Abstract
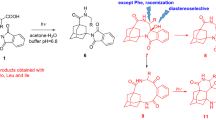
A variety of N-(selenomethyl)alkyl-phthalimides (alkyl =–(CH2)n–; n = 2–5, 1a, b, d, e) and N-(selenobenzyl)propyl phthalimide (1c) were synthesized and their photochemistry was studied at λ = 300 nm. Steady-state photolysis and laser time-resolved spectroscopy studies confirmed that these reactions proceeded by direct or acetone-sensitized excitation followed by intramolecular electron transfer (ET) between Se atom and the phthalimide moiety. Two main pathways are possible after ET: proton transfer to the ketyl radical anion from the CH3Se+˙ or the–CH2Se+˙–moieties, yielding the corresponding biradicals. Collapse of these biradicals yields cyclization products with the respective endo or exo selenium-containing heterocycles. Competition between both proton transfer processes depends on the chain length of the alkyl spacer between the phthalimide and Se groups as well as the size of the cycle being formed.
Similar content being viewed by others
References
G. J. Kovarnos, in Fundamentals of Photoinduced Electron Transfer, VCH Pub., New York, 1993.
A. G. Griesbeck and J. Mattay, in Synthetic Organic Photochemistry, Molecular and Supramolecular Photochemistry, Marcel-Dekker, New York, 2005, vol. 12.
R. A. Rossi, A. B. Peñéñory, Strategies in synthetic radical organic chemistry. Recent advances on cyclization and SRN1 reactions, Curr. Org. Synth., 2006, 3, 121–158.
N. Hoffmann, Efficient photochemical electron transfer sensitization of homogeneous organic reactions, J. Photochem. Photobiol., C, 2008, 9, 43–60.
M. Oelgemöeller and A. G. Griesbeck, Photoinduced electron transfer chemistry of phthalimides: an efficient tool for C–C-bond formation, J. Photochem. Photobiol., C, 2002, 3, 109–127.
A. G. Griesbeck, N. Hoffmann, K.-D. Warzecha, Photoinduced-Electron-Transfer Chemistry: from studies on PET processes to applications in natural product synthesis, Acc. Chem. Res., 2007, 40, 128–140.
Y. Kanaoka, Photoreactions of cyclic imides. Examples of synthetic organic photochemistry, Acc. Chem. Res., 1978, 11, 407–413.
U. C. Yoon and P. S. Mariano, The synthetic potential of phthalimide SET photochemistry, Acc. Chem. Res., 2001, 34, 523–533.
M. Horvat, K. Mlinarić-Majerski, N. Basarić, Photochemistry of N-alkyl and N-aryl substituted phthalimides: H-abstractions, Single Electron Transfer and cycloadditions, Croat. Chem. Acta, 2010, 83, 179–188.
Y. Sato, H. Nakai, M. Wada, T. Mizoguchi, Y. Hatanaka, Y. Migita and Y. Kanaoka, Photochemistry of the phthalimide system, 37. Thiazacycloalkanols by photocyclization of S-substituted N-(thioalkyl)phthalimides, Liebigs Ann. Chem., 1985, 1099–1118.
Y. Hatanaka, Y. Sato, H. Nakai, M. Wada, T. Mizoguchi and Y. Kanaoka, Photochemistry of the phthalimide system, 44. Photoinduced reactions, 122. Regioselective remote photocyclization: examples of a photochemical macrocyclic synthesis with sulfide-containing phthalimides, Liebigs Ann. Chem., 1992, 1113–1123.
Y. Sato, H. Nakai, M. Wada, T. Mizoguchi, Y. Hatanaka and Y. Kanaoka, Application of remote photocyclization with a pair system of phthalimide and methylthio groups. A photochemical synthesis of cyclic peptide models, Chem. Pharm. Bull., 1992, 40, 3174–3180.
Y. Sato, H. Nakai, T. Mizoguchi and Y. Kanaoka, A synthetic approach to cyclic peptide models by regioselective remote photocyclization of sulfide-containing phthalimides, Tetrahedron Lett., 1976, 17, 1889–1890.
Y. Sato, H. Nakai, T. Mizoguchi, Y. Hatanaka and Y. Kanaoka, Photochemistry of the phthalimide system. VII. Regioselective remote photocyclization. Examples of a photochemical macrocyclic synthesis with sulfide-containing phthalimides, J. Am. Chem. Soc., 1976, 98, 2349–2351.
