Abstract
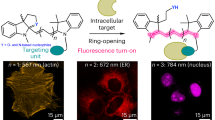
This study presents the full theoretical optical and biological characteristics of a new fluorescent probe based on the phenanthroimidazole backbone (PB5). The aldehyde group was selected as the active group to bind to the protein during conjugation. The new fluorescent probe is based on the phenanthroimidazole backbone; however, unlike previously presented works, as the chromophore part, it contains the first introduction of the 4-chloro-2H-chromen-2-one part. In order to achieve the best cognitive aspect, the study included not only the dye itself but also the concanavalin A conjugate. The linear and non-linear optical properties and biological activities described in this study clearly indicate that the presented dye is a promising material as a fluorescent probe in medical imaging.
Similar content being viewed by others
Abbreviations
- GP:
-
Gas phase
- MCH:
-
MethylCycloHexane
- 1,4-Dx:
-
1,4-Dioxane
- Et2O:
-
DiethylEther
- EtAc:
-
EthylEthanoate
- THF:
-
TetraHydroFuran
- MeAc:
-
Acetone
- MeCN:
-
Acetonitrile
- DMF:
-
N,N-DiMethylFormamide
- DMSO:
-
DiMethylSulfoxide
References
P. Mitra, M. Banerjee, S. Biswas and S. Basu, J. Photochem. Photobiol., B, 2013, 121, 46–56, DOI: 10.1016/j. jphotobiol.2013.02.010.
N. Romanov, C. Anastasi and X. Liu, Cyanine dyes for labeling molecular ligands with improved fluorescence intensity and photostability, WO2013041117A1, 2013.
M. Fernández-Suárez and A. Y. Ting, Nat. Rev. Mol. Cell Biol., 2008, 9, 929–943, DOI: 10.1038/nrm2531.
C. Basford, N. Forraz and C. McGuckin, Nat. Protoc., 2010, 5(7), 1337–1346, DOI: 10.1038/nprot.2010.88.
R. Weissleder and V. Pittet, Nature, 2008, 452, 580–589, DOI: 10.1038/nature06917.
L. E. Jennings and N. J. Long, Chem. Commun., 2009, 24, 3511–3524, DOI: 10.1039/B821903F.
J.-M. Liu, J.-T. Chen and X.-P. Yan, Anal. Chem., 2013, 85, 3238–3245, DOI: 10.1021/ac303603f.
H. Guo, N. M. Idris and Y. Zhang, Langmuir, 2011, 27, 2854–2860, DOI: 10.1021/la102872v.
Z. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765–4769, DOI: 10.1002/adma.200801056.
F. Rijke, H. Zijlmans, S. Li, V. Tim, A. K. Raap and R. S. Niedbala, Nat. Biotechnol., 2001, 19, 273–276, DOI: 10.1038/85734.
J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47(44), 8438–8441, DOI: 10.1002/anie.200802469.
D. Janczewski, Y. Zhang, G. K. Das, D. K. Yi, P. Padmanabhan, K. K. Bhakoo, T. T. Tan and S. T. Selvan, Microsc. Res. Tech., 2011, 74, 563–576, DOI: 10.1002/jemt.20912.
M. Tsuji, S. Ueda, T. Hirayama, K. Okuda, Y. Sakaguchi, A. Isono and H. Nagasawa, Org. Biomol. Chem., 2013, 11(18), 3030–3037, DOI: 10.1039/C3OB27445D.
F. Auzel, Chem. Rev., 2004, 104(1), 139–174, DOI: 10.1021/cr020357g.
M. Brinkley, Bioconjugate Chem., 1992, 3(1), 2–13, DOI: 10.1021/bc00013a001.
D. Zhang, J. Tang, Ch. Cao, Z. Ma, Y. Wang, Y. Ma, H. Wang, H. Yin, J. Liu and B. Jia, J. Lumin., 2019, 205, 299–303, DOI: 10.1016/j.jlumin.2018.09.037.
Q. Li, J. Nie, Y. Shan, Y. Li, J. Du, L. Zhu, Q. Yang and F. Bai, Anal. Biochem., 2020, 15, 113539, DOI: 10.1016/j. ab.2019.113539.
G. Yin, Y. Gan, T. Yu, T. Niu, P. Yin, H. Chen, Y. Zhang, H. Li and S. Yao, Talanta, 2019, 191, 428–434, DOI: 10.1016/j.talanta.2018.08.059.
M. Xiaoyu, Ch. Shanyong, Y. Hong, G. Youwei, L. Junjun, Y. Xingwu and Z. Zhenghao, Nanoscale Res. Lett., 2019, 14(1), 318, DOI: 10.1186/s11671–019–3149-x.
