Abstract
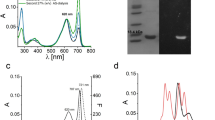
Cyanobacterial phycobilisomes funnel the harvested light energy to the reaction centers via two terminal emitters, allophycocyanin B and the core–membrane linker. ApcD is the a-subunit of allophycocyanin B responsible for its red-shifted absorbance (?max 665 nm). Far-red photo-acclimated cyanobacteria contain certain allophycocyanins that show even further red-shifted absorbances (?max > 700 nm). We studied the chromophorylation of the three far-red induced ApcD subunits ApcD2, ApcD3 and ApcD4 from Chroococcidiopsis thermalis sp. PCC7203 during the expression in E. coli. The complex behavior emphasizes that a variety of factors contribute to the spectral red-shift. Only ApcD2 bound phycocyanobilin covalently at the canonical position C81, while ApcD3 and ApcD4 gave only traces of stable products. The product of ApcD2 was, however, heterogeneous. The major fraction had a broad absorption around 560 nm and double-peaked fluorescence at 615 and 670 nm. A minor fraction was similar to the product of conventional ApcD, with maximal absorbance around 610 nm and fluorescence around 640 nm. The heterogeneity was lost in C65 and C132 variants; in these variants only the conventional product was formed. With ApcD4, a red-shifted product carrying non-covalently bound phycocyanobilin could be detected in the supernatant after cell lysis. While this chromophore was lost during purification, it could be stabilized by co-assembly with a far-red light-induced ß-subunit, ApcB3.
Similar content being viewed by others
References
A. N. Glazer, Phycobilisome–A macromolecular complex optimized for light energy transfer, Biochim. Biophys. Acta, 1984, 768, 29–51.
E. Gantt, B. Grabowski and F. X. Cunningham, in Light-harvesting antennas in photosynthesis, ed. B. Green and W. Parson, Kluwer, Dordrecht, 2003, pp. 307–322.
W. A. Sidler, in The molecular biology of cyanobacteria, ed. D. A. Bryant, Kluwer, Dordrecht, 1994, pp. 139–216.
A. Ducret, S. A. Müller, K. N. Goldie, A. Hefti, W. A. Sidler, H. Zuber and A. Engel, Reconstitution, characterisation and mass analysis of the pentacylindrical allophycocyanin core complex from the cyanobacterium Anabaena, sp. PCC 7120, J. Mol. Biol., 1998, 278, 369–388.
H. Liu, H. Zhang, D. M. Niedzwiedzki, M. Prado, G. He, M. L. Gross and R. E. Blankenship, Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria, Science, 2013, 342, 1104–1107.
L. Chang, X. Liu, Y. Li, C. C. Liu, F. Yang, J. Zhao and S. F. Sui, Structural organization of an intact phycobilisome and its association with photosystem II, Cell Res., 2015, 25, 726–737.
F. Gan, G. Shen and D. A. Bryant, Occurrence of Far-Red Light Photoacclimation (FaRLiP) in Diverse Cyanobacteria, Life, 2015, 5, 4–24.
F. Gan, S. Zhang, N. C. Rockwell, S. S. Martin, J. C. Lagarias, D. A. Bryant, Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light, Science, 2014, 345, 1312–1317.
M. Chen, M. Schliep, R. D. Willows, Z. L. Cai, B. A. Neilan and H. Scheer, A red-shifted chlorophyll, Science, 2010, 329, 1318–1319.
Y. Li, Y. Lin, C. J. Garvey, D. Birch, R. W. Corkery, P. C. Loughlin, H. Scheer, R. D. Willows and M. Chen, Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris, Biochim. Biophys. Acta, 2015, 1857, 107–114.
E. A. Rodriguez, G. N. Tran, L. A. Gross, J. L. Crisp, X. Shu, J. Y. Lin and R. Y. Tsien, A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein, Nat. Methods, 2016, 13, 763–769.
D. M. Shcherbakova, M. Baloban and V. V. Verkhusha, Near-infrared fluorescent proteins engineered from bacterial phytochromes, Curr. Opin. Chem. Biol., 2015, 27, 52–63.
X. L. Zeng, K. Tang, N. Zhou, M. Zhou, H. J. Hou, H. Scheer, K. H. Zhao and D. Noy, Bimodal intramolecular excitation energy transfer in a multichromophore photosynthetic model system: hybrid fusion proteins comprising natural phycobilin- and artificial chlorophyll-binding domains, J. Am. Chem. Soc., 2013, 135, 13479–13487.
D. Miao, W. L. Ding, B. Q. Zhao, L. Lu, Q. Z. Xu, H. Scheer and K. H. Zhao, Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC7335, Biochim. Biophys. Acta, 2016, 1857, 688–694.
