Abstract
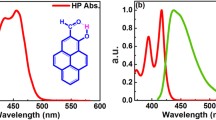
We report on the photodynamics of 2-(2′-hydroxyphenyl)benzoxazole (HBO), compared to its amino derivatives, 6-amino-2-(2′-hydroxypheny)benzoxazole (6A-HBO) and 5-amino-2-(2′-hydroxypheny)- benzoxazole (5A-HBO) in N,N-dimethylformamide (DMF) solutions. HBO at S0 shows a reversible deprotonation reaction leading to the production of anionic forms. However, for 6A-HBO and 5A-HBO, DMF containing KOH is necessary to produce the anions. Excited HBO in DMF exhibits intra- as well as intermolecular proton transfer (ESIPT and ESPT) reactions. With excitation at 330 nm, we observed the openenol, anti-enol and keto forms with different emission and lifetimes (620 ps, 1.5 ns, and 74 ps, respectively), while with the excitation at 433 nm, only the anionic species emission was detected (3.7 ns). Contrary to HBO, 6A-HBO and 5A-HBO do not exhibit any proton transfer process, and only the emissions of the open-enol charge-transferred forms (open-ECT) were observed, which are comparable to those of their methylated derivatives (6A-MBO and 5A-MBO). Femtosecond studies of 6A-MBO and 6A-HBO in DMF indicate that an intramolecular charge-transfer (ICT) reaction (≈80 fs) and solvent relaxation process (2 ps) take place at S1. Remarkably, the photoinduced breaking of the intramolecular hydrogen bond of 6A-HBO and the formation of an intermolecular hydrogen bond with DMF molecules occurs in 80 ps, while for 5A-HBO, this process occurs in less than 10 ps. In this study, we have demonstrated that the presence and position of the amino group in the HBO framework change both the S0 and S1 behaviours of the intramolecular H-bonds; a result which might be useful for the design and better understanding of supramolecular systems based on intra- and intermolecular H-bonds.
Similar content being viewed by others
References
G. J. Woolfe, M. Melzig, S. Schneider, F. Dörr, Chem. Phys., 1983, 77, 213–221.
C. A. S. Potter, R. G. Brown, Chem. Phys. Lett., 1988, 153, 7–12.
K. Das, N. Sarkar, D. Majumdar, K. Bhattacharyya, Chem. Phys. Lett., 1992, 198, 443–448.
A. Douhal, F. Amat-Guerri, M. P. Lillo, A. U. Acuna, J. Photochem. Photobiol., A, 1994, 78, 127–138.
M. A. Rios, M. C. Rios, J. Phys. Chem., 1995, 99, 12456–12460.
D. P. Zhong, A. Douhal, A. H. Zewail, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14056–14061.
P. Purkayastha, N. Chattopadhyay, Phys. Chem. Chem. Phys., 2000, 2, 203–210.
O. K. Abou-Zied, R. Jimenez, E. H. Z. Thompson, D. P. Millar, F. E. Romesberg, J. Phys. Chem. A, 2002, 106, 3665–3672.
H. Wang, H. Zhang, O. K. Abou-Zied, C. Yu, F. E. Romesberg, M. Glasbeek, Chem. Phys. Lett., 2003, 367, 599–608.
M. Rini, J. Dreyer, E. T. J. Nibbering, T. Elsaesser, Chem. Phys. Lett., 2003, 374, 13–19.
Y.-M. Cheng, S.-C. Pu, C.-J. Hsu, C.-H. Lai, P.-T. Chou, ChemPhysChem, 2006, 7, 1372–1381.
O. F. Mohammed, S. Luber, V. S. Batista, E. T. J. Nibbering, J. Phys. Chem. A, 2011, 115, 7550–7558.
O. K. Abou-Zied, Phys. Chem. Chem. Phys., 2012, 14, 2832–2839.
Y. Houari, S. Chibani, D. Jacquemin, A. D. Laurent, J. Phys. Chem. B, 2015, 119(6), 2180–2192.
N. Alarcos, B. Cohen, A. Douhal, J. Phys. Chem. C, 2014, 118, 19431–19443.
P. Wnuk, G. Burdzinski, M. Sliwa, M. Kijak, A. Grabowska, J. Sepiol, J. Kubicki, Phys. Chem. Chem. Phys., 2014, 16, 2542–2552.
