Abstract
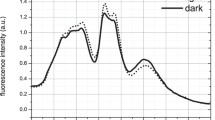
The light-driven conversions between the dark-adapted and the photoproduct state were recorded for bacteriophytochromes (BphP) carrying biliverdin IXα (BV) as chromophore by time-resolved absorption spectroscopy. BphPs can be photoswitched between a red absorbing (Pr, maximum at ca. 700 nm) and a far-red/near-infrared (Pfr, maximum at ca. 750 nm) absorbing state, thereby showing a considerable red-shift with respect to plant phytochromes. Representatives for BphPs studied here are: PstBphP1 from Pseudomonas syringae pv. tomato, for which Pfr is the photoproduct; the bathy-phytochrome PaBphP from Pseudomonas aeruginosa for which instead Pfr is the thermally stable parental state. The third BphP-like protein was FphA from the fungus Aspergillus nidulans, a eukaryotic protein also carrying BV as a chromophore, for which Pr is considered to be the dark-adapted state. All three BphPs show a canonical modular arrangement with a three-domain photosensory module (PAS-GAF-PHY) and a histidine-kinase (HK) signalling domain. The quantum yields for Pr-to-Pfr photoconversion are in the range 0.02–0.12, and 0.04–0.08 for the Pfr-to-Pr route. Photoproducts of both bacterial phytochromes thermally recovered in the dark, whereas for the fungal protein (FphA) both Pr and Pfr forms are thermally stable for days and could be interconverted only by selective irradiation. The photoinduced reactions of all three BV-phytochromes are in general kinetically less complex than those of plant phytochromes, with the notable exception of the Pr-to-Pfr route for PstBphP1. By contrast in the Pfr-to-Pr conversion of FphAN753 the final product is already formed during the very early steps of the process, without formation of any further intermediates: to our knowledge it is the first phytochrome showing this behavior. All three proteins investigated are weakly fluorescent in the Pr form, with a maximum fluorescence quantum yield of 0.02 (PaBphP), and have undetectable fluorescence in the Pfr state.
Similar content being viewed by others
Notes and references
K. Anders and L.-O. Essen, The family of phytochrome-like photoreceptors: diverse, complex and multi-colored, but very useful, Curr. Opin. Struct. Biol., 2015, 35, 7–16.
S. Nagano, From photon to signal in phytochromes: similarities and differences between prokaryotic and plant phytochromes, J. Plant Res., 2016, 129, 123–135.
C. Mandalari, A. Losi and W. Gärtner, Distance-tree analysis, distribution and co-presence of bilin- and flavinbinding prokaryotic photoreceptors for visible light, Photochem. Photobiol. Sci., 2013, 12, 1144–1157.
A. L. Mitchell, T. K. Attwood, P. C. Babbitt, M. Blum, P. Bork, A. Bridge, S. D. Brown, H.-Y. Chang, S. El-Gebali, M. I. Fraser, J. Gough, D. R. Haft, H. Huang, I. Letunic, R. Lopez, A. Luciani, F. Madeira, A. Marchler-Bauer, H. Mi, D. A. Natale, M. Necci, G. Nuka, C. Orengo, A. P. Pandurangan, T. Paysan-Lafosse, S. Pesseat, S. C. Potter, M. A. Qureshi, N. D. Rawlings, N. Redaschi, L. J. Richardson, C. Rivoire, G. A. Salazar, A. Sangrador- Vegas, C. J. A. Sigrist, I. Sillitoe, G. G. Sutton, N. Thanki, P. D. Thomas, S. C. E. Tosatto, S.-Y. Yong and R. D. Finn, InterPro in 2019: improving coverage, classification and access to protein sequence annotations, Nucleic Acids Res., 2019, 47, D351–D360.
K. Fushimi and R. Narikawa, Cyanobacteriochromes: photoreceptors covering the entire UV-to-visible spectrum, Curr. Opin. Struct. Biol., 2019, 57, 39–46.
G. Gourinchas, S. Etzl and A. Winkler, Bacteriophytochromes –from informative model systems of phytochrome function to powerful tools in cell biology, Curr. Opin. Struct. Biol., 2019, 57, 72–83.
