Abstract
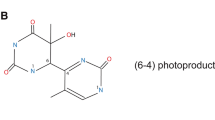
There is ample evidence demonstrating that solar ultraviolet light (UV) induces human skin cancers. First, epidemiological studies have demonstrated a negative correlation between the latitude of residence and incidence and mortality rates of both melanoma and non-melanoma skin cancers in homogeneous populations. Second, skin cancer can be produced in mice by UV irradiation; the action spectrum of photocarcinogenesis falls into UVB (280–320 nm). Third, patients with genetic disorders that lead to deficiencies in repairing UV-induced DNA damage are prone to develop cancers in sun-exposed areas of the skin. Photocarcinogenesis is a multistage process that involves initiation, promotion, and progression. In addition UV induced immunosuppression is closely involved in photocarcinogenesis. Accumulation of DNA lesions caused by UV in several cancer related genes plays a crucial role in carcinogenesis. Indeed, even in actinic keratosis, precancerous lesions, genetic alterations can be observed. A conventional knowledge demonstrated that UVB induced DNA lesion causes genetic mutation (initiation) and UVB-inflammation (sunburn) induces promotion. However recent findings revealed that the photocarcinogenesis pathway is more complex consequences where each of these processes, mediated by various cellular, biochemical, and molecular changes, are closely related to each other. The pyrimidine photoproducts that result from direct DNA damage induced by UV are involved in developing skin cancer through mutations that lead to the upregulation or downregulation of signal transduction pathways, cell cycle dysregulation, and depletion of antioxidant defenses. In addition pyrimidine dimers have been shown to trigger UV induced immunosuppression, which also plays an important role in photocarcinogenesis, partly by upregulation of IL-10, an immunosuppressive cytokine. UV also produces oxidative stress and oxidative DNA damage in skin cells, which cause alteration of the genes involved in the cell cycle, apoptosis and modification of cell signaling by redox regulation, resulting in inflammation. It has been shown that in Ogg1 knockout mice which are deficient in repairing 8-oxo-7, 8-dihydroguanine (8-oxoG), UVB irradiation up-regulates the inflammatory gene, implying that 8-oxoG is involved in triggering inflammation. In this review I summarize the state of the art knowledge regarding photocarcinogenesis including experimental data and implication for clinical viewpoints.
Similar content being viewed by others
References
B. K. Armstrong and A. Kricker, The epidemiology of UV induced skin cancer, J. Photochem. Photobiol., B, 2001, 63, 8–18.
M. Das, D. R. Bickers, R. M. Santella and H. Mukhtar, Altered patterns of cutaneous xenobiotic metabolism in UVB-induced squamous cell carcinoma in SKH-1 hairless mice, J. Invest. Dermatol., 1985, 84, 532–536.
C. Nishigori, M. Tanaka, S. Moriwaki, S. Imamura and H. Takebe, Accelerated appearance of skin tumors in hairless mice by repeated UV irradiation with initial intense exposure and characterization of the tumors, Jpn. J. Cancer Res., 1992, 83, 1172–1178.
C. Nishigori, S. Moriwaki, H. Takebe, T. Tanaka and S. Imamura, Gene alterations and clinical characteristics of xeroderma pigmentosum group A patients in Japan, Arch. Dermatol., 1994, 130, 191–197.
A. Ziegler, A. S. Jonason, D. J. Leffell, J. A. Simon, H. W. Sharma, J. Kimmelman, L. Remington, T. Jacks and D. E. Brash, Sunburn and p53 in the onset of skin cancer, Nature, 1994, 372, 773–776.
C. Nishigori, Y. Hattori and S. Toyokuni, Role of reactive oxygen species in skin carcinogenesis, Antioxid. Redox Signaling, 2004, 6, 561–570.
M. L. Kripke, Antigenicity of murine skin tumors induced by ultraviolet light, J. Natl. Cancer Inst., 1974, 53, 1333–1336.
C. Nishigori, D. B. Yarosh, S. E. Ullrich, A. A. Vink, C. D. Bucana, L. Roza and M. L. Kripke, Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10354–10359.
M. Kunisada, F. Yogianti, K. Sakumi, R. Ono, Y. Nakabeppu and C. Nishigori, Increased expression of versican in the inflammatory response to UVB- and reactive oxygen species-induced skin tumorigenesis, Am. J. Pathol., 2011, 179, 3056–3065.
E. Kvam and R. M. Tyrrell, Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation, Carcinogenesis, 1997, 18, 2379–2384.
