Abstract
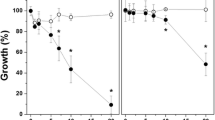
The extensive and repetitive use of antifungal drugs has led to the development of drug-resistant Candida albicans. Antimicrobial photodynamic therapy (aPDT) has received considerable attention as an emerging and promising approach to combat drug-resistant microbes. This study evaluated the photodynamic effects mediated by aloe emodin (AE), a natural compound isolated from Aloe vera and Rheum palmatum, on azole-sensitive and azole-resistant C. albicans in vitro. AE exhibited no significant dark toxicity, but in the presence of light, effectively inactivated C. albicans cells in a concentration-dependent manner. The uptake of AE by fungal cells was investigated by confocal laser scanning microscopy (CLSM), and the results showed that AE possessed stronger ability to enter into C. albicans cells following light irradiation. Transmission electron microscopy analysis suggested that AE-mediated aPDT could induce damage to the cell wall, cytoplasm, and nucleus. Damage to the surface of C. albicans was observed by scanning electron microscopy. These results suggest that AE is a potential PS for use in aPDT of drug-resistant C. albicans strains, and AE-mediated aPDT shows promise as an antifungal treatment.
Similar content being viewed by others
Notes and references
J. C. Sardi, L. Scorzoni, T. Bernardi, A. M. Fusco-Almeida and M. J. Mendes Giannini, Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options J. Med. Microbiol., 2013, 62, 10–24.
C. T. Chien, Y. C. Chen, Y. C. Liu, S. H. Liang, H. H. Lin and C. H. Lin, The antimicrobial photodynamic inactivation resistance of Candida albicans is modulated by the Hog1 pathway and the Cap1 transcription factor, Med. Mycol., 2018, 1–10.
M. G. Alvarez, M. N. Montes de Oca, M. E. Milanesio, C. S. Ortiz and E. N. Durantini, Photodynamic properties and photoinactivation of Candida albicans mediated by brominated derivatives of triarylmethane and phenothiazinium dyes Photodiagn. Photodyn. Ther., 2014, 11, 148–155.
M. P. Paz-Cristobal, D. Royo, A. Rezusta, E. Andres-Ciriano, M. C. Alejandre, J. F. Meis, M. J. Revillo, C. Aspiroz, S. Nonell and Y. Gilaberte, Photodynamic fungicidal efficacy of hypericin and dimethyl methylene blue against azole-resistant Candida albicans strains, Mycoses, 2014, 57, 35–42.
G. S. Barbério, S. V. da Costa, M. dos Santos Silva, T. M. de Oliveira, T. C. Silva, M. A. de and A. M. Machado, Photodynamic inactivation of Candida albicans mediated by a low density of light energy, Lasers Med. Sci., 2014, 29, 907–910.
I. B. Rosseti, L. R. Chagas and M. S. Costa, Photodynamic antimicrobial chemotherapy (PACT) inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability, Lasers Med. Sci., 2014, 29, 1059–1064.
M. R. Ke, J. M. Eastel, K. L. Ngai, Y. Y. Cheung, P. K. Chan, M. Hui, D. K. Ng and P. C. Lo, Photodynamic inactivation of bacteria and viruses using two monosubstituted zinc(II) phthalocyanines, Eur. J. Med. Chem., 2014, 84, 278–283.
T. G. St Denis, T. Dai, L. Izikson, C. Astrakas, R. R. Anderson, M. R. Hamblin and G. P. Tegos, All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease, Virulence, 2011, 2, 509–520.
A. Rineh, N. K. Dolla, A. R. Ball, M. Magana, J. B. Bremner, M. R. Hamblin, G. P. Tegos and M. J. Kelso, Attaching the NorA efflux pump inhibitor INF55 to methylene blue enhances antimicrobial photodynamic inactivation of methicillin-resistant Staphylococcus aureus in vitro and in vivo, ACS Infect. Dis., 2017, 3, 756–766.
M. Wainwright, Photodynamic antimicrobial chemotherapy (PACT), J. Antimicrob. Chemother., 1998, 42, 13–28.
N. Kashef and M. R. Hamblin, Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation?, Drug Resist. Updates, 2017, 31, 31–42.
C. Giuliani, B. Altieri, C. Bombelli, L. Galantini, G. Mancini and A. Stringaro, Remote loading of aloe emodin in gemini-based cationic liposomes, Langmuir, 2015, 31, 76–82.
K. Y. Lin and Y. H. Uen, Aloe-emodin, an anthraquinone, in vitro, inhibits proliferation and induces apoptosis in human colon carcinoma cells, Oncol. Lett., 2010, 1, 541–547.
H. D. Lin, K. T. Li, Q. Q. Duan, Q. Chen, S. Tian, E. S. M. Chu and D. Q. Bai, The effect of aloe-emodininduced photodynamic activity on the apoptosis of human gastric cancer cells: A pilot study, Oncol. Lett., 2017, 13, 3431–3436.
