Abstract
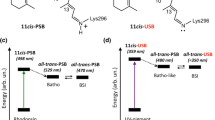
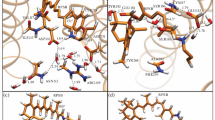
During the photoreaction of rhodopsin, retinal isomerizes, rotating the C11=C12 π-bond from cis to an all-trans configuration. Unprotonated (UR) or protonated (PR) retinal in the Schiff’s base (SB) is related to UV and light vision. Because the UR and PR have important differences in their physicochemical reactivities, we compared the atomic and molecular properties of these molecules using DFT calculations. The C10-C11=C12-C13 dihedral angle was rotated from 0° to 180° in 45° steps, giving five conformers, and the following were calculated from them: atomic orbital (AO) contributions to the HOMO and LUMO, atomic charges, bond length, bond order, HOMO, LUMO, hardness, electronegativity, polarizability, electrostatic potential, UV-vis spectra and dipole moment (DM). Similarly, the following were analyzed: the energy profile, hybridization, pyramidalization and the hydrogen-out-of-plane (HOOP) wagging from the H11-C11=C12-H12 dihedral angle. In addition, retinal with a water H-bond (HR) in the SB was included for comparison. Interestingly, in the PR, C11 and C12 are totally the LUMO and the HOMO, respectively, and have a large electronegativity difference, which predicts an electron jump in these atoms during photoexcitation. At the same time, the PR showed a longer bond length and lower bond order, with a larger DM, lower HOMO-LUMO gap, lower hardness and higher electronegativity. In addition, the AOs of −45° and −90° conformers changed significantly, from pz to py, during the rotation concomitantly with marked hybridization, smooth pyramidalization and lower HOOP activity. Clearly, the atomic and molecular differences between the UR and PR are overwhelming, including the rotational energy profile and light absorption spectra, which indicates that light absorption of UR and PR is already determined by the retinal characteristics of the SB protonation. The HR-model compared with UR shows a lower energy barrier and a discreet bathochromic effect in the UV region.
Similar content being viewed by others
References
Y. Shichida and T. Matsuyama, Evolution of opsins and phototransduction, Philos. Trans. R. Soc. London, Ser. B, 2009, 364, 2881–2895.
J. McCarren and E. F. DeLong, Proteorhodopsin photosystem gene clusters exhibit co-evolutionary trends and shared ancestry among diverse marine microbial phyla, Environ. Microbiol., 2007, 9, 846–858.
J. L. Spudich, C. S. Yang, K. H. Jung and E. N. Spudich, Retinylidene proteins: structures and functions from archaea to humans, Annu. Rev. Cell Dev. Biol., 2000, 16, 365–392.
J. K. Lanyi, Bacteriorhodopsin, Annu. Rev. Physiol., 2004, 66, 665–688.
K. Palczwski, T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. L. Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto and M. Miyano, Crystal structure of rhodopsin: A G protein-coupled receptor, Science, 2000, 289, 739–745.
J. A. Gascón, E. M. Sproviero and V. S. Batista, Computational studies of the primary phototransduction event in visual rhodopsin, Acc. Chem. Res., 2006, 39, 184–193.
L. M. Frutos, T. Andruniów, F. Santoro, N. Ferré and M. Olivucci, Tracking the excited-state time evolution of the visual pigment with multiconfigurational quantum chemistry, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7764–7769.
R. Send and D. Sundholm, Stairway to the conical intersection: A computational study of the retinal isomerization, J. Phys. Chem. A, 2007, 111, 8766–8773.
A. Yabushita, T. Kobayashi and M. Tsuda, Time-resolved spectroscopy of ultrafast photoisomerization of octopus rhodopsin under photoexcitation, J. Phys. Chem. B, 2012, 116, 1920–1926.
S. Gozem, A. I. Krylov and M. Olivucci, Conical intersection and potential energy surface features of a model retinal chromophore: Comparison of EOM-CC and multireference methods, J. Chem. Theory Comput., 2013, 9, 284–292.