G. Zhang, H. Wang, Y. Yu, F. Xiong, G. Tang, W. Chen, Appl. Phys. B, 2003, 76, 677–681.
O. K. Abou-Zied, Chem. Phys., 2007, 337, 1–10.
M. Krishnamurthy, S. K. Dogra, J. Photochem., 1986, 32, 235–242.
R. S. Becker, C. Lenoble, A. Zein, J. Phys. Chem., 1987, 91, 3509–3517.
T. Elsaesser, B. Schmetzer, Chem. Phys. Lett., 1987, 140, 293–299.
W. Frey, F. Laermer, T. Elsaesser, J. Phys. Chem., 1991, 95, 10391–10395.
S. Lochbrunner, A. J. Wurzer, E. Riedle, J. Phys. Chem. A, 2003, 107, 10580–10590.
S. M. Aly, A. Usman, M. AlZayer, G. A. Hamdi, E. Alarousu, O. F. Mohammed, J. Phys. Chem. B, 2015, 119(6), 2596–2603.
H. K. Sinha, S. K. Dogra, Chem. Phys., 1986, 102, 337–347.
T. Elsaesser, W. Kaiser, Chem. Phys. Lett., 1986, 128, 231–237.
A. P. Fluegge, F. Waiblinger, M. Stein, J. Keck, H. E. A. Kramer, P. Fischer, M. G. Wood, A. D. DeBellis, R. Ravichandran, D. Leppard, J. Phys. Chem. A, 2007, 111, 9733–9744.
C. H. Kim, J. Park, J. Seo, S. Y. Park, T. Joo, J. Phys. Chem. A, 2010, 114, 5618–5629.
C.-C. Hsieh, Y.-M. Cheng, C.-J. Hsu, K.-Y. Chen, P.-T. Chou, J. Phys. Chem. A, 2008, 112, 8323–8332.
M. Gutierrez, N. Alarcos, M. Liras, F. Sánchez, A. Douhal, J. Phys. Chem. B, 2015, 119, 552–562.
N. Alarcos, M. Gutiérrez, M. Liras, F. Sánchez, A. Douhal, Phys. Chem. Chem. Phys., 2015 10.1039/C5CP00577A.
N. Alarcos, M. Gutiérrez, M. Liras, F. Sánchez, M. Moreno, A. Douhal, Phys. Chem. Chem. Phys., 2015, 17(22), 14569–14581.
A. Douhal, M. Sanz, M. A. Carranza, J. A. Organero, L. Santos, Chem. Phys. Lett., 2004, 394, 54–60.
A. D. Roshal, J. A. Organero, A. Douhal, Chem. Phys. Lett., 2003, 379, 53–59.
J. Seo, S. Kim, S. Y. Park, J. Am. Chem. Soc., 2004, 126, 11154–11155.
M. J. Kamlet, J. L. M. Abboud, M. H. Abraham, R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887.
R. A. Velapoldi, K. D. Mielenz, Appl. Opt., 1981, 20, 1718–1718.
J. A. Organero, L. Tormo, A. Douhal, Chem. Phys. Lett., 2002, 363, 409–414.
M. Gil, A. Douhal, Chem. Phys. Lett., 2006, 428, 174–177.
E. L. Roberts, J. Dey, I. M. Warner, J. Phys. Chem., 1996, 100, 19681–19686.
M. L. Horng, J. A. Gardecki, A. Papazyan, M. Maroncelli, J. Phys. Chem., 1995, 99, 17311–17337.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Reversible model explanation and thermodynamic constants calculations (ΔH°, ΔS° and ΔG°); Fig. S1 shows the emission fluorescence spectra for HBO in DMF and DMF/KOH solutions; Fig. S2 is the emission spectra for 6A-MBO, 6A-HBO, 5A-MBO, and 5A-HBO in neutral water solution; Fig. S3 and S4 present the UV-visible absorption and emission spectra for 5A-HBO in different pH solutions; Fig. S5 and S6 display the UV-visible absorption and fluorescence spectra for 6A-MBO and 5A-MBO in DMF and DMF/KOH solutions, respectively; Fig. S7 and S8 show the excitation fluorescence and absorption spectra for HBO, 6A-HBO, 6A-MBO, 5A-HBO and 5A-MBO in DMF and DMF/KOH solutions, respectively; Fig. S9 presents the magic-angle emission decay for 6A-HBO in DMF solution; Fig. S10 and S11 show the UV-visible absorption and fluorescence spectra for HBO and 5A-HBO in DMF solution upon fs-pulse irradiation; Fig. S12–S17 give the 1H (A) and 13C (B) NMR spectra of the different molecules in DMSO-d6. See DOI: 10.1039/c5pp00079c
Rights and permissions
About this article
Cite this article
Alarcos, N., Gutiérrez, M., Liras, M. et al. From intra- to inter-molecular hydrogen bonds with the surroundings: steady-state and timeresolved behaviours. Photochem Photobiol Sci 14, 1306–1318 (2015). https://doi.org/10.1039/c5pp00079c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00079c