Abstract
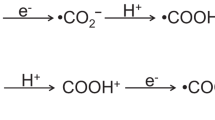
Using the EPR spin trapping technique, we prove that simultaneous reactions take place in illuminated suspensions of TiO2 in aqueous carbonate solutions (pH ≈ 7). The adsorbed HCO3− is reduced to formate as directly made evident by the detection of formate radicals (˙CO2−). In addition, the amount of OH˙ radicals from the photo-oxidation of water shows a linear dependence on the concentration of bicarbonate, indicating that electron scavenging by HCO3− increases the lifetime of holes. In a weakly alkaline medium, photo-oxidation of HCO3−/CO32− to ˙CO3− interferes with the oxidation of water. A comparative analysis of different TiO2 samples shows that formation of ˙CO2− is influenced by factors related to the nature of the surface, once expected surface area effects are accounted for. Modification of the TiO2 surface with noble metal nanoparticles does not have unequivocal benefits: the overall activity improves with Pd and Rh but not with Ru, which favours HCO3− photo-oxidation even at pH = 7. In general, identification of radical intermediates of oxidation and reduction reactions can provide useful mechanistic information that may be used in the development of photocatalytic systems for the reduction of CO2 also stored in the form of carbonates.
Similar content being viewed by others
References
M. Aresta, in Carbon Dioxide as Chemical Feedstock, Wiley-VCH Verlag, Weinheim, 2010.
G. Centi and S. Perathoner, Catal. Today, 2009, 148, 19.
E. Benson, P. Kubiak, A. Clifford, J. Sathrum and J. M. Smieja, Chem. Soc. Rev., 2009, 38, 89.
E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazabal and J. Perez-Ramirez, Energy Environ. Sci., 2013, 6, 3112.
S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Angew. Chem., Int. Ed., 2013, 52, 2.
Y. Hori, in Modern Aspects of Electrochemistry, ed. C. Vayenas, et al., Springer, New York, 2008, vol.42, 89–189.
P. Indrakanti, J. D. Kubickib and H. H. Schobert, Energy Environ. Sci., 2009, 2, 745.
H. He, P. Zapol and L. A. Curtiss, J. Phys. Chem. C, 2010, 114, 21474.
G. Centi, S. Perathoner, G. Winè and M. Gangeri, Green Chem., 2007, 9, 671.
Y. Izumi, Coord. Chem. Rev., 2013, 257, 171.
G. Mele, C. Annese, A. De Riccardis, C. Fusco, L. Palmisano, G. Vasapollo and L. D’Accolti, Appl. Catal., A, 2014, 481, 169.
K. Li, A. D. Handoko, M. Khraisheh and J. Tang, Nanoscale, 2014, 6, 9767.
C. J. Stalder, S. Chao and M. S. Wrighton, J. Am. Chem. Soc., 1984, 106, 3673.
H. S. Freund and M. W. Roberts, Surf. Sci. Rep., 1996, 25, 225.
Z. Goren, I. Willner, A. J. Nelson and A. J. Frank, J. Phys. Chem., 1990, 94, 3784.
C. C. Yang, J. Vernimmen, V. Meynen, P. Cool and G. Mul, J. Catal., 2011, 284, 1.
R. Kortlever, K. H. Tan, Y. Kwon and M. T. M. Koper, J. Solid State Electrochem., 2013, 17, 1843.
X. G. Meng, S. X. Ouyang, T. Kako, P. Li, Q. Yu, T. Wang and J. H. Ye, Chem. Commun., 2014, 50, 11517.
N. Sreekanth and K. L. Phani, Chem. Commun., 2014, 50, 11143.
N. M. Dimitrijevic, B. K. Vijayan, O. G. Poluektov, T. Rajh, K. A. Gray, H. He and P. Zapol, J. Am. Chem. Soc., 2011, 133, 3964.
H. Fu, L. Zhang, S. Zhang, Y. Zhu and J. Zhao, J. Phys. Chem. B, 2006, 110, 3061.
A. Di Paola, M. Bellardita, L. Palmisano, Z. Barbierikova and V. Brezova, J. Photochem. Photobiol., A, 2014, 273, 59.
A. Molinari, R. Argazzi and A. Maldotti, J. Mol. Catal. A: Chem., 2013, 372, 23.
A. Molinari, G. Magnacca, G. Papazzoni and A. Maldotti, Appl. Catal., B, 2013, 138–139, 446.
A. Molinari, A. Maldotti and R. Amadelli, Chem.–Eur. J., 2014, 20, 7759.
G. R. Buettner, Free Radicals Biol. Med., 1987, 3, 259.
F. A. Villamena, E. J. Locigno, A. Rockenbauer, C. M. Hadad and J. L. Zweier, J. Phys. Chem. A, 2006, 110, 13253.
R. Amadelli, A. Maldotti, C. Bartocci and V. Carassiti, J. Phys. Chem., 1989, 93, 6448.
B. Aurian-Blajeni, M. Halmann and J. Manassen, Photochem. Photobiol., 1982, 35, 157.
K. Sayama and H. Arakawa, J. Chem. Soc., Faraday Trans., 1997, 93, 1647.
H. Czili and A. Horvath, Appl. Catal., B, 2008, 81, 295.
U. Černigoj, U. Lavrenčič Štangar, P. Trebšea and M. Sarakhab, J. Photochem. Photobiol., A, 2009, 201, 142.
E. Guerrini and S. Trasatti, Russ. J. Electrochem., 2006, 42, 1017.
K. Kobayakawa, Y. Nakazawa, M. Ikeda and A. Fujishima, Ber. Bunsen-Ges. Phys. Chem., 1990, 94, 1439.
D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, J. Phys. Chem. B, 2003, 107, 4545.
J. T. Carneiro, T. J. Savenije, J. A. Moulijn and G. Mul, J. Phys. Chem. C, 2011, 115, 2211.
D. D’Elia, C. Beauger, J. F. Hochepied, A. Rigacci, M. H. Berger, N. Keller, V. Keller-Spitzer, Y. Suzuki, J. C. Valmalette, M. Benabdesselam and P. Achard, J. Hydrogen Energy, 2011, 36, 14360.
R. Amadelli, et al., unpublished work.
M. Pourbaix, Atlas of electrochemical equilibria in aqueous solutions, National Association of Corrosion Engineers, Houston, 1974.
S. Trasatti in Interfacial Electrochemistry: Theory, Experiment, and Applications, ed. A. Wieckowski, Marcel Dekker, Inc., New York, 1999, pp.769–792.
A. Borgschulte and L. Schlapbach in Hydrogen as a Future Energy Carrier, ed. A. Züttel, A. Borgschulte and L. Schlapbach, Wiley-VCH Verlag, Weinheim, 2008, pp.94–108.
H. Kramer, M. Levy and A. Warshawsky, Int. J. Hydrogen Energy, 1995, 20, 229.
P. Panagiotopoulou, D. I. Kondarides and X. E. Verykios, J. Phys. Chem. C, 2011, 115, 1220.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c4pp00467a
Rights and permissions
About this article
Cite this article
Molinari, A., Samiolo, L. & Amadelli, R. EPR spin trapping evidence of radical intermediates in the photo-reduction of bicarbonate/CO2 in TiO2 aqueous suspensions. Photochem Photobiol Sci 14, 1039–1046 (2015). https://doi.org/10.1039/c4pp00467a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00467a