D. Polli, P. Altoe, O. Weingart, K. M. Spillane, C. Manzoni, D. Brida, G. Tomasello, G. Orlandi, P. Kukura, R. A. Mathies, M. Garavelli and G. Cerullo, Conical intersection dynamics of the primary photoisomerization event in vision, Nature, 2010, 467, 440–443.
O. P. Ernst and F. J. Bartl, Active states of rhodopsin, ChemBioChem, 2002, 3, 968–974.
A. V. Struts, G. F. Salgado, K. Martínez-Mayorga and M. F. Brown, Retinal dynamics underlie its switch from inverse agonist to agonist during rhodopsin activation, Nat. Struct. Mol. Biol., 2011, 18, 392–394.
S. Yokoyama, Molecular evolution of vertebrate visual pigments, Prog. Retin. Eye Res., 2000, 19, 385–419.
A. K. Kusnetzow, A. Dukkipati, K. R. Babu, L. Ramos, B. E. Knox and R. R. Birge, Vertebrate ultraviolet visual pigments: Protonation of the retinylidene Schiff base and a counterion switch during photoactivation, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 941–946.
A. Dukkipati, A. Kusnetzow, K. R. Babu, L. Ramos, D. Singh, B. E. Knox and R. R. Birge, Phototransduction by vertebrate ultraviolet visual pigments: Protonation of the retinylidene Schiff base following photobleaching, Biochemistry, 2002, 41, 9842–9851.
A. Altun, K. Morokuma and S. Yokoyama, H-Bond network around retinal regulates the evolution of ultraviolet and violet vision, ACS Chem. Biol., 2011, 6, 775–780.
Y. Shi, F. B. Radlwimmer and S. Yokoyama, Molecular genetics and the evolution of ultraviolet vision in vertebrates, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11731–11736.
B. M. Cabrera-Vivas, J. C. Ramírez, L. M. R. Martínez-Aguilera and C. Kubli-Garfias, Theoretical assessment of the mechanism involved in the cholesterol biosynthesis from lanosterol, J. Mol. Struct. (THEOCHEM), 2002, 584, 5–14.
K. Sharma, R. Vázquez-Ramírez and C. Kubli-Garfias, A theoretical model of the catalytic mechanism of the Δ5-3-ketosteroid isomerase reaction, Steroids, 2006, 71, 549–557.
J. A. Cogordan, M. Mayoral, E. Angeles, R. A. Toscano and R. Martínez, Neuroleptic and antidepressant tricyclic compounds: Theoretical study for predicting their biological activity by semiempirical, density functional and Hartree-Fock methods, Int. J. Quantum Chem., 1999, 71, 415–432.
G. E. Scuseria and P. Y. Ayala, Linear scaling coupled cluster and perturbation theories in the atomic orbital basis, J. Chem. Phys., 1999, 111, 8330–8343.
Y. Yang, F. H. Wang and Y. S. Zhou, Density functional calculations of the polarizability and second-order hyperpolarizability of C50Cl10, Phys. Rev., 2005, 71, 13202–13205.
R. C. Haddon, Comment on the relationship of the pyramidalization angle at a conjugated carbon atom to the sigma bond angles, J. Phys. Chem. A, 2001, 105, 4164–4165.
O. Weingart, The twisted C11=C12 bond of the rhodopsin chromophores-A photochemical hot spot, J. Am. Chem. Soc., 2007, 129, 10618–10619.
M. Garavelli, F. Bernardi, M. A. Roob and M. Olivucci, Computer simulation of photoinduced molecular motion and reactivity, Int. J. Photoenergy, 2002, 4, 57–68.
A. Cehovin, H. Mera, J. H. Jensen, K. Stokbro and T. B. Pedersen, Role of the virtual orbitals and HOMO-LUMO gap in mean-field approximations to the conductance of molecular junctions, Phys. Rev. B: Condens. Matter, 2008, 77, 195432.