Q. Gao, Y. Jiao, C. He and C. Duan, Molecules, 2019, 12, 2268.
Y. Ning, J. Cui, Y. Lu, X. Wang, Ch. Xiao, S. Wu, J. Li and Y. Zhang, Sens. Actuators, B, 2018, 269, 322–330, DOI: 10.1016/j.snb.2018.04.156.
W. Xiaomei, L. Yong, H. Xu, L. Dan and W. Yuhong, Aust. J. Chem., 2018, 71, 971–977, DOI: 10.1071/CH18207; K. Bo-Yeon, P. Anup, Ch. Sik Cho and K. Hong-Seok, Bull. Korean Chem. Soc., 2019, 40, 163–168, DOI: 10.1002/bkcs.11663.
Z. Lu, Y. Lu, X. Sun, C. Fan, Z. Long and L. Gao, Bioorg. Chem., 2019, 92, 103215. PMID: 31541803.
P. Siqi, Z. Tianya, G. Tiantong, S. Dehua, M. Defen, L. Haoran and G. Dongcai, New J. Chem., 2018, 42, 5185–5192.
G. Yunyan, Y. Na, O. Zhize, L. Zhiyuan, M. Tuotuo, J. Hongdan, X. Wenli, Y. Guoqiang and L. Yi, Sens. Actuators, B, 2018, 267, 136–144, DOI: 10.1016/j. snb.2018.04.017.
K. Shantaram, B. Sulochana and S. Nagaiyan, Dyes Pigm., 2018, 159, 209–222, DOI: 10.1016/j.dyepig.2018.06.020.
U. Uçucu, N. G. Karaburun and I. Işikdağ, Farmaco, 2020, 56, 285–290, DOI: 10.1016/S0014–827X(01)01076-X.
A. Yeşilada, S. Koyunoğlu, N. Saygili, E. Kupeli, E. Yeşilada, E. Bedir and I. Khanc, Arch. Pharm. Pharm. Med. Chem., 2004, 337, 96–104, DOI: 10.1002/ ardp.200200752.
L. Quattara, M. Debaert and R. Cavier, Farmaco (sci.), 1987, 42(6), 449–456, PMID:2904887.
S. Dutta, Acta Pharm., 2010, 60(2), 229–235, DOI: 10.2478/v10007-010-0011-1.
A. K. Sengupta and T. Bhattacharya, J. Indian Chem. Soc., 1983, 60, 373–376, DOI: 10.1002/chin.198349246.
L. Navidpour, H. Shadnia, H. Shafaroodi, M. Amini, R. Dehpour and A. Shafiee, Bioorg. Med. Chem., 2007, 15, 1976–1982, DOI: 10.1016/j.bmc.2006.12.041.
G. Fluoret, J. Med. Chem., 1970, 13, 843–845, DOI: 10.1021/jm00299a011.
R. N. Brogden, R. C. Heel, T. M. Speight and G. S. Avery, Drugs, 1978, 16(5), 387–417, PMID: 363399.
R. W. Brimblecombe, W. A. M. Duncan, G. J. Durant, J. C. Emmett, C. R. Ganellin, M. E. Parsons and J. W. Black, J. Int. Med. Res., 1975, 3(2), 86–92, DOI: 10.1111/j.1476-5381.2010.00854.x.
W. Hunkeler, H. Möhler, L. Pieri, P. Polc, E. P. Bonetti, R. Cumin, R. Schaffner and W. Haefely, Nature, 1981, 290, 514–516, DOI: 10.1038/290514a0.
X. Cheng, H. Jia, J. Feng, J. Qin and Z. Li, Sens. Actuators, B, 2013, 184, 274–280, DOI: 10.1016/j.snb.2013.04.070.
W. Lin, L. Long, L. Yuan, Z. Cao, B. Chen and W. Tan, Org. Lett., 2008, 10(24), 5577–5580, DOI: 10.1021/ol802436j.
Jawaharmal, H. S. Lamba, S. Narwal, G. Singh, D. R. Saini, A. Kaur and S. Narwal, Indo Global J. Pharm. Sci., 2012, 2(2), 147–156. ISSN 2249-1023.
L. Long, L. Zhou, L. Wang, S. Meng, A. Gong, F. Du and C. Zhang, Anal. Methods, 2013, 5, 6605–6610, DOI: 10.1039/C3AY41475B.
M.-S. Tsai, Y.-C. Hsu, J. T. Lin, H.-C. Chen and C.-P. Hsu, J. Phys. Chem. C, 2007, 111(50), 18785–18793, DOI: 10.1021/jp075653h.
J. Liu, J. Qiu, M. Wang, L. Wang, L. Su, J. Gao, Q. Gua, S. L. Huang, L. Q. Gu, Z. S. Huang and D. Li, Biochim. Biophys. Acta, 2014, 1840(9), 2886–2903, DOI: 10.1016/j.bbagen.2014.05.005.