Y. Q. Liu, P. S. Meng, H. C. Zhang, X. Liu, M. X. Wang, W. W. Cao, Z. Hu and Z. G. Zhang, Inhibitory effect of aloe emodin mediated photodynamic therapy on humanoral mucosa carcinoma in vitro and in vivo, Biomed. Pharmacother., 2018, 97, 697–707.
P. Tu, Q. Huang, Y. Ou, X. Du, K. Li, Y. Tao and H. Yin, Aloe-emodin-mediated photodynamic therapy induces autophagy and apoptosis in human osteosarcoma cell line MG-63 through the ROS/JNK signaling pathway, Oncol. Rep., 2016, 35, 3209–3215.
M. H. Gold, Photodynamic therapy in dermatology, Springer, 2004.
P. Grosjean, G. Wagnieres, C. Fontolliet, H. van den Bergh and P. Monnier, Clinical Photodynamic Therapy for Superficial Cancer in the Oesophagus and the Bronchi: 514 Nm Compared with 630 Nm Light Irradiation After Sensitization with Photofrin II, Br. J. Cancer, 1998, 77, 1989–1995.
P. Monnier, M. Savary, C. Fontolliet, G. Wagnieres, A. Chatelain, P. Cornaz, C. Depeursinge and H. V. D. Bergh, Photodetection and Photodynamic Therapy of ‘Early’ Squamous Cell Carcinomas of the Pharynx, Oesophagus and Tracheo-Bronchial Tree, Lasers Med. Sci., 1990, 5, 149–169.
K. Morimoto, T. Ozawa, K. Awazu, N. Ito, N. Honda, S. Matsumoto and D. Tsuruta, Photodynamic Therapy Using Systemic Administration of 5-Aminolevulinic Acid and a 410 nm Wavelength light-emitting Diode for Methicillin-Resistant Staphylococcus aureus-Infected Ulcers in Mice, PLoS One, 2014, 9, e105173.
L. Zang, H. Zhao, X. Ji, W. Cao, Z. Zhang and P. Meng, Photophysical properties, singlet oxygen generation efficiency and cytotoxic effects of aloe emodin as a blue light photosensitizer for photodynamic therapy in dermatological treatment, Photochem. Photobiol. Sci., 2017, 16, 1088–1094.
H. Z. Lee, W. H. Yang, M. J. Hour, C. Y. Wu, W. H. Peng, B. Y. Bao, P. H. Han and D. T. Bau, Photodynamic activity of aloe-emodin induces resensitization of lung cancer cells to anoikis, Eur. J. Pharmacol., 2010, 648, 50–58.
G. Monfrecola, E. M. Procaccini, M. Bevilacqua, A. Manco, G. Calabro and P. Santoianni, In vitro effect of 5-aminolaevulinic acid plus visible light on Candida albicans, Photochem. Photobiol. Sci., 2004, 3, 419–422.
R. C. Souza, J. C. Junqueira, R. D. Rossoni, C. A. Pereira, E. Munin and A. O. Jorge, Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite red and low-power laser irradiation alone against Candida albicans, Lasers Med. Sci., 2010, 25, 385–389.
G. S. Barberio, S. V. da Costa, M. dos Santos Silva, T. M. de Oliveira, T. C. Silva and M. A. de Andrade Moreira Machado, Photodynamic inactivation of Candida albicans mediated by a low density of light energy, Lasers. Med. Sci., 2014, 29, 907–910.
F. Freire, A. C. Costa, C. A. Pereira, M. Beltrame Junior, J. C. Junqueira and A. O. Jorge, Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans, Lasers Med. Sci., 2014, 29, 949–955.
S. Beirao, S. Fernandes, J. Coelho, M. A. Faustino, J. P. Tome, M. G. Neves, A. C. Tome, A. Almeida and A. Cunha, Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin, Photochem. Photobiol., 2014, 90, 1387–1396.
M. A. Di Palma, M. G. Alvarez, A. L. Ochoa, M. E. Milanesio and E. N. Durantini, Optimization of cellular uptake of zinc(II) 2, 9, 16, 23-tetrakis [4-(N-methylpyridyloxy)] phthalocyanine for maximal photoinactivation of Candida albicans, Fungal Biol., 2013, 117, 744–751.
T. S. Mang, L. Mikulski and R. E. Hall, Photodynamic inactivation of normal and antifungal resistant Candida species, Photodiagn. Photodyn. Ther., 2010, 7, 98–105.
J. Cabrini Carmello, F. Alves, F. G. Basso, C. A. de Souza Costa, A. C. Tedesco, F. Lucas Prinmo, E. G. de, O. Mima and A. C. Pavarina, Antimicrobial photodynamic therapy reduces adhesion capacity and biofilm formation of Candida albicans from induced oral candidiasis in mice Photodiagn. Photodyn. Ther., 2019, 27, 402–407.
R. M. Machado-de-Sena, L. Correa, I. T. Kato, R. A. Prates, A. M. Senna, C. C. Santos, D. A. Picanco and M. S. Ribeiro, Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis Photodiagn. Photodyn. Ther., 2014, 11, 275–282.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, W., Liu, C., Li, J. et al. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem Photobiol Sci 19, 485–494 (2020). https://doi.org/10.1039/c9pp00352e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00352e