Oil.
Degrémont, Mémento technique de l’eau, 2 vols., 10th Ed. (Lavoisier, Paris, 2005), Vol. 1.
Government Decree of April 15, 2014 No 321 On approval of the Russian State Program “Energy Efficiency and Energy Development” (as amended on December 7, 2015).
H. J. Huang and S. Ramaswamy, Sep. Purif. Technol. 132, 513 (2014).
L. M. Vane, J. Chem. Technol. Biotechnol. 80, 603 (2005).
G. Liu, W. Wei, and W. Jin, ACS Sustain. Chem. Eng. 2, 546 (2013).
I. L. Borisov, N. V. Ushakov, V. V. Volkov, and E. Sh., Finkel’shtein, Izv. Akad. Nauk, Ser. Khim., No. 4, 1020 (2016).
W. A. Piccoli, G. G. Haberland, and R. L. Merker, J. Am. Chem. Soc. 82, 1883 (1960).
US Patent No. 3 041 362 (1962).
D. Wittenberg and H. Gilman, J. Am. Chem. Soc. 74, 5091 (1952).
C. R. Hauser and C. R. Hance, J. Am. Chem. Soc. 80, 2677 (1958).
G. Greber and L. Metzinger, Makromol. Chem. 39, 226 (1960).
L. V. Interrante, Q. Shen, and J. Li, Macromolecules 34, 1545 (2001).
M. Samara and D. A. Loy, Am. Chem. Soc. Polym. Prepr., Polym. Chem. 39, 599 (1998).
L. Li, Z. Xiao, S. Tan, et al., J. Membr. Sci. 243, 177 (2004).
I. L. Borisov and V. V. Volkov, Sep. Purif. Technol. 146, 33 (2015).
J. Pump, E. G. Rochow, and U. Wannagat, Angew. Chem. 75, 374 (1963).
J. J. Eisch and G. R. Hask, J. Org. Chem. 29, 254 (1964).
Q. T. Nguyen, Z. Bendjama, R. Clément, and Z. Ping, Phys. Chem. Chem. Phys. 2, 395 (2000).
S. A. Stern, V. M. Shah, and B. J. Hardy, J. Polym. Sci., Part B: Polym. Phys. 25, 1263 (1987).
I. L. Borisov, V. V. Volkov, V. A. Kirsh, and V. I. Roldugin, Pet. Chem. 51, 542 (2011).
R. W. Baker, J. G. Wijmans, and Y. Huang, J. Membr. Sci. 348, 346 (2010).
W. Wei and W. Jin, ACS Sustain. Chem. Eng. 2, 546 (2013).
M. H. V. Mulder, Basic Principles of Membrane Technology (Kluwer Academic, Dordrecht, 1990).
S. F. Li, F. Qin, P. Y. Qin, et al., Green Chem. 15, 2180 (2013).
Z. Dong, G. Liu, S. Liu, et al., J. Membr. Sci. 450, 38 (2014).
J. Niemisto, W. Kujawski, and R. L. Keiski, J. Membr. Sci. 434, 55, (2013).
A. Kujawska, J. Kujawski, M. Bryjak, and W. Kujawski, Chem. Eng. Process.: Proc. Intens. 94, 62 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.L. Borisov, N.V. Ushakov, V.V. Volkov, E.Sh. Finkel’shtein, 2016, published in Neftekhimiya, 2016, Vol. 56, No. 6, pp. 578–583.
Rights and permissions
About this article
Cite this article
Borisov, I.L., Ushakov, N.V., Volkov, V.V. et al. Polydimethylsilalkylene-dimethylsiloxanes as advanced membrane materials for thermopervaporative recovery of oxygenates from aqueous reaction media. Pet. Chem. 56, 798–804 (2016). https://doi.org/10.1134/S0965544116090024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544116090024