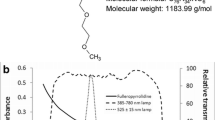
Abstract
Hypericin (Hyp) is one of the most effective, naturally occurring photodynamic agents, which proved effective against a wide array of microorganisms. One limitation of its large scale application as a disinfectant is the high production cost of the pure compound. The availability of photoactive materials at a lower cost may be highly beneficial to the actual implementation of photodisinfection also at the industrial level. In this work we report the use of a lyophilized extract from Hypericum perforatum as a photosensitizing material. We show that optical absorption in the green-red region of the visible spectrum of ethanol or DMSO solutions of the lyophilized extract contains bands arising from Hyp. When excited with light in the main Hyp absorption bands, fluorescence emission and triplet state formation occur as in pure Hyp solutions. We show that ethanol or DMSO solutions of the lyophilized extract from Hypericum perforatum are highly efficient photodynamic agents against Gram-positive Staphylococcus aureus, chosen as a model. The performance is indistinguishable from that of the pure compound. Using fluorescence microscopy, we demonstrate that upon incubation of S. aureus with lyophilized extract solutions, Hyp is found on the bacterial wall, as previously reported for the pure compound.
Similar content being viewed by others
References
J. Kadariya, T. C. Smith and D. Thapaliya, BioMed Res. Int., 2014, 2014, 9.
F. D. Lowy, N. Engl. J. Med., 1998, 339, 520–532.
Y. Ie Loir, F. Baron and M. Gautier, GMR, Genet. Mol. Res., 2003, 2, 63–76.
P. Chaibenjawong and S. J. Foster, Arch. Microbiol., 2011, 193, 125–135.
C. Cortimiglia, M. Luini, V. Bianchini, L. Marzagalli, F. Vezzoli, D. Avisani, M. Bertoletti, A. Ianzano, A. Franco and A. Battisti, Epidemiol. Infect., 2016, 144, 3046–3051.
T. Ronco, I. C. Klaas, M. Stegger, L. Svennesen, L. B. Astrup, M. Farre and K. Pedersen, Vet. Microbiol., 2018, 215, 35–42.
R. C. Neyra, J. A. Frisancho, J. L. Rinsky, C. Resnick, K. C. Carroll, A. M. Rule, T. Ross, Y. You, L. B. Price and E. K. Silbergeld, Environ. Health Perspect., 2014, 122, 471–477.
D. Sergelidis, T. Papadopoulos, D. Komodromos, E. Sergelidou, T. Lazou, M. Papagianni, A. Zdragas and A. Papa, Lett. Appl. Microbiol., 2015, 61, 498–503.
A. Marek, E. Pyzik, D. Stępień-Pyśniak, R. Urban-Chmiel and L. S. Jarosz, Curr. Microbiol., 2018, 75, 1256–1266.
D. Thomas, S. Chou, O. Dauwalder and G. Lina, Superantigens and Superallergens, 2007, 93, 24–41.
N. Balaban and A. Rasooly, Int. J. Food Microbiol., 2000, 61, 1–10.
D.-L. Hu and A. Nakane, Eur. J. Pharmacol., 2014, 722, 95–107.
G. K. Paterson, E. M. Harrison and M. A. Holmes, Trends Microbiol., 2014, 22, 42–47.
W. A. McGuinness, N. Malachowa and F. R. DeLeo, Yale J. Biol. Med., 2017, 90, 269–281.
M. Aires-de-Sousa, Clin. Microbiol. Infect., 2017, 23, 373–380.
H. W. Boucher, G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg and J. Bartlett, Clin. Infect. Dis., 2009, 48, 1–12.
M. Wainwright, T. Maisch, S. Nonell, K. Plaetzer, A. Almeida, G. P. Tegos and M. R. Hamblin, Lancet Infect. Dis., 2017, 17, e49–e55.
