Abstract
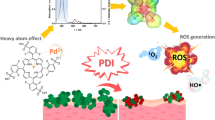
Photodynamic therapy (PDT) is a promising alternative approach particularly attractive for treatment of localized fungal infections. It is based on compounds, photosensitizers (PSs), which when excited with visible light, generate reactive species that ultimately cause cell death. Such species have short lifespans; as a consequence, efficiency and selectivity of the PDT treatment depend mainly on the properties of the PSs. This study is the first to explore the effect of cationic porphyrin-based photosensitizers on Saccharomyces cerevisiae, a member of the fungus kingdom. The study investigates which properties of the PS are essential for efficient antifungal PDT. Cationic Zn(ii) meso-tetrakis(N-alkylpyridinium-2-yl)porphyrins (ZnP) with identical tetrapyrrole core and photo-physical properties, but with different substituents at the meso positions of the porphyrin ring were studied. Attaching six-carbon aliphatic chains to the four pyridyl nitrogens at all meso positions to the porphyrin ring produced a highly photo-efficient amphiphilic, water soluble PS, with minimal dark toxicity. It was taken up by the yeast cells and upon illumination suppressed metabolism by inactivating cytoplasmic and mitochondrial enzymes, and compromising plasma membrane barrier function. At low concentrations (up to 5 μM) the tetrahexyl derivative was a much more powerful antifungal agent than the commercially available chlorin e6. The more lipophilic tetraoctyl analog was also highly photo-efficient but displayed strong dark toxicity, presumably due to higher lipophilicity which might affect the lipid bilayer of membranes. Results presented here can assist the design of antifungal agents whose biological action depends on efficient and rapid uptake by the cells.
Similar content being viewed by others
References
M. E. Rodrigues, S. Silva, J. Azeredo, and M. Henriques, Novel strategies to fight Candida species infection, Crit. Rev. Microbiol., 2016, 42, 594–606.
M. R. Hamblin, Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes, Curr. Opin. Microbiol., 2016, 33, 67–73.
M. Wainwright, and K. B. Crossley, Photosensitising agents - Circumventing resistance and breaking down biofilms: A review, Int. Biodeterior. Biodegrad., 2004, 53, 119–126.
L. Benov, Photodynamic therapy: Current status and future directions, Med. Princ. Pract., 2015, 24, 14–28.
M. R. Hamblin, Antimicrobial Photodynamic Therapy and Photodynamic Inactivation, or Killing Bugs with Dyes and Light-A Symposium-in-Print, Photochem. Photobiol., 2012, 88, 496–498.
M. R. Hamblin, and T. Hasan, Photodynamic therapy: A new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci., 2004, 3, 436–450.
M. Wainwright, In defence of ‘dye therapy’, Int. J. Antimicrob. Agents., 2014, 44, 26–29.
M. Wainwright, ‘Safe’ photoantimicrobials for skin and soft-tissue infections, Int. J. Antimicrob. Agents., 2010, 36, 14–18.
T. Dai, B. B. Fuchs, J. J. Coleman, R. A. Prates, C. Astrakas, T. G. St Denis, M. S. Ribeiro, E. Mylonakis, M. R. Hamblin, and G. P. Tegos, Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform, Front. Microbiol., 2012, 3, 120.
R. F. Donnelly, P. A. McCarron, and M. M. Tunney, Antifungal photodynamic therapy, Microbiol. Res., 2008, 163, 1–12.
E. B. Gralla, and J. S. Valentine, Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates, J. Bacteriol., 1991, 173, 5918–5920.
A. Tovmasyan, J. S. Reboucas, and L. Benov, Simple biological systems for assessing the activity of superoxide dismutase mimics, Antioxid. Redox Signaling., 2014, 20, 2416–2436.
M. M. Awad, A. Tovmasyan, J. D. Craik, I. Batinic-Haberle, and L. T. Benov, Important cellular targets for antimicrobial photodynamic therapy, Appl. Microbiol. Biotechnol., 2016, 100, 7679–7688.
K. Alenezi, A. Tovmasyan, I. Batinic-Haberle, and L. T. Benov, Optimizing Zn porphyrin-based photosensitizers for efficient antibacterial photodynamic therapy, Photodiagn. Photodyn. Ther., 2017, 17, 154–159.