B.-G. Kim, X. Ma, C. Chen, Y. Ie, E. W. Coir, H. Hashemi, Y. Aso, P. F. Green, J. Kieffer and J. Kim, Energy level modulation of HOMO, LUMO, and band-gap in conjugated polymers for organic photovoltaic applications, Adv. Funct. Mater., 2013, 23, 439–445.
F. Plasser and H. Lischka, Analysis of excitonic and charge transfer interactions from quantum chemical calculations, J. Chem. Theor. Comput., 2012, 8, 2777–2789.
F. Plasser and H. Lischka, Electronic excitation and structural relaxation of the adenine dinucleotide in gas phase and solution, Photochem. Photobiol. Sci., 2013, 12, 1440–1452.
B. Honig, A. D. Greenberg, U. Dinur and T. G. Ebrey, Visual-pigment spectra: Implications of the protonation of the retinal Schiff base, Biochemistry, 1976, 15, 4593–4599.
R. A. Poirier, A. Yadav and P. R. Surján, Effect of protonation on the ground state properties of retinal analogs: An ab initio study, J. Chem., 1987, 65, 892–897.
R. G. Parr and R. G. Pearson, Absolute hardness: Companion parameter to absolute electronegativity, J. Am. Chem. Soc., 1983, 105, 7512–7516.
J. E. Gready, The value of the π-bond order-bond length relationship in geometry prediction and chemical bonding interpretation, J. Comput. Chem., 1984, 5, 411–426.
P. Politzer and S. Ranganathan, Bond-order-bond-energy correlations, Chem. Phys. Lett., 1986, 124, 527–530.
C. Kubli-Garfias, K. Salazar-Salinas, E. C. Perez-Angel and J. M. Seminario, Light activation of the isomerization and deprotonation of the protonated Schiff base retinal, J. Mol. Model, 2011, 17, 2539–2547.
G. Lendvay, Characterization of the progress of chemical reactions by ab initio bond orders, J. Phys. Chem., 1994, 98, 6098–6104.
R. Ponec, G. Yuzhakov and D. L. Cooper, Electron reorganization in chemical reactions. Structural changes from the analysis of bond order profiles, J. Phys. Chem. A, 2003, 107, 2100–2105.
V. R. Kaila, R. Send and D. Sundholm, The effect of protein environment on photoexcitation properties of retinal, J. Phys. Chem. B, 2012, 116, 2249–2258.
K. Bravaya, A. Bochenkova, A. Granovsky and A. Nemukhin, An opsin shift in rhodopsin: Retinal S0-S1 excitation in protein, in solution, and in the gas phase, J. Am. Chem. Soc., 2007, 129, 13035–13042.
I. Schapiro, M. N. Ryazantsev, L. M. Frutos, N. Ferré, R. Lindh and M. Olivucci, The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects, J. Am. Chem. Soc., 2011, 133, 3354–3364.
P. Kukura, D. W. McCamant, S. Yoon, D. B. Wandschneider and R. A. Mathies, Structural observation of the primary isomerization in vision with femtosecond-stimulated raman, Science, 2005, 310, 1006–1009.
N. Klaffki, O. Weingart, M. Garavelli and E. Spohr, Sampling excited state dynamics: Influence of HOOP mode excitations in a retinal model, Phys. Chem. Chem. Phys., 2012, 14, 14299–14305.
W. T. Borden, Pyramidalized alkenes, Chem. Rev., 1989, 89, 1095–1109.
S. Vázquez and P. Camps, Chemistry of pyramidalized alkenes, Tetrahedron, 2005, 61, 5147–5208.
L. S. Ramos, M. H. Chen, B. E. Knox and R. R. Birge, Regulation of photoactivation in vertebrate short wavelength visual pigments: Protonation of the retinylidene Schiff base and a counterion switch, Biochemistry, 2007, 46, 5330–5340.
K. Tsutsui, H. Imai and Y. Shichida, E113 is required for the efficient photoisomerization of the unprotonated chromophore in a UV-absorbing visual pigment, Biochemistry, 2008, 47, 10829–10833.