Y. Sato, H. Nakai, H. Ogiwara, T. Mizoguchi, Y. Migita and Y. Kanaoka, Photochemistry of the phthalimide system. V photocyclization of the phthalimides with a sulfide chain: synthesis of aza-cyclols by δ, ε, and ς hydrogen abstraction, Tetrahedron Lett., 1973, 14, 4565–4568.
Y. Sato, H. Nakai, M. Wada, H. Ogiwara, T. Mizoguchi, Y. Migita, Y. Hatanaka and Y. Kanaoka, Photocyclization of N-alkoxyalkylphthalimides with favored δ-hydrogen abstraction: syntheses of oxazolo [4, 3-α] isoindoles and oxazolo [4, 3-α]-isoindole-1-spiro-1′-cycloalkane ring systems, Chem. Pharm. Bull., 1982, 30, 1639–1645.
K. Maruyama, Y. Kubo, M. Machida, K. Oda, Y. Kanaoka and K. Fukuyama, Photochemical cyclization of N-2-alkenyl- and N-3-alkenylphthalimides, J. Org. Chem., 1978, 43, 2303–2304.
K. Maruyarna and Y. Kubo, Solvent-incorporated medium to macrocyclic compounds by the photochemical cyclization of N-alkenylphthalimides, J. Am. Chem. Soc., 1978, 100, 7772–7773.
K. Maruyama and Y. Kubo, Photochemistry of N-(2-alkenyl)phthalimides. Photoinduced cyclization and elimination reaction, J. Org. Chem., 1981, 46, 3612–3622.
P. H. Mazzocchi and G. J. Fritz, Photolysis of N-(2-methyl-2-propenyl)phthalimide in methanol. Evidence supporting radical-radical coupling of a photochemically generated radical ion pair, J. Am. Chem. Soc., 1986, 108, 5362–5364.
A. G. Griesbeck, A. Henz, J. Ptatschek, V. Hirt, T. Engel, D. Löffler and F. W. Schneider, Photochemistry of N-phthaloyl derivatives of electron-donor-substituted amino acids, Tetrahedron, 1994, 50, 701–714.
Y. Kanaoka; and Y. Migita, Photocyclization of N-aralkylphthalimides: An example of possible synthetic control in a heterocyclic series, Tetrahedron Lett., 1974, 42, 3693–3696.
A. G. Griesbeck, A. Henz, W. Kramer, J. Lex, F. Nerowski, M. Oelgemöller, 70. Synthesis of medium- and large-Ring compounds initiated by photochemical decarboxylation of ω-phthalimidoalkanoates, Helv. Chim. Acta, 1997, 80, 912–933.
A. G. Griesbeck, T. Heinrich, M. Oelgemöller, A. Molis and A. Heidtmann, Synthesis of cyclic peptides by photochemical decarboxylation of N-phthaloyl peptides in aqueous solution, Helv. Chim. Acta, 2002, 85, 4561–4578.
A. G. Griesbeck, T. Heinricht, M. Oelgemöller, J. Lex and A. Molis, A photochemical route for efficient cyclopeptide formation with a minimum of protection and activation chemistry, J. Am. Chem. Soc., 2002, 124, 10972–10973.
A. G. Griesbeck, W. Kramer, M. Oelgemöller, Synthetic applications of photoinduced electron transfer decarboxylation reactions, Synlett, 1999, 1169–1178.
A. G. Griesbeck, F. Nerowski and J. Lex, Decarboxylative photocyclization: synthesis of benzopyrrolizidines and macrocyclic lactones, J. Org. Chem., 1999, 64, 5213–5217.
A. G. Griesbeck, W. Kramer, M. Oelgemöller, Photoinduced decarboxylation reactions, Green Chem., 1999, 1, 205–207.
A. G. Griesbeck, M. Oelgemöller, J. Lex, A. Haeuseler and M. Schmittel, Synthesis of sulfur-containing tricyclic ring systems by means of photoinduced decarboxylative cyclizations, Eur. J. Org. Chem., 2001, 1831–1843.
U. C. Yoon, Y. X. Jin, S. W. Oh, D. W. Cho, K. N. Park and P. S. Mariano, Comparison of photomacrocyclization reactions of trimethylsilyl- and tributylstannyl-terminated phthalimido- and maleimido-polyethers, J. Photochem. Photobiol., A, 2002, 150, 77–84.
D. W. Cho, J. H. Choi, S. W. Oh, C. Quan, U. C. Yoon, R. Wang, S. Yang and P. S. Mariano, Single electron transfer-promoted photocyclization reactions of linked acceptor–polydonor systems: effects of chain length and type on the efficiencies of macrocyclic ring-forming photoreactions of tethered α-silyl ether phthalimide substrates, J. Am. Chem. Soc., 2008, 130, 2276–2284.