M. J. Ellison and J. D. Childs, Pyrimidine dimers induced in Escherichia coli DNA by ultraviolet radiation present in sunlight, Photochem. Photobiol., 1981, 34, 465–469.
R. Ono, T. Masaki, S. Dien, X. Yu, A. Fukunaga, J. Yodoi and C. Nishigori, Suppressive effect of recombinant human thioredoxin on ultraviolet light-induced inflammation and apoptosis in murine skin, J. Dermatol., 2012, 39, 843–851.
M. Kunisada, K. Sakumi, Y. Tominaga, A. Budiyanto, M. Ueda, M. Ichihashi, Y. Nakabeppu and C. Nishigori, 8-Oxoguanine formation induced by chronic ultraviolet B exposure makes ogg1 knockout mice susceptible to skin carcinogenesis, Cancer Res., 2005, 65, 6006–6010.
K. Ito, S. Inoue, K. Yamamoto and S. Kawanishi, 8-Hydroxydeoxyguanosine formation at the 5′ site of 5′-GG-3′ sequences in double-stranded DNA by UV radiation with riboflavin, J. Biol. Chem., 1993, 268, 13221–13227.
A. Besaratinia, T. W. Synold, B. Xi and G. P. Pfeifer, G-to-T transversions and small tandem base deletions are the hallmark of mutations induced by ultraviolet A radiation in mammalian cells, Biochemistry, 2004, 43, 8169–8177.
E. A. Drobetsky, J. Turcotte and A. Châteauneuf, A role for ultraviolet A in solar mutagenesis, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 2350–2354.
T. Douki, A. Reynaud-Angelin, J. Cadet and E. Sage, Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation, Biochemistry, 2003, 43, 9221–9226.
S. Courdavault, C. Baudouin, M. Charveron, A. Favier, J. Cadet and T. Douki, Larger yield of cyclobutane dimers than 8-oxo-7,8-dihydroguanine in the DNA of UVA-irradiated human skin cells, Mutat. Res., 2004, 556, 135–142.
S. Mouret, C. Baudouin, M. Charveron, A. Favier, J. Cadet and T. Douki, Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13765–13770.
M. Kunisada, T. Masaki, R. Ono, H. Morinaga, E. Nakano, F. Yogianti, K. Okunishi, H. Sugiyama and C. Nishigori, Hydrochlorothiazide enhances UVA-induced DNA damage, Photochem. Photobiol., 2013, 89, 649–654.
F. de Gruijl, Action spectrum for photocarcinogenesis, Recent. Results, Cancer Res., 1995, 139, 21–30.
J. Miller, Mutagenic specificity of ultraviolet light, J. Mol. Biol., 1985, 182, 45–65.
W. E. Pierceall, L. H. Goldberg, M. A. Tainsky, T. Mukhopadhyay and H. N. Ananthaswamy, Ras gene mutation and amplification in human nonmelanoma skin cancers, Mol. Carcinog., 1991, 4, 196–202.
C. Nishigori, S. Wang, J. Miyakoshi, M. Sato, T. Tsukada, T. Yagi, S. Imamura and H. Takebe, Mutations in ras genes in cells cultured from mouse skin tumors induced by ultraviolet irradiation, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 7189–7193.
H. Takebe, C. Nishigori and Y. Satoh, Genetics and skin cancer of xeroderma pigmentosum in Japan, Jpn. J. Cancer Res., 1987, 78, 1135–1143.
M. Sato, C. Nishigori, M. Zghal, T. Yagi and H. Takebe, Ultraviolet specific mutations in p53 gene in skin tumors in xeroderma pigmentosum patients, Cancer Res., 1993, 53, 2944–2946.
A. Spatz, G. Giglia-Mari, S. Benhamou and A. Sarasin, Association between DNA repair-deficiency and high level of p53 mutations in melanoma of Xeroderma pigmentosum, Cancer Res., 2001, 61, 2480–2486.
Y. Matsumura, M. Sato, C. Nishigori, M. Zghal, T. Yagi, S. Imamura and H. Takebe, High prevalence of mutations in the p53 gene in poorly differentiated squamous cell carcinomas in xeroderma pigmentosum patients, J. Invest. Dermatol., 1995, 105, 399–401.
M. Sato, C. Nishigori, Y. Lu, M. Zghal, T. Yagi and H. Takebe, Far less frequent mutations in ras genes than in the p53 gene in skin tumors of xeroderma pigmentosum patients, Mol. Carcinog., 1994, 11, 98–105.