P. Krawczyk, B. Jędrzejewska, M. Pietrzak and T. Janek, J. Photochem. Photobiol., B, 2016, 164, 112–122, DOI: 10.1016/j.jphotobiol.2016.07.044.
P. Krawczyk, B. Jędrzejewska, M. Pietrzak and T. Janek, J. Photochem. Photobiol., B, 2017, 166, 74–85, DOI: 10.1016/j.jphotobiol.2016.11.008.
P. Krawczyk, T. Wybranowski, Ł. Kaźmierski, I. Hołyńska-Iwan, M. Bratkowska, P. Cysewski and B. Jędrzejewska, Spectrochim. Acta, Part A, 228, 117757, DOI: 10.1016/j. saa.2019.117757.
P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871, DOI: 10.1103/physrev.136.b864.
W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138, DOI: 10.1103/physrev.140.a113.
R. G. Parr and W. Yang, Density-functional theory of atoms and molecules, Oxford Univ. Press, Oxford, 1989.
The Challenge of d and f Electrons, ed. D. R. Salahub and M. C. Zerner, ACS, Washington DC, 1989.
C. Sosa and C. Lee, J. Chem. Phys., 1993, 98, 8004–8011, DOI: 10.1063/1.464554.
G. E. Scuseria, J. Chem. Phys., 1992, 97, 7528–7530, DOI: 10.1063/1.463977.
Density Functional Methods in Chemistry, ed. J. K. Labanowski and J. W. Andzelm, Springer-Verlag, New York, 1991.
M. Kurt, T. R. Sertbakan and M. Ozduran, Spectrochim. Acta, Part A, 2008, 70(3), 664–673, DOI: 10.1016/j. saa.2007.08.019.
C. Adamo, G. E. Scuseria and V. Barone, J. Chem. Phys., 1999, 111, 2889–2899, DOI: 10.1063/1.479571.
C. J. Jamorski-Jödicke and H. P. Lüthi, J. Chem. Phys., 2002, 117, 4146–4156, DOI: 10.1063/1.1498817.
V. Cavillot and B. Champagne, Chem. Phys. Lett., 2002, 354, 449–457, DOI: 10.1016/S0009-2614(02)00161-6.
C. Ravikumar, I. H. Joe and V. S. Jayakumar, Chem. Phys. Lett., 2008, 460, 552–558, DOI: 10.1016/j. cplett.2008.06.047.
R. Zhang, B. Du, G. Sun and Y. Sun, Spectrochim. Acta, Part A, 2010, 75, 1115–1124, DOI: 10.1016/j.saa.2009.12.067.
F. J. A. Ferrer, F. Santoro and R. Improta, Comput. Theor. Chem., 2014, 1040–1041, 186–194, DOI: 10.1016/j. comptc.2014.03.010.
N. Sekar, P. G. Umape, V. S. Padalkar, R. P. Tayade and P. Ramasami, J. Lumin., 2014, 150, 8–18, DOI: 10.1016/j. jlumin.2014.01.060.
H. Wang, J. Shi, et al., Spectrochim. Acta, Part A, 2013, 103, 62–67, DOI: 10.1016/j.saa.2012.10.075.
H. Wang, L. F. Chen, et al., Spectrochim. Acta, Part A, 2014, 121, 355–362, DOI: 10.1016/j.saa.2013.10.087.
P. Krawczyk, P. Czeleń and P. Cysewski, Org. Biomol. Chem., 2018, 16, 3788–3800, DOI: 10.1039/c8ob00729b.
M. J. Frisch, G. W. Trucks, G. B. Schlegel, et al., Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009.
C. Adamo, G. E. Scuseria and V. Barone, J. Chem. Phys., 1999, 111, 2889–2899, DOI: 10.1063/1.479571.
C. Guido and S. Caprasecca, 2016, https://www1.dcci.unipi.it/molecolab/tools/white-papers/pisalr/, DOI: 10.13140/RG.2.1.1903.7845.
J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868, DOI: 10.1103/PhysRevLett.77.3865.
J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396, DOI: 10.1103/PhysRevLett.78.1396.
T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57, DOI: 10.1016/j.cplett.2004.06.011.
J. Heyd and G. E. Scuseria, J. Chem. Phys., 2004, 120, 7274, DOI: 10.1063/1.1668634.
J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2006, 124, 219906, DOI: 10.1063/1.2204597.
C. Adamo and V. Barone, J. Chem. Phys., 1998, 108, 664–675, DOI: 10.1063/1.475428.
J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620, DOI: 10.1039/B810189B.