C. W. Nogueira, G. Zeni and J. B. T. Rocha, Organoselenium and organotellurium compounds: toxicology and pharmacology, Chem. Rev., 2004, 104, 6255–6285.
A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Organoselenium chemistry: role of intramolecular interactions, Chem. Rev., 2010, 110, 4357–4416.
S. Hazebrouck, L. Camoin, Z. Faltin, A. D. Strosberg and Y. Eshdat, Substituting selenocysteine for catalytic cysteine 41 enhances enzymatic activity of plant phospholipid hydroperoxide glutathione peroxidase expressed in Escherichia coli, J. Biol. Chem., 2000, 275, 28715–28721.
Y. Eshdat, D. Holland, Z. Faltin, G. Ben-Hayyim, Plant glutathione peroxidases, Physiol. Plant., 1997, 100, 234–240.
K. C. Nicolaou and N. A. Petasi, in Selenium in Natural Products Synthesis, CIS, Philadelphia, 1984.
C. Paulmier, in Selenium Reagents and Intermediates in Organic Synthesis, Pergamon, Oxford, 1986.
S. Patai and Z. Rappoport, in The Chemistry of Organic Selenium and Tellurium Compounds, Wiley, New York, 1986, vol. 1.
D. L. Klayman and W. H. H. Günther, in Organic Selenium Compounds: Their Chemistry and Biology, Wiley Interscience, New York, 1973.
R. J. Shamberger, in Biochemistry of Selenium, Plenum Press, New York, 1983.
V. A. Potapov, M. V. Musalov and S. V. Amosova, Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring, Tetrahedron Lett., 2011, 52, 4606–4610.
F. V. Singh and T. Wirth, in PATAI’S Chemistry of Functional Groups; The Chemistry of Organic Selenium and Tellurium Compounds, John Wiley & Sons, Ltd, 2011.
K. C. Nicolaou, D. A. Claremon, W. E. Barnette and S. P. Seitz, N-Phenylselenophthalimide (N-PSP) and N-phenylselenosuccinimide (N-PSS). Two versatile carriers of the phenylseleno group. Oxyselenation of olefins and a selenium-based macrolide synthesis, J. Am. Chem. Soc., 1979, 101, 3704–3706.
A. Temperini and L. Minuti, N-(phenylselenomethyl)phthalimide as new reagent for mild protection of alcohols as Pim-ethers, Tetrahedron Lett., 2012, 53, 2709–2711.
B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, in Vogel’s: Texbook of Practical Organic Chemistry, John Wiley & Sons, Inc., New York, 5th edn, 1989, p. 780.
N. L. Drake and J. A. Garman, Some N1-(6-methoxy-8-quinolylaminoalkyl)-guanidines, J. Am. Chem. Soc., 1949, 71, 2425–2427.
The generation in situ of the alkaneselenate ions by reduction of dialkyldiselenide with Na in NH3(l) can be replaced by reduction with NaBH4 in tert-BuOH (A. B. Peñéñory, R. A. Rossi, Photostimulated reactions of haloarenes with benzeneselenate ions by the SRN1 mechanism. Competition between electron transfer and fragmentation of radical anion intermediates, J. Phys. Org. Chem., 1990, 3, 266–272). With this methodology, tert-BuOH must be distilled off and subsequent addition of DMF allows the substitution reaction with the bromo phthalimide derivative.
H. Komatsu, M. Iwaoka and S. Tomoda, Intramolecular non-bonded interaction between selenium and oxygen as revealed by 17O and 77Se NMR spectroscopy and natural bond orbital analysis, Chem. Commun., 1999, 205–206.
M. Iwaoka, H. Komatsu, T. Katsuda and S. Tomoda, Nature of nonbonded Se⋯O interactions characterized by 17O NMR spectroscopy and NBO and AIM analyses, J. Am. Chem. Soc., 2004, 126, 5309–5317.
G. Pandey, B. B. V. S. Sekhar and U. T. Bhalerao, Photoinduced single electron transfer initiated heterolytic carbon-selenium bond dissociation. Sequential one-pot selenenylation and deselenenylation reaction, J. Am. Chem. Soc., 1990, 112, 5650–5651.
G. Pandey and S. R. Gadre, Generation and mesolytic dynamics of organoselenane and selenosilane radical ions: development of mechanistically interesting and synthetically useful chemistry, Acc. Chem. Res., 2004, 37, 201–210.