C. Nishigori, Cellular aspects of photocarcinogenesis, Photochem. Photobiol. Sci., 2006, 5, 208–214.
Y. Devary, R. A. Gottlieb, T. Smeal and M. Karin, The mammalian ultraviolet response is triggered by activation of src tyrosine kinases, Cell, 1992, 71, 1081–1091.
W. Köpcke and J. Krutmann, Protection from sunburn with beta-carotene–a meta-analysis, Photochem. Photobiol., 2008, 84, 284–288.
Y. Sun and L. W. Oberley, Redox regulation of transcriptional activators, Free Radicals Biol. Med., 1996, 21, 335–348.
L. Rittié and G. J. Fisher, UV-light-induced signal cascades and skin aging, Ageing Res. Rev., 2002, 1, 705–720.
J. Hildesheim, T. A. Rania and A. J. Fornace, p38 Mitogen-activated protein kinase inhibitor protects the epidermis against the acute damaging effects of ultraviolet irradiation by blocking apoptosis and inflammatory responses, J. Invest. Dermatol., 2004, 122, 497–502.
A. L. Kim, J. M. Labasi, Y. Zhu, X. Tang, K. McClure, C. A. Gabel, M. Athar and D. R. Bickers, Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice, J. Invest. Dermatol., 2005, 124, 1318–1325.
R. J. Moore, D. M. Owens, G. Stamp, C. Arnott, F. Burke, N. East, H. Holdsworth, L. Turner, B. Rollins, M. Pasparakis, G. Kollias and F. Balkwill, Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis, Nat. Med., 1999, 5, 828–831.
C. H. Arnott, K. A. Scott, R. J. Moore, A. Hewer, D. H. Phillips, P. Parker, F. R. Balkwill and D. M. Owens, Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway, Oncogene, 2002, 21, 4728–4738.
S. Toyokuni, K. Okamoto, J. Yodoi and H. Hiai, Persistent oxidative stress in cancer, FEBS Lett., 1995, 358, 1–3.
D. Crawford, I. Zbinden, P. Amstad and P. Cerutti, Oxidant stress induces the proto-oncogenes c-fos and c-myc in mouse epidermal cells, Oncogene, 1988, 3, 27–32.
Y. Hattori, C. Nishigori, T. Tanaka, K. Uchida, O. Nikaido, T. Osawa, H. Hiai, S. Imamura and S. Toyokuni, 8-Hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure, J. Invest. Dermatol., 1996, 107, 733–737.
J. Sheu, E. B. Hawryluk, D. Guo, W. B. London and J. T. Huang, Voriconazole phototoxicity in children: a retrospective review, J. Am. Acad. Dermatol., 2015, 72, 314–320.
U. Giri, S. D. Sharma, M. Abdulla and M. Athar, Evidence that in situ generated reactive oxygen species act as a potent stage I tumor promoter in mouse skin, Biochem. Biophys. Res. Commun., 1995, 209, 698–705.
Y. Bai, H. Edamatsu, S. Maeda, H. Saito, N. Suzuki, T. Satoh and T. Kataoka, Crucial role of phospholipase Cepsilon in chemical carcinogen-induced skin tumor development, Cancer Res., 2004, 64, 8808–8810.
M. Oka, H. Edamatsu, M. Kunisada, L. Hu, N. Takenaka, S. Dien, M. Sakaguchi, R. Kitazawa, K. Norose, T. Kataoka and C. Nishigori, Enhancement of ultraviolet B-induced skin tumor development in phospholipase Cε-knockout mice is associated with decreased cell death, Carcinogenesis, 2010, 31, 1897–1902.
R. Gopalakrishna and S. Jaken, Protein kinase C signaling and oxidative stress, Free Radicals Biol. Med., 2000, 28, 1349–1361.
F. Yogianti, M. Kunisada, R. Ono, K. Sakumi, Y. Nakabeppu and C. Nishigori, Skin tumours induced by narrowband UVB have higher frequency of p53 mutations than tumours induced by broadband UVB independent of Ogg1 genotype, Mutagenesis., 2012, 27, 637–643.
F. Rodier, J. P. Coppé, C. K. Patil, W. A. Hoeijmakers, D. P. Muñoz, S. R. Raza, A. Freund, E. Campeau, A. R. Davalos and J. Campisi, Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion, Nat. Cell Biol., 2009, 11, 973–979.
