Abstract
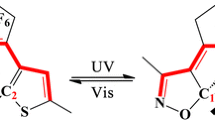
1,2-Diarylbenzenes (DABs) have been developed as a new family of fast T-type photochromic switches. However, the molecular design strategy for DABs with desired optical and thermal properties is not established. In this work, we explored the best functional in quantum chemical calculations to predict the properties of DABs. Furthermore, we newly designed and synthesized DABs based on the calculation using the best functional, resulting in the improvement of the photosensitivity in the UV-A region (i.e. a shift of absorption to lower energies and an increase in the absorption coefficient) without changing the thermal back-reaction rate.
Similar content being viewed by others
References
Y. Inagaki, Y. Kobayashi, K. Mutoh and J. Abe, A simple and versatile strategy for rapid color fading and intense coloration of photochromic naphthopyran families, J. Am. Chem. Soc., 2017, 139, 13429–13441.
Y. Kobayashi and J. Abe, Real-time dynamic hologram of a 3D object with fast photochromic molecules, Adv. Opt. Mater., 2016, 4, 1354–1357.
N. Ishii, T. Kato and J. Abe, A real-time dynamic holographic material using a fast photochromic molecule, Sci. Rep., 2012, 2, 819.
J. Cusido, S. S. Ragab, E. R. Thapaliya, S. Swaminathan, J. Garcia-Amorós, M. J. Roberti, B. Araoz, M. M. A. Mazza, S. Yamazaki, A. M. Scott, F. M. Raymo and M. L. Bossi, A photochromic bioconjugate with photoactivatable fluorescence for superresolution imaging, J. Phys. Chem. C, 2016, 120, 12860–12870.
E. Deniz, M. Tomasulo, J. Cusido, I. Yildiz, M. Petriella, M. L. Bossi, S. Sortino and F. M. Raymo, Photoactivatable fluorophores for super-resolution imaging based on oxazine auxochromes, J. Phys. Chem. C, 2012, 116, 6058–6068.
F. Tong, M. P. Hanson and C. J. Bardeen, Analysis of reaction kinetics in the photomechanical molecular crystal 9-methylanthracene using an extended Finke-Watzky model, Phys. Chem. Chem. Phys., 2016, 18, 31936–31945.
L. Zhu, F. Tong, C. Salinas, M. K. Al-Muhanna, F. S. Tham, D. Kisailus, R. O. Al-Kaysi and C. J. Bardeen, Improved solid-state photomechanical materials by fluorine substitution of 9-anthracene carboxylic acid, Chem. Mater., 2014, 26, 6007–6015.
M. Tomasulo, S. Sortino, A. J. P. White and F. M. Raymo, Fast and stable photochromic oxazines, J. Org. Chem., 2005, 70, 8180–8189.
M. Tomasulo, S. Sortino and F. M. Raymo, A fast and stable photochromic switch based on the opening and closing of an oxazine ring, Org. Lett., 2005, 7, 1109–1112.
M. Tomasulo, S. Sortino and F. M. Raymo, Amplification of the coloration efficiency of photochromic oxazines, Adv. Mater., 2008, 20, 832–835.
Y. Zhang, S. Tang, E. R. Thapaliya, L. Sansalone and F. M. Raymo, Fluorescence activation with switchable oxazines, Chem. Commun., 2018, 54, 8799–8809.
C. M. Sousa, J. Berthet, S. Delbaere, A. Polónia and P. J. Coelho, Fast color change with photochromic fused naphthopyrans, J. Org. Chem., 2015, 80, 12177–12181.
K. Fujita, S. Hatano, D. Kato and J. Abe, Photochromism of a radical diffusion-inhibited hexaarylbiimidazole derivative with intense coloration and fast decoloration performance, Org. Lett., 2008, 10, 3105–3108.
Y. Kishimoto and J. Abe, A fast photochromic molecule that colors only under UV light, J. Am. Chem. Soc., 2009, 131, 4227–4229.
H. Yamashita, T. Ikezawa, Y. Kobayashi and J. Abe, Photochromic phenoxyl-imidazolyl radical complexes with decoloration rates from tens of nanoseconds to seconds, J. Am. Chem. Soc., 2015, 137, 4952–4955.
T. Ikezawa, K. Mutoh, Y. Kobayashi and J. Abe, Thiophene-substituted phenoxyl-imidazolyl radical complexes with high photosensitivity, Chem. Commun., 2016, 52, 2465–2468.
M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Photochromism of diarylethene molecules and crystals: memories, switches, and actuators, Chem. Rev., 2014, 114, 12174–12277.
K. Uchida, T. Matsuoka, K. Sayo, M. Iwamoto, S. Hayashi and M. Irie, Thermally reversible photochromic systems. photochromism of a dipyrrolylperfluorocyclopentene, Chem. Lett., 1999, 28, 835–836.
S. Kawai, T. Nakashima, K. Atsumi, T. Sakai, M. Harigai, Y. Imamoto, H. Kamikubo, M. Kataoka and T. Kawai, Novel photochromic molecules based on 4,5-dithienyl thiazole with fast thermal bleaching rate, Chem. Mater., 2007, 19, 3479–3483.