Q. Z. Xu, J. X. Han, Q. Y. Tang, W. L. Ding, D. Miao, M. Zhou, H. Scheer and K. H. Zhao, Far-red light photoacclimation: Chromophorylation of FR induced alpha- and beta-subunits of allophycocyanin from Chroococcidiopsis thermalis sp. PCC7203, Biochim. Biophys. Acta, 2016, 1857, 1607–1616.
J. Sambrook, E. Fritsch and T. Maniatis, Molecular cloning: a laboratory manual, Cold Spring Harbour Laboratory Press, New York, 2nd edn, 1989.
K. H. Zhao, P. Su, J. Li, J. M. Tu, M. Zhou, C. Bubenzer and H. Scheer, Chromophore attachment to phycobiliprotein ß-subunits: phycocyanobilin:cysteine-ß84 phycobiliprotein lyase activity of CpeS-like protein from Anabaena sp. PCC7120, J. Biol. Chem., 2006, 281, 8573–8581.
K. H. Zhao, P. Su, J. M. Tu, X. Wang, H. Liu, M. Plöscher, L. Eichacker, B. Yang, M. Zhou and H. Scheer, Phycobilin:cystein-84 biliprotein lyase, a near-universal lyase for cysteine-84-binding sites in cyanobacterial phycobiliproteins, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14300–14305.
J. Zhang, X. J. Wu, Z. B. Wang, Y. Chen, X. Wang, M. Zhou, H. Scheer and K. H. Zhao, Fused-gene approach to photoswitchable and fluorescent biliproteins, Angew. Chem., Int. Ed., 2010, 49, 5456–5458.
M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254.
U. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, 227, 680–685.
T. Berkelman and J. C. Lagarias, Visualization of bilin-linked peptides and proteins in polyacrylamide gels, Anal. Biochem., 1986, 156, 194–201.
P. H. Brown and P. Schuck, Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation, Biophys. J., 2006, 90, 4651–4661.
A. N. Glazer and S. Fang, Chromophore content of blue-green algal phycobiliproteins, J. Biol. Chem., 1973, 248, 659–662.
Y. A. Cai, J. T. Murphy, G. J. Wedemayer and A. N. Glazer, Recombinant phycobiliproteins. Recombinant C-phycocyanins equipped with affinity tags, oligomerization, and biospecific recognition domains, Anal. Biochem., 2001, 290, 186–204.
R. MacColl, Allophycocyanin and energy transfer, Biochim. Biophys. Acta, 2004, 1657, 73–81.
A. Biswas, Y. M. Vasquez, T. M. Dragomani, M. L. Kronfel, S. R. Williams, R. M. Alvey, D. A. Bryant and W. M. Schluchter, Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002, Appl. Environ. Microbiol., 2010, 76, 2729–2739.
K. Tang, W.-L. Ding, A. Höppner, C. Zhao, L. Zhang, Y. Hontani, J. T. M. Kennis, W. Gärtner, H. Scheer, M. Zhou and K.-H. Zhao, The terminal phycobilisome emitter, LCM: a light-harvesting pigment with a phytochrome chromophore, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 15880–15885.
K. H. Zhao and H. Scheer, Type I and type II reversible photochemistry of phycoerythrocyanin a-subunit from Mastigocladus laminosus, both involve Z, E isomerization of phycoviolobilin chromophore and are controlled by sulfhydryls in apoprotein, Biochim. Biophys. Acta, Bioenerg., 1995, 1228, 244–253.
K. H. Zhao, R. Haessner, E. Cmiel and H. Scheer, Type I reversible of phycoerythrocyanin involves Z/E-isomerization of a-84 phycoviolobilin chromophore, Biochim. Biophys. Acta, 1995, 1228, 235–243.
N. C. Rockwell, S. S. Martin, A. G. Gulevich and J. C. Lagarias, Phycoviolobilin formation and spectral tuning in the DXCF cyanobacteriochrome subfamily, Biochemistry, 2012, 51, 1449–1463.
Q. Ma, H. H. Hua, Y. Chen, B. B. Liu, A. L. Kramer, H. Scheer, K. H. Zhao and M. Zhou, A rising tide of blue-absorbing biliprotein photoreceptors: characterization of seven such bilin-binding GAF domains in Nostoc, sp. PCC7120, FEBS J., 2012, 279, 4095–4108.
M. Ikeuchi and T. Ishizuka, Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria, Photochem. Photobiol. Sci., 2008, 7, 1159–1167.
P. P. Peng, L. L. Dong, Y. F. Sun, X. L. Zeng, W. L. Ding, H. Scheer, X. Yang and K. H. Zhao, The structure of allophycocyanin B from Synechocystis PCC 6803 reveals the structural basis for the extreme redshift of the terminal emitter in phycobilisomes, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, 70, 2558–2569.
A. Marx and N. Adir, Allophycocyanin and phycocyanin crystal structures reveal facets of phycobilisome assembly, Biochim. Biophys. Acta, 2013, 1827, 311–318.