F. Yogiant, M. Kunisada, E. Nakano, R. Ono, K. Sakumi, S. Oka, Y. Nakabeppu and C. Nishigori, Inhibitory effects of dietary Spirulina platensis on UVB-induced skin inflammatory responses and carcinogenesis, J. Invest. Dermatol., 2014, 134, 2610–2619.
Y. Sato, Y. Goto, N. Narita and D. S. Hoon, Cancer cells expressing Toll-like receptors and the tumor microenvironment, Cancer Microenviron., 2009, 2suppl 1, 205–214.
F. P. Noonan, J. A. Recio, H. Takayama, P. Duray, M. R. Anver, W. L. Rush, E. C. De Fabo and G. Merlino, Neonatal sunburn and melanoma in mice, Nature, 2001, 413, 271–272.
P. Autier and J. F. Doré, Influence of sun exposures during childhood and during adulthood on melanoma risk. EPIMEL and EORTC Melanoma Cooperative Group. European Organisation for Research and Treatment of Cancer, Int. J. Cancer, 1998, 77, 533–537.
D. C. Whiteman, C. A. Whiteman and A. C. Green, Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies, Cancer Causes Control, 2001, 12, 69–82.
M. R. Zaidi, S. Davis, F. P. Noonan, C. Graff-Cherry, T. S. Hawley, R. L. Walker, L. Feigenbaum, E. Fuchs, L. Lyakh, H. A. Young, T. J. Hornyak, H. Arnheiter, G. Trinchieri, P. S. Meltzer, E. C. De Fabo and G. Merlino, Interferon-γ links ultraviolet radiation to melanomagenesis in mice, Nature, 2011, 469, 548–553.
R. B. Setlow, E. Grist, K. Thompson and A. D. Woodhead, Wavelengths effective in induction of malignant melanoma, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 6666–6670.
D. L. Mitchell, A. A. Fernandez, R. S. Nairn, R. Garcia, L. Paniker, D. Trono, H. D. Thames and I. Gimenez-Conti, Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9329–9334.
F. P. Noonan, M. R. Zaidi, A. Wolnicka-Glubisz, M. R. Anver, J. Bahn, A. Wielgus, J. Cadet, T. Douki, S. Mouret, M. A. Tucker, A. Popratiloff, G. Merlino and E. C. De Fabo, Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment, Nat. Commun., 2012, 3, 884.
H. Z. Hill and G. J. Hill, UVA, pheomelanin and the carcinogenesis of melanoma, Pigm. Cell Res., 2000, 13Suppl 8, 140–144.
M. T. Glover, N. Niranjan, J. T. Kwan and I. M. Leigh, Non-melanoma skin cancer in renal transplant recipients: the extent of the problem and a strategy for management, Br. J. Plast. Surg., 1994, 47, 86–89.
L. Naldi, Malignancy concerns with psoriasis treatments using phototherapy, methotrexate, cyclosporin, and biologics: facts and controversies, Clin. Dermatol., 2010, 28, 88–92.
J. M. Rivas and S. E. Ullrich, The role of IL-4, IL-10, and TNF-α in the immune suppression induced by ultraviolet radiation, J. Leukocyte Biol., 1994, 56, 769–775.
V. Shreedhar, T. Giese, V. W. Sung and S. E. Ullrich, A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression, J. Immunol., 1998, 160, 3783–3789.
K. Loser, J. Apelt, M. Voskort, M. Mohaupt, S. Balkow, T. Schwarz, S. Grabbe and S. Beissert, IL-10 controls ultraviolet-induced carcinogenesis in mice, J. Immunol., 2007, 179, 365–371.
T. Nagano, M. Kunisada, X. Yu, T. Masaki and C. Nishigori, Involvement of interleukin-10 promoter polymorphism in non-melanoma skin cancers–A case study in non-carcinoma skin cancer patients, Photochem. Photobiol., 2008, 84, 63–66.
E. Alamartine, P. Berthoux, C. Mariat, F. Cambazard and F. Berthoux, Interleukin-10 promoter polymorphisms and susceptibility to skin squamous cell carcinoma after renal transplantation, J. Invest. Dermatol., 2003, 120, 99–103.
G. M. Halliday, Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis, Mutat. Res., 2005, 571, 107–120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishisgori, C. Current concept of photocarcinogenesis. Photochem Photobiol Sci 14, 1713–1721 (2015). https://doi.org/10.1039/c5pp00185d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00185d