M. G. Müller, I. Lindner, I. Martin, W. Gärtner and A. R. Holzwarth, Femtosecond Kinetics of Photoconversion of the Higher Plant Photoreceptor Phytochrome Carrying Native and Modified Chromophores, Biophys. J., 2008, 94, 4370–4382.
J. A. Ihalainen, H. Takala and H. Lehtivuori, Fast Photochemistry of Prototypical Phytochromes-A Species vs. Subunit Specific Comparison, Front. Mol. Biosci., 2015, 2, 75.
A. Björling, O. Berntsson, H. Lehtivuori, H. Takala, A. J. Hughes, M. Panman, M. Hoernke, S. Niebling, L. Henry, R. Henning, I. Kosheleva, V. Chukharev, N. V. Tkachenko, A. Menzel, G. Newby, D. Khakhulin, M. Wulff, J. A. Ihalainen and S. Westenhoff, Structural photoactivation of a full-length bacterial phytochrome, Sci. Adv., 2016, 2, e1600920.
D. J. Heyes, S. J. O. Hardman, M. N. Pedersen, J. Woodhouse, E. De La Mora, M. Wulff, M. Weik, M. Cammarata, N. S. Scrutton and G. Schirò, Light-induced structural changes in a full-length cyanobacterial phytochrome probed by time-resolved X-ray scattering, Commun. Biol., 2019, 2, 1.
I. Chizhov, B. Zorn, D. J. Manstein and W. Gärtner, Kinetic and Thermodynamic Analysis of the Light-induced Processes in Plant and Cyanobacterial Phytochromes, Biophys. J., 2013, 105, 2210–2220.
A. Remberg, I. Lindner, T. Lamparter, J. Hughes, C. Kneip, P. Hildebrandt, S. E. Braslavsky, W. Gärtner and K. Schaffner, Raman Spectroscopic and Light-Induced Kinetic Characterization of a Recombinant Phytochrome of the Cyanobacterium Synechocystis, Biochemistry, 1997, 36, 13389–13395.
S. E. Braslavsky, W. Gärtner and K. Schaffner, Phytochrome photoconversion, Plant, Cell Environ., 1997, 20, 700–706.
J. J. van Thor, B. Borucki, W. Crielaard, H. Otto, T. Lamparter, J. Hughes, K. J. Hellingwerf and M. P. Heyn, Light-induced proton release and proton uptake reactions in the cyanobacterial phytochrome Cph1., Biochemistry, 2001, 40, 11460–11471.
B. Borucki, D. von Stetten, S. Seibeck, T. Lamparter, N. Michael, M. A. Mroginski, H. Otto, D. H. Murgida, M. P. Heyn and P. Hildebrandt, Light-induced Proton Release of Phytochrome Is Coupled to the Transient Deprotonation of the Tetrapyrrole Chromophore, J. Biol. Chem., 2005, 280, 34358–34364.
B. Borucki, S. Seibeck, M. P. Heyn and T. Lamparter, Characterization of the Covalent and Noncovalent Adducts of Agp1 Phytochrome Assembled with Biliverdin and Phycocyanobilin by Circular Dichroism and Flash Photolysis, Biochemistry, 2009, 48, 6305–6317.
B. Zienicke, I. Molina, R. Glenz, P. Singer, D. Ehmer, F. V. Escobar, P. Hildebrandt, R. Diller and T. Lamparter, Unusual spectral properties of bacteriophytochrome Agp2 result from a deprotonation of the chromophore in the redabsorbing form Pr., J. Biol. Chem., 2013, 288, 31738–31751.
F. Velazquez Escobar, D. von Stetten, M. Günther-Lütkens, A. Keidel, N. Michael, T. Lamparter, L.-O. Essen, J. Hughes, W. Gärtner, Y. Yang, K. Heyne, M. A. Mroginski and P. Hildebrandt, Conformational heterogeneity of the Pfr chromophore in plant and cyanobacterial phytochromes, Front. Mol. Biosci., 2015, 2, 37.
F. Velazquez Escobar, P. Piwowarski, J. Salewski, N. Michael, M. Fernandez Lopez, A. Rupp, B. M. Qureshi, P. Scheerer, F. Bartl, N. Frankenberg-Dinkel, F. Siebert, M. Andrea Mroginski and P. Hildebrandt, A protonationcoupled feedback mechanism controls the signalling process in bathy phytochromes, Nat. Chem., 2015, 7, 423–430.