J. Y. Liu, T. Jiang, J. P. Zhang and D. C. Liang, Crystal structure of allophycocyanin from red algae Porphyra yezoensis at 2.2-A resolution, J. Biol. Chem., 1999, 274, 16945–16952.
K. Brejc, R. Ficner, R. Huber and S. Steinbacher, Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution, J. Mol. Biol., 1995, 249, 424–440.
W. Reuter, G. Wiegand, R. Huber and M. E. Than, Structural analysis at 2.2 Å of orthorhombic crystals presents the asymmetry of the allophycocyanin-linker complex, AP.LC7.8, from phycobilisomes of Mastigocladus laminosus, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1363–1368.
M. Y. Ho, F. Gan, G. Shen and D. A. Bryant, Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II.Characterization of phycobiliproteins produced during acclimation to far-red light, Photosynth. Res., 2017, 131, 187–202.
N. Tandeau de Marsac and G. Cohen-bazire, Molecular composition of cyanobacterial phycobilisomes, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 1635–1639.
A. N. Glazer, Adaptive variations in Phycobilisome structure, Adv. Mol. Cell Biol., 1994, 10, 119–149.
N. C. Rockwell, D. Duanmu, S. S. Martin, C. Bachy, D. C. Price, D. Bhattacharya, A. Z. Worden and J. C. Lagarias, Eukaryotic algal phytochromes span the visible spectrum, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3871–3876.
C. Six, J. C. Thomas, L. Garczarek, M. Ostrowski, A. Dufresne, N. Blot, D. J. Scanlan and F. Partensky, Diversity and evolution of phycobilisomes in marine Synechococcus spp.: a comparative genomics study, Genome Biol., 2007, 8, R259.
A. Shukla, A. Biswas, N. Blot, F. Partensky, J. A. Karty, L. A. Hammad, L. Garczarek, A. Gutu, W. M. Schluchter and D. M. Kehoe, Phycoerythrin-specific bilin lyase-isomerase controls blue-green chromatic acclimation in marine Synechococcus, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 20136–20141.
N. Blot, X. J. Wu, J. C. Thomas, J. Zhang, L. Garczarek, S. Bohm, J. M. Tu, M. Zhou, M. Plöscher, L. Eichacker, F. Partensky, H. Scheer and K. H. Zhao, Phycourobilin in trichromatic phycocyanin from oceanic cyanobacteria is formed post-translationally by a phycoerythrobilin lyase-isomerase, J. Biol. Chem., 2009, 284, 9290–9298.
K. H. Zhao, M. G. Deng, M. Zheng, M. Zhou, A. Parbel, M. Storf, M. Meyer, B. Strohmann and H. Scheer, Novel activity of a phycobiliprotein lyase: both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon, FEBS Lett., 2000, 469, 9–13.
R. M. Alvey, A. Biswas, W. M. Schluchter and D. A. Bryant, Attachment of noncognate chromophores to CpcA of Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 by heterologous expression in Escherichia coli, Biochemistry, 2011, 50, 4890–4902.
C. D. Fairchild and A. N. Glazer, Oligomeric structure, enzyme kinetics, and substrate specificity of the phycocyanin alpha subunit phycocyanobilin lyase, J. Biol. Chem., 1994, 269, 8686–8694.
M. Storf, A. Parbel, M. Meyer, B. Strohmann, H. Scheer, M. G. Deng, M. Zheng, M. Zhou and K. H. Zhao, Chromophore attachment to biliproteins: specificity of PecE/PecF, a lyase-isomerase for the photoactive 31-cys-a84-phycoviolobilin chromophore of phycoerythrocyanin, Biochemistry, 2001, 40, 12444–12456.
A. J. Tooley, Y. A. Cai and A. N. Glazer, Biosynthesis of a fluorescent cyanobacterial C-phycocyanin holo-a subunit in a heterologous host, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 10560–10565.
H. Scheer and K. H. Zhao, Biliprotein maturation: the chromophore attachment, Mol. Microbiol., 2008, 68, 263–276.
W. M. Schluchter, G. Shen, R. M. Alvey, A. Biswas, N. A. Saunee, S. R. Williams, C. A. Mille and D. A. Bryant, Phycobiliprotein biosynthesis in cyanobacteria: structure and function of enzymes involved in post-translational modification, Adv. Exp. Med. Biol., 2010, 675, 211–228.
Author information
Authors and Affiliations
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c7pp00066a
Rights and permissions
About this article
Cite this article
Xu, QZ., Tang, QY., Han, JX. et al. Chromophorylation (in Escherichia coli) of allophycocyanin B subunits from far-red light acclimated Chroococcidiopsis thermalis sp. PCC7203. Photochem Photobiol Sci 16, 1153–1161 (2017). https://doi.org/10.1039/c7pp00066a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00066a