R. Ezzeddine, A. Al-Banaw, A. Tovmasyan, J. D. Craik, I. Batinic-Haberle, and L. T. Benov, Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(II) N-alkylpyridylporphyrins, J. Biol. Chem., 2013, 288, 36579–36588.
A. M. Odeh, J. D. Craik, R. Ezzeddine, A. Tovmasyan, I. Batinic-Haberle, and L. T. Benov, Targeting mitochondria by Zn(II)NAlkylpyridylporphyrins: The impact of compound sub-mitochondrial partition on cell respiration and overall photodynamic efficacy, PLoS One., 2014, 9, e108238.
M. Thomas, J. D. Craik, A. Tovmasyan, I. Batinic-Haberle, and L. T. Benov, Amphiphilic cationic Zn-porphyrins with high photodynamic antimicrobial activity, Future Microbiol., 2015, 10, 709–724.
T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods., 1983, 65, 55–63.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275.
I. Batinic-Haberle, and L. T. Benov, An SOD mimic protects NADP+-dependent isocitrate dehydrogenase against oxidative inactivation, Free Radical Res., 2008, 42, 618–624.
D. Deere, J. Shen, G. Vesey, P. Bell, P. Bissinger, and D. Veal, Flow cytometry and cell sorting for yeast viability assessment and cell selection, Yeast., 1998, 14, 147–160.
R. Yin, and M. R. Hamblin, Antimicrobial photosensitizers: Drug discovery under the spotlight, Curr. Med. Chem., 2015, 22, 2159–2185.
T. N. Demidova, and M. R. Hamblin, Effect of cell-photo sensitizer binding and cell density on microbial photoinactivation, Antimicrob. Agents Chemother., 2005, 49, 2329–2335.
G. Jori, C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and G. Roncucci, Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications, Lasers Surg. Med., 2006, 38, 468–481.
A. M. Li, J. Martins, A. Tovmasyan, J. S. Valentine, I. Batinic-Haberle, I. Spasojevic, and E. B. Gralla, Differential localization and potency of manganese porphyrin superoxide dismutase-mimicking compounds in Saccharomyces cerevisiae, Redox Biol., 2014, 3, 1–6.
S. Oriel, and Y. Nitzan, Mechanistic aspects of photoinactivation of Candida albicans by exogenous porphyrins, Photochem. Photobiol., 2012, 88, 604–612.
M. P. Cormick, M. G. Alvarez, M. Rovera, and E. N. Durantini, Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives, Eur. J. Med. Chem., 2009, 44, 1592–1599.
V. Contreras-Shannon, and L. McAlister-Henn, Influence of compartmental localization on the function of yeast NADP+-specific isocitrate dehydrogenases, Arch. Biochem. Biophys., 2004, 423, 235–246.
D. A. Al-Mutairi, J. D. Craik, I. Batinic-Haberle, and L. T. Benov, Photosensitizing action of isomeric zinc N-methylpyridyl porphyrins in human carcinoma cells, Free Radical Res., 2006, 40, 477–483.
I. Batinić-Haberle, I. Spasojević, R. D. Stevens, P. Hambright, P. Neta, A. Okado-Matsumoto, and I. Fridovich, New class of potent catalysts of O2.- Dismutation. Mn(III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethyl- imidazolylporphyrins, Dalton Trans., 2004, 1696–1702.
I. Batinić-Haberle, I. Spasojevic, R. D. Stevens, P. Hambright, and I. Fridovich, Manganese(III) meso-tetrakis(ortho-Nalkylpyridyl) porphyrins. Synthesis, characterization, and catalysis of O2.- dismutation, J. Chem. Soc., Dalton Trans., 2002, 2689–2696.
T. N. Demidova, and M. R. Hamblin, Photodynamic therapy targeted to pathogens, Int. J. Immunopathol. Pharmacol., 2004, 17, 245–254.
Acknowledgments
This work was supported by grants YM03/13 and SRUL02/13 from Kuwait University. The authors are grateful to Milini Thomas for excellent technical assistance and to Dr Edith Gralla, University of California at Los Angeles for the yeast strain used in this study. IBH and AT are thankful for IBH general research funds. IBH is thankful to the Department of Radiation Oncology for the support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghnie, S., Tovmasyan, A., Craik, J. et al. Cationic amphiphilic Zn-porphyrin with high antifungal photodynamic potency. Photochem Photobiol Sci 16, 1709–1716 (2017). https://doi.org/10.1039/c7pp00143f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00143f