G. Pandey, R. Sochanchingwung, S. Kant Tiwari, Diastereoselective oxyselenylation of 1,n-diolefins utilizing PET generated [PhSeSePh]+˙ as an electrophilic species: An efficient and general strategy for the synthesis of α,α′-trans-dialkyl cyclic ethers, J. Indian Inst. Sci., 2001, 81, 87–93.
S. Hu and D. C. Neckers, Photochemical reactions of sulfide-containing alkyl phenylglyoxylates, Tetrahedron, 1997, 53, 7165–7180.
A. G. Griesbeck, J. H. Mauder, I. Müller, E.-M. Peters, K. Peters, H. G. von Schnering, Photochemistry of N-phthaloyl derivatives of methionine, Tetrahedron Lett., 1993, 34, 453–456.
M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, in Handbook of Photochemistry, CRC Press Taylor & Francis Group, New York, 3rd edn, 2006.
H. Göner, A. G. Griesbeck, T. Heinrich, W. Kramer, M. Oelgemöller, Time-resolved spectroscopy of sulfur- and carboxy-substituted N-alkylphthalimides, Chem.–Eur. J., 2001, 7, 1530–1538.
A. G. Griesbeck, H. Görner, Laser flash photolysis study of N-alkylated phthalimides, J. Photochem. Photobiol., A, 1999, 129, 111–119, and reference therein.
R. Pérez-Ruiz, S. Gil and M. A. Miranda, Stereodifferentiation in the photochemical cycloreversion of diastereomeric methoxynaphthalene-oxetane dyads, J. Org. Chem., 2005, 70, 1376–1381.
D. Rehm and A. Weller, Kinetics of fluorescence quenching by electron and H-atom transfer, Isr. J. Chem., 1970, 8, 259–271.
G. Pandey, B. B. V. S. Sekhar, Photoinduced electron transfer initiated activation of organoselenium substrates as carbocation equivalents: sequential one-pot selenylation and deselenylation reaction, J. Org. Chem., 1994, 59, 7367–7372.
D. W. Leedy and D. L. Muck, Cathodic reduction of phthalimide systems in nonaqueous solutions, J. Am. Chem. Soc., 1971, 93, 4264–4270.
K.-D. Warzecha, H. Görner and A. G. Griesbeck, Photoinduced decarboxylative benzylation of phthalimide triplets with phenyl acetates: a mechanistic study, J. Phys. Chem. A, 2006, 110, 3356–3363.
V. Wintgens, P. Valat, J. Kossanyi, L. Biczok, A. Demeter and T. Berces, Spectroscopic properties of aromatic dicarboximides. Part1.-N-H and N-methyl-substituted naphthalimides, J. Chem. Soc., Faraday Trans., 1994, 90, 411–421.
A. G. Griesbeck, J. Hirt, K. Peters, E.-M. Peters, H. G. von Schnering, Photochemistry of N-phthaloylcysteine derivatives: multiplicity-directed regioselective CH activation, Chem.–Eur. J., 1996, 2, 1388–1394.
A. G. Griesbeck, M. Oelgemöller and J. Lex, Photochemistry of MTM- and MTE-esters of ω-phthalimido carboxylic acids: macrocyclization versus deprotection, J. Org. Chem., 2000, 65, 9028–9032.
O. Shvydkiv, K. Nolan, M. Oelgemöller, Microphotochemistry: 4,4′-dimethoxybenzophenone mediated photodecarboxylation reactions involving phthalimides, Beilstein J. Org. Chem., 2011, 7, 1055–1063.
O. Shvydkiv, S. Gallagher, K. Nolan, M. Oelgemöller, From conventional to microphotochemistry: photodecarboxylation reactions involving phthalimides, Org. Lett., 2010, 12, 5170–5173.
R. Grigg, V. Sridharan, P. Stevenson, S. Sukirthalingam and T. Worakun, The synthesis of fused ring nitrogen heterocycles via regiospecific intramolecular Heck reactions, Tetrahedron, 1990, 46, 4003–4018.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Electronic supplementary information (ESI) available: Spectra (1H and 13C NMR) for all the substrates 1a-e, and products 2a, 2b, 2e, 3b, and 3d. See DOI: 10.1039/c4pp00452c
Rights and permissions
About this article
Cite this article
Oksdath-Mansilla, G., Heredia, A.A., Argüello, J.E. et al. Photochemistry of N-(selenoalkyl)-phthalimides. Formation of N, Se-heterocyclic systems. Photochem Photobiol Sci 14, 726–736 (2015). https://doi.org/10.1039/c4pp00452c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00452c