S.-H. Bhoo, S. J. Davis, J. Walker, B. Karniol and R. D. Vierstra, Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore, Nature, 2001, 414, 776–779.
S. I. Beale, Biosynthesis of phycobilins, Chem. Rev., 1993, 93, 785–802.
M. E. Auldridge and K. T. Forest, Bacterial phytochromes: More than meets the light, Crit. Rev. Biochem. Mol. Biol., 2011, 46, 67–88.
J. R. Wagner, J. S. Brunzelle, K. T. Forest and R. D. Vierstra, A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome, Nature, 2005, 438, 325–331.
T. Lamparter, N. Krauß and P. Scheerer, Phytochromes from Agrobacterium fabrum, Photochem. Photobiol., 2017, 93, 642–655.
D. Buhrke, U. Kuhlmann, N. Michael and P. Hildebrandt, The Photoconversion of Phytochrome Includes an Unproductive Shunt Reaction Pathway, ChemPhysChem, 2018, 19, 566–570.
G. A. Beattie, B. M. Hatfield, H. Dong and R. S. McGrane, Seeing the Light: The Roles of Red- and Blue-Light Sensing in Plant Microbes, Annu. Rev. Phytopathol., 2018, 56, 41–66.
K. G. Chernov, T. A. Redchuk, E. S. Omelina and V. V. Verkhusha, Near-Infrared Fluorescent Proteins, Biosensors, and Optogenetic Tools Engineered from Phytochromes, Chem. Rev., 2017, 117, 6423–6446.
R. Tasler, T. Moises and N. Frankenberg-Dinkel, Biochemical and spectroscopic characterization of the bacterial phytochrome of Pseudomonas aeruginosa, FEBS J., 2005, 272, 1927–1936.
R. Shah, J. Schwach, N. Frankenberg-Dinkel and W. Gärtner, Complex formation between heme oxygenase and phytochrome during biosynthesis in Pseudomonas syringae pv. tomato, Photochem. Photobiol. Sci., 2012, 11, 1026.
S. Brandt, D. von Stetten, M. Günther, P. Hildebrandt and N. Frankenberg-Dinkel, The fungal phytochrome FphA from Aspergillus nidulans., J. Biol. Chem., 2008, 283, 34605–34614.
J. Mailliet, G. Psakis, K. Feilke, V. Sineshchekov, L.-O. Essen and J. Hughes, Spectroscopy and a High-Resolution Crystal Structure of Tyr263 Mutants of Cyanobacterial Phytochrome Cph1, J. Mol. Biol., 2011, 413, 115–127.
F. Pennacchietti, A. Losi, X.-L. Xu, K.-H. Zhao, W. Gärtner, C. Viappiani, F. Cella, A. Diaspro and S. Abbruzzetti, Photochromic conversion in a red/green cyanobacteriochrome from Synechocystis PCC6803: Quantum yields in solution and photoswitching dynamics in living E. coli cells, Photochem. Photobiol. Sci., 2015, 14, 229–237.
A. Losi, H. R. Bonomi, N. Michael, K. Tang and K.-H. Zhao, Time-Resolved Energetics of Photoprocesses in Prokaryotic Phytochrome-Related Photoreceptors, Photochem. Photobiol., 2017, 93, 733–740.
J. M. Beechem, Global analysis of biochemical and biophysical data, Methods Enzymol., 1992, 210, 37–54.
I. Chizhov, D. S. Chernavskii, M. Engelhard, K. H. Mueller, B. V. Zubov and B. Hess, Spectrally silent transitions in the bacteriorhodopsin photocycle, Biophys. J., 1996, 71, 2329–2345.
T. Lamparter, F. Mittmann, W. Gärtner, T. Börner, E. Hartmann and J. Hughes, Characterization of recombinant phytochrome from the cyanobacterium Synechocystis, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 11792–11797.