Y. H. Yang, Y. S. Xie, Q. Zhang, K. Nakatani, H. Tian and W. H. Zhu, Aromaticity-controlled thermal stability of photochromic systems based on a six-membered ring as ethene bridges: photochemical and kinetic studies, Chem. –Eur. J., 2012, 18, 11685–11694.
V. Z. Shirinian, A. G. Lvov, E. Y. Bulich, A. V. Zakharov and M. M. Krayushkin, Novel photochromic diarylethenes bearing an imidazole moiety, Tetrahedron Lett., 2015, 56, 5477–5481.
D. Kitagawa and S. Kobatake, Strategy for molecular design of photochromic diarylethenes having thermal functionality, Chem. Rec., 2016, 16, 2005–2015.
K. Inaba, R. Iwai, M. Morimoto and M. Irie, Thermally reversible photochromism of dipyrrolylethenes, Photochem. Photobiol. Sci., 2019, 18, 2136–2141.
Y. Sato, D. Kitagawa and S. Kobatake, Molecular design for a write-by-light/erase-by-heat recording system using photo-chromic diarylethenes with thermal cycloreversion, Tetrahedron, 2019, 75, 130487.
D. Kitagawa, T. Nakahama, Y. Nakai and S. Kobatake, 1,2-Diarylbenzene as fast T-type photochromic switch, J. Mater. Chem. C, 2019, 7, 2865–2870.
T. Nakahama, D. Kitagawa and S. Kobatake, Tuning of optical properties and thermal cycloreversion reactivity of photochromic diarylbenzene by introducing electrondonating substituents, J. Phys. Chem. C, 2019, 123, 31212–31218.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009.
A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652.
C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789.
J. P. Perdew and Y. Wang, Accurate and simple analytic representation of the electron-gas correlation energy, Phys. Rev. B: Condens. Matter, 1992, 45, 13244–13249.
A. D. Boese and J. M. Martin, Development of density functionals for thermochemical kinetics, J. Chem. Phys., 2004, 121, 3405–3416.
Y. Zhao, N. E. Schultz and D. G. Truhlar, Exchange-correlation functional with broad accuracy for metallic and non-metallic compounds, kinetics, and noncovalent interactions, J. Chem. Phys., 2005, 123, 161103.
Y. Zhao, N. E. Schultz and D. G. Truhlar, Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions, J. Chem. Theory Comput., 2006, 2, 364–382.
Y. Zhao and D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 2008, 120, 215–241.
J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620.
R. Ditchfield, W. J. Hehre and J. A. Pople, Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules, J. Chem. Phys., 1971, 54, 724–728.
T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57.
C. Adamo and V. Barone, Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models, J. Chem. Phys., 1998, 108, 664–675.
N. Brimhall, T. L. Andrew, R. V. Manthena and R. Menon, Breaking the far-field diffraction limit in optical nanopatterning via repeated photochemical and electrochemical transitions in photochromic molecules, Phys. Rev. Lett., 2011, 107, 205501.
S. Kobatake and M. Irie, Synthesis and photochromism of diarylethenes with isopropyl groups at the reactive carbons and long and π-conjugated heteroaryl groups, Chem. Lett., 2003, 32, 1078–1079.
P. D. Patel and A. E. Masunov, Theoretical study of photo-chromic compounds: Part 3. Prediction of thermal stability, J. Phys. Chem. C, 2011, 115, 10292–10297.
X. Li, Q. Zou and H. Ågren, Photochromic diarylethenes with heterocyclic aromatic rings: Correlation between thermal bistability and geometrical characters of transition states, J. Phys. Chem. A, 2015, 119, 9140–9147.
P. D. Patel and A. E. Masunov, Theoretical study of photochromic compounds. 1. Bond length alternation and absorption spectra for the open and closed forms of 29 diarylethene derivatives, J. Phys. Chem. A, 2009, 113, 8409–8414.
R. Li, H. Arai, Y. Kobayashi, K. Mutoh and J. Abe, Molecular design to increase the photosensitivity of photo-chromic phenoxyl–imidazolyl radical complexes, Mater. Chem. Front., 2019, 3, 2380–2387.
G. Pariani, M. Quintavalla, L. Colella, L. Oggioni, R. Castagna, F. Ortica, C. Bertarelli and A. Bianco, New insight into the fatigue resistance of photochromic 1,2-diarylethenes, J. Phys. Chem. C, 2017, 121, 23592–23598.
A. Perrier, F. Maurel and D. Jacquemin, Interplay between electronic and steric effects in multiphotochromic diarylethenes, J. Phys. Chem. C, 2011, 115, 9193–9203.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Detailed experimental data (Fig. S1–S7 and Tables S1–S17). See DOI: 10.1039/d0pp00024h
Rights and permissions
About this article
Cite this article
Kitagawa, D., Takahashi, N., Nakahama, T. et al. Improving photosensitivity without changing thermal reactivity in photochromic diarylbenzenes based on accurate prediction by DFT calculations. Photochem Photobiol Sci 19, 644–653 (2020). https://doi.org/10.1039/d0pp00024h
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/d0pp00024h