H. Iikura, T. Tsuneda, T. Yanai and K. Hirao, J. Chem. Phys., 2001, 115, 3540–3544, DOI: 10.1063/1.1383587.
O. A. Vydrov and G. E. Scuseria, J. Chem. Phys., 2006, 125, 234109–234109, DOI: 10.1063/1.2409292.
O. A. Vydrov, G. E. Scuseria and J. P. Perdew, J. Chem. Phys., 2007, 126, 1541009–1541009, DOI: 10.1063/1.2723119.
N. Minezawa, Chem. Phys. Lett., 2014, 608, 140–144, DOI: j.cplett.2014.05.104.
G. S. Ming Tong, K. T. Chan, X. Chang and Ch.-M. Che, Chem. Sci., 2015, 6, 3026–3037, DOI: 10.1039/c4sc03697b.
C. Guido and S. Caprasecca, https://www1.dcci.unipi.it/molecolab/tools/white-papers/pisalr/, DOI: 10.13140/RG.2.1.1903.7845.
L. V. Slipchenko, J. Phys. Chem. A, 2010, 114, 8824–8830, DOI: 10.1021/jp101797a.
K. Sneskov, T. Schwabe, O. Christiansen and J. Kongsted, Phys. Chem. Chem. Phys., 2011, 13, 18551–18560, DOI: 10.1039/C1CP22067E.
M. Caricato, J. Chem. Phys., 2013, 139, 044116, DOI: 10.1063/1.4816482.
T. Le Bahers, C. Adamo and I. Ciofini, J. Chem. Theory Comput., 2011, 7, 2498–2506, DOI: 10.1021/ct200308m.
M. T. Cancés, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041, DOI: 10.1063/1.474659.
M. Arivazhagan, P. Muniappan, R. Meenakshi and G. Rajavel, Spectrochim. Acta, Part A, 2013, 105, 497–508, DOI: 10.1016/j.saa.2012.11.033.
R. W. Boyd, in Nonlinear Optics, Academic, London, 2nd edn, 2003, p. 521.
D. P. Craig and T. Thirunamachandran, Molecular quantum electrodynamics: an introduction to radiation-molecule interaction, Dover Publications, Inc, Mineola, New York, 1st edn, 1998, ch. 5.
K. Ohta, L. Antonov, S. Yamada and K. Kamada, J. Chem. Phys., 2007, 127, 084504–084515, DOI: 10.1063/1.2753490.
R. Zaleśny, W. Bartkowiak, S. Styrcz and J. Leszczynski, J. Phys. Chem. A, 2002, 106, 4032–4037, DOI: 10.1021/jp0142684.
J. Olsen and P. Jorgensen, J. Chem. Phys., 1985, 82, 3235, DOI: 10.1063/1.448223.
P. Sałek, O. Vahtras, J. D. Guo, Y. Luo, T. Helgaker and H. Ågren, Chem. Phys. Lett., 2003, 374, 446–452, DOI: 10.1016/S0009-2614(03)00681-X.
DALTON A molecular electronic structure program. Release Dalton 2011, 2011, see http://daltonprogram.org/.
LSDALTON, A linear scaling molecular electronic structure program. Release Dalton 2011, 2011, see http://daltonpro-gram.org.
G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785–2791, DOI: 10.1002/jcc.21256.
S. Cosconati, S. Forli, A. L. Perryman, R. Harris, D. S. Goodsell and A. J. Olson, Expert Opin. Drug Discovery, 2010, 5, 597–607, DOI: 10.1517/17460441.2010.484460.
S. Forli and A. J. Olson, J. Med. Chem., 2012, 55, 623–638, DOI: 10.1021/jm2005145.
C. S. Panjikar, P. A. Tucker and M. S. Weiss, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2005, 61, 1263–1272, DOI: 10.2210/pdb2a7e/pdb.
O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461, DOI: 10.1002/jcc.21334.
V. Potemkin and M. Grishina, Drug Discovery Today, 2008, 13(21–22), 952–959, DOI: 10.1016/j.drudis.2008.07.006.
V. Potemkin and M. Grishina, J. Comput.-Aided Mol. Des., 2008, 22(6–7), 489–505, DOI: 10.1007/s10822-008-9203-x.
V. Potemkin, A. A. Pogrebnoy and M. A. Grishina, J. Chem. Inf. Model., 2009, 49(6), 1389–1406, DOI: 10.1021/ci800405n.
G. Snatzke, Angew. Chem., Int. Ed. Engl., 1979, 18(5), 363–377, DOI: 10.1002/anie.197903631.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Molecular structure and spectroscopic properties for investigated compound. See DOI: 10.1039/c9pp00478e
Rights and permissions
About this article
Cite this article
Krawczyk, P. 4-(4-Chloro-2-oxo-3(1H-phenanthro[9,10-d] imidazol-2-yl)-2H-chromen-6-yl)benzaldehyde as a fluorescent probe for medical imaging: linear and nonlinear optical properties. Photochem Photobiol Sci 19, 473–484 (2020). https://doi.org/10.1039/c9pp00478e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00478e