L. H. Pratt, Photochemistry of high molecular weight phytochrome in vitro, Photochem. Photobiol., 1975, 22, 33–36.
L. H. Otero, S. Klinke, J. Rinaldi, F. Velázquez-Escobar, M. A. Mroginski, M. Fernández López, F. Malamud, A. A. Vojnov, P. Hildebrandt, F. A. Goldbaum and H. R. Bonomi, Structure of the Full-Length Bacteriophytochrome from the Plant Pathogen Xanthomonas campestris Provides Clues to its Long-Range Signaling Mechanism, J. Mol. Biol., 2016, 428, 3702–3720.
C. Schumann, R. Groß, N. Michael, T. Lamparter and R. Diller, Sub-Picosecond Mid-Infrared Spectroscopy of Phytochrome Agp1 fromAgrobacterium tumefaciens, ChemPhysChem, 2007, 8, 1657–1663.
C. Schumann, R. Gross, M. M. N. Wolf, R. Diller, N. Michael and T. Lamparter, Subpicosecond midinfrared spectroscopy of the Pfr reaction of phytochrome Agp1 from Agrobacterium tumefaciens., Biophys. J., 2008, 94, 3189–3197.
H. Lehtivuori, I. Rissanen, H. Takala, J. Bamford, N. V. Tkachenko and J. A. Ihalainen, Fluorescence Properties of the Chromophore-Binding Domain of Bacteriophytochrome from Deinococcus radiodurans, J. Phys. Chem. B, 2013, 117, 11049–11057.
P. Eilfeld and W. Rüdiger, Absorption Spectra of Phytochrome Intermediates, Z. Naturforsch., C: J. Biosci., 1985, 40, 109–114.
J. R. Wagner, J. Zhang, D. von Stetten, M. Günther, D. H. Murgida, M. A. Mroginski, J. M. Walker, K. T. Forest, P. Hildebrandt and R. D. Vierstra, Mutational Analysis of Deinococcus radiodurans Bacteriophytochrome Reveals Key Amino Acids Necessary for the Photochromicity and Proton Exchange Cycle of Phytochromes, J. Biol. Chem., 2008, 283, 12212–12226.
J. J. van Thor, B. Borucki, W. Crielaard, H. Otto, T. Lamparter, J. Hughes, K. J. Hellingwerf and M. P. Heyn, Light-induced proton release and proton uptake reactions in the cyanobacterial phytochrome Cph1., Biochemistry, 2001, 40, 11460–11471.
M. Biasini, S. Bienert, A. Waterhouse, K. Arnold, G. Studer, T. Schmidt, F. Kiefer, T. Gallo Cassarino, M. Bertoni, L. Bordoli and T. Schwede, SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information., Nucleic Acids Res., 2014, 42, W252–W258.
S. Altschul, T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller and D. J. Lipman, Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res., 1997, 25, 3389–3402.
L.-O. Essen, J. Mailliet and J. Hughes, The structure of a complete phytochrome sensory module in the Pr ground state., Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14709–14714.
H. Takala, A. Björling, O. Berntsson, H. Lehtivuori, S. Niebling, M. Hoernke, I. Kosheleva, R. Henning, A. Menzel, J. A. Ihalainen and S. Westenhoff, Signal amplification and transduction in phytochrome photosensors., Nature, 2014, 509, 245–248.
H. Takala, H. K. Lehtivuori, O. Berntsson, A. Hughes, R. Nanekar, S. Niebling, M. Panman, L. Henry, A. Menzel, S. Westenhoff and J. A. Ihalainen, On the (un)coupling of the chromophore, tongue interactions, and overall conformation in a bacterial phytochrome, J. Biol. Chem., 2018, 293, 8161–8172.
A. T. Ulijasz and R. D. Vierstra, Phytochrome structure and photochemistry: recent advances toward a complete molecular picture, Curr. Opin. Plant Biol., 2011, 14, 498–506.
X. Yang, J. Kuk and K. Moffat, Conformational differences between the Pfr and Pr states in Pseudomonas aeruginosa bacteriophytochrome., Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15639–15644.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/ c9pp00264b
Present affiliation of L. S.: Department of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany.
Rights and permissions
About this article
Cite this article
Consiglieri, E., Gutt, A., Gärtner, W. et al. Dynamics and efficiency of photoswitching in biliverdin-binding phytochromes†. Photochem Photobiol Sci 18, 2484–2496 (2019). https://doi.org/10.1039/c9pp00264b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00264b