R. S. Becker and K. Freedman, A comprehensive investigation of the mechanism and photophysics of isomerization of a protonated and unprotonated Schiff base of 11-cis-retinal, J. Am. Chem. Soc., 1985, 107, 1477–1485.
J. E. Kim, M. J. Tauber and R. A. Mathies, Wavelength dependent cis-trans isomerization in vision, Biochemistry, 2001, 40, 13774–13778.
K. Tsutsui, H. Imai and Y. Shichida, Photoisomerization efficiency in UV-absorbing visual pigments: Protein-directed isomerization of an unprotonated retinal Schiff base, Biochemistry, 2007, 46, 6437–6445.
J. Li, P. C. Edwards, M. Burghammer, C. Villa and G. F. Schertler, Structure of bovine rhodopsin in a trigonal crystal form, J. Mol. Biol., 2004, 343, 1409–1438.
J. Liu, M. Y. Liu, J. B. Nguyen, A. Bhagat, V. Mooney and E. C. Yan, Thermal properties of rhodopsin, insight into the molecular mechanism of dim-light vision, J. Biol. Chem., 2011, 286, 27622–27629.
G. Tomasello, G. Olaso-González, P. Altoè, M. Stenta, L. Serrano-Andrés, M. Merchán, G. Orlandi, A. Bottoni and M. Garavelli, Electrostatic control of the photoisomerization efficiency and optical properties in visual pigments: On the role of counterion quenching, J. Am. Chem. Soc., 2009, 131, 5172–5186.
C. Kubli-Garfias and R. Vázquez-Ramírez, Free energy of cis and all-trans retinal conformers in the rhodopsin binding pocket, presented at 3rd Latin-American Congress of Photocatalysis, San Luis Potosí, México, October, 2014.
F. J. Bartl and R. Vogel, Structural and functional properties of metarhodopsin III: Recent spectroscopic studies on deactivation pathways of rhodopsin, Phys. Chem. Chem. Phys., 2007, 9, 1648–1658.
M. Heck, S. A. Schädel, D. Maretzki, F. J. Bartl, E. Ritter, K. Palczewski and K. P. Hofmann, Signaling states of rhodopsin formation of the storage form, metarhodopsin III, from active metarhodopsin II, J. Biol. Chem., 2003, 278, 3162–3169.
K. Zimmermann, E. Ritter, F. J. Bartl, K. P. Hofmann and M. H. Heck, Interaction with transducin depletes metarhodopsin III: A regulated retinal storage in visual signal transduction?, J. Biol. Chem., 2004, 279, 48112–48119.
M. H. Chen, C. Kuemmel, R. R. Birge and B. E. Knox, Rapid release of retinal from a cone visual pigment following photoactivation, Biochemistry, 2012, 51, 4117–4125.
T. Wolter, K. Welke, P. Phatak, A. N. Bondar and M. Elstner, Excitation energies of a water-bridged twisted retinal structure in the bacteriorhodopsin proton pump: A theoretical investigation, Phys. Chem. Chem. Phys., 2013, 15, 12582–12590.
H. Abramczyk, Femtosecond primary events in bacteriorhodopsin and its retinal modified analogs: Revision of commonly accepted interpretation of electronic spectra of transient intermediates in the bacteriorhodopsin photocycle, J. Chem. Phys., 2004, 120, 11120–11132.
P. Tavan, K. Schulten and D. Oesterhelt, The effect of protonation and electrical interactions on the stereochemistry of retinal Schiff bases, Biophys. J., 1985, 47, 415–430.
D. W. McCamant, P. Kukura and R. A. Mathies, Femtosecond stimulated Raman study of excited-state evolution in bacteriorhodopsin, local-access model for proton transfer in bacteriorhodopsin, J. Phys. Chem. B, 2005, 109, 10449–10457.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubli-Garfias, C., Vázquez-Ramírez, R., Cabrera-Vivas, B.M. et al. Atomic and molecular analysis highlights the biophysics of unprotonated and protonated retinal in UV and scotopic vision. Photochem Photobiol Sci 14, 1660–1672 (2015). https://doi.org/10.1039/c5pp00091b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00091b