A. Darmanyan, L. Burel, D. Eloy and P. Jardon, J. Chim. Phys., 1994, 91, 1774–1785.
M. Roslaniec, H. Weitman, D. Freeman, Y. Mazur and B. Ehrenberg, J. Photochem. Photobiol., B, 2000, 57, 149–158.
A. Losi, Photochem. Photobiol., 1997, 65, 791–801.
J. M. Jacobson, L. Feinman, L. Liebes, N. Ostrow, V. Koslowski, A. Tobia, B. E. Cabana, D. H. Lee, J. Spritzler and A. M. Prince, Antimicrob. Agents Chemother., 2001, 45, 517–524.
A. Kubin, F. Wierrani, U. Burner, G. Alth and W. Grünberger, Curr. Pharm. Des., 2005, 11, 233–253.
K. Kairyte, S. Lapinskas, V. Gudelis and Z. Luksiene, J. Appl. Microbiol., 2012, 112, 1144–1151.
C. M. N. Yow, H. M. Tang, E. S. M. Chu and Z. Huang, Photochem. Photobiol., 2012, 88, 626–632.
N. Nafee, A. Youssef, H. El-Gowelli, H. Asem and S. Kandil, Int. J. Pharm., 2013, 454, 249–258.
J. Comas-Barceló, B. Rodríguez-Amigo, S. Abbruzzetti, P. d. Rey-Puech, M. Agut, S. Nonell and C. Viappiani, RSC Adv., 2013, 3, 17874–17879.
B. Rodríguez-Amigo, P. Delcanale, G. Rotger, J. Juárez Jiménez, S. Abbruzzetti, A. Summer, M. Agut, F. J. Luque S. Nonell and C. Viappiani, J. Dairy Scl., 2015, 98, 89–94.
P. Delcanale, F. Pennacchietti, G. Maestrini, B. Rodríguez-Amigo, P. Bianchini, A. Diaspro, A. Iagatti, B. Patrizi, P. Foggi, M. Agut, S. Nonell, S. Abbruzzetti and C. Viappiani, Sci. Rep., 2015, 5, 15564.
P. Delcanale, B. Rodríguez-Amigo, J. Juárez-Jiménez, J. Luque, S. Abbruzzetti, M. Agut, S. Nonell and C. Viappiani, J. Mater. Chem. B, 2017, 5, 1633–1641.
D. Pezzuoli, M. Cozzolino, C. Montali, L. Brancaleon, P. Bianchini, M. Zantedeschi, S. Bonardi, C. Viappiani and S. Abbruzzetti, Food Control, 2018, 94, 254–262.
A. Rezusta, P. López-Chicón, M. P. Paz-Cristobal, M. Alemany-Ribes, D. Royo-Díez, M. Agut, C. Semino, S. Nonell, M. J. Revillo, C. Aspiroz and Y. Gilaberte, Photochem. Photobiol., 2012, 88, 613–619.
P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, CA-Cancer J. Clin., 2011, 61, 250–281.
P. Agostinis, A. Vantieghema, W. Merlevede and P. A. M. deWitte, Int. J. Biochem. Cell Biol., 2002, 34, 221–241.
E. Buytaert, G. Callewaert, N. Hendrickx, L. Scorrano, D. Hartmann, L. Missiaen, J. R. Vandenheede, I. Heirman, J. Grooten and P. Agostinis, FASEB J., 2006, 20, 756–758.
A. Garg, D. Krysko, P. Vandenabeele and P. Agostinis, Cancer Immunol. Immunother., 2012, 61, 215–221.
A. Vantieghem, Z. Assefa, P. Vandenabeele, W. Declercq, S. Courtois, J. R. Vandenheede, W. Merlevede, P. de Witte and P. Agostinis, FEES Lett, 1998, 440, 19–24.
A. Karioti and A. R. Bilia, Int. J. Mol Sci., 2010, 11, 562–594.
H. Brockmann, M. N. Haschad, K. Maier and F. Pohl, Naturwissenschaften, 1939, 32, 550–555.
N. Duràn and P. S. Song, Photochem. Photobiol., 1986, 43, 677–680.
Z. Saddiqe, I. Naeem and A. Maimoona, J. Ethnopharmacol., 2010, 131, 511–521.
C. M. Schempp, K. Pelz, A. Wittmer, E. Schöpf and J. C. Simon, Lancet, 1999, 353, 2129.
S. S. Chatterjee, S. K. Bhattacharya, M. Wonnemann, A. Singer and W. E. Müller, Life Scl, 1998, 63, 499–510.
J. Greeson, B. Sanford and D. Monti, Psychopharmacology, 2001, 153, 402–414.
H. Brockmann, F. Kluge and H. Muxfeldt, Chem. Ber., 1957, 90, 2302–2318.
H. Falk, J. Meyer and M. Oberreiter, Monatsh. Chem., 1993, 124, 339–341.
L.-F. Huang, Z.-H. Wang and S.-L. Chen, Chin. J. Nat. Medicines, 2014, 12, 81–88.
K. Aponiene, E. Paskeviciute, I. Reklaitis and Z. Luksiene, J. FoodEng., 2015, 144, 29–35.
Z. Luksiene and L. Brovko, Food Eng. Rev., 2013, 5, 185–199.
A. Nahrstedt and V. Butterweck, Pharmacopsychiatry, 1997, 30, 129–134.
A. L. Vandenbogaerde, A. Kamuhabwa, E. Delaey, B. E. Himpens, W. J. Merlevede and P. A. de Witte, J. Photochem. Photobiol., B, 1998, 45, 87–94.
A. RossiFanelli, E. Antonini and A. Caputo, Biochim. Biophys. Acta, 1958, 30, 608–615.
S. Abbruzzetti, E. Crema, L. Masino, A. Vecli, C. Viappiani, J. R. Small, L. J. Libertini and E. W. Small, Biophys. J., 2000, 78, 405–415.
S. Abbruzzetti, S. Bruno, S. Faggiano, E. Grandi, A. Mozzarelli and C. Viappiani, Photochem. Photobiol Scl, 2006, 5, 1109–1120.
T. N. Demidova and M. R. Hamblin, App.l Environ. Microbiol., 2005, 71, 6918–6925.
P. López-Chicón, M. P. Paz-Cristobal, A. Rezusta, C. Aspiroz, M. Royo-Canas, E. Andres-Ciriano, Y. Gilaberte, M. Agut and S. Nonell, Photochem. Photobiol Scl, 2012, 11, 1099–1107.
D. S. English, K. Das, J. M. Zenner, W. Zhang, G. A. Kraus, R. C. Larock and J. W. Petrich, J. Phys. Chem. A, 1997, 101, 3235–3240.
a. G. H. Krause and E. Weis, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1991, 42, 313–349.
T. Yamazaki, N. Ohta, I. Yamazaki and P. S. Song, J. Phys. Chem., 1993, 97, 7870–7875.
P. Bianchini, M. Cozzolino, M. Oneto, L. Pesce, F. Pennacchietti, M. Tognolini, C. Giorgio, S. Nonell, L. Cavanna, P. Delcanale, S. Abbruzzetti, A. Diaspro and C. Viappiani, Biomacromolecules, 2019, 20, 2024–2033.
L. A. Schmitt, Y. Liu, P. A. Murphy, J. W. Petrich, P. M. Dixon and D. F. Birt, J. Photochem. Photobiol., B, 2006, 85, 118–130.
C. Cecchini, A. CreSci, M. M. Coman, M. Ricciutelli, G. Sagratini, S. Vittori, D. Lucarini and F. Maggi, Planta Med., 2007, 73, 564–566.
Author information
Authors and Affiliations
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00428a
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Delcanale, P., Hally, C., Nonell, S. et al. Photodynamic action of Hypericum perforatum hydrophilic extract against Staphylococcus aureus. Photochem Photobiol Sci 19, 324–331 (2020). https://doi.org/10.1039/c9pp00428a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00428a