Abstract
Dengue virus (DENV) infects 400 million people worldwide annually. Infection of more than one serotype of DENV highly corresponds to dengue hemorrhagic fever and dengue shock syndrome, which are the leading causes of high mortality. Due to lack of effective vaccines and unavailable therapies against DENV, discovery of anti-DENV agents is urgently needed. We first characterize that Schisandrin A can inhibit the replication of four serotypes of DENV in a concentration- and time-dependent manner, with an effective half-maximal effective concentration 50% (EC50) value of 28.1 ± 0.42 μM against DENV serotype type 2 without significant cytotoxicity. Furthermore, schisandrin A can effectively protect mice from DENV infection by reducing disease symptoms and mortality of DENV-infected mice. We demonstrate that STAT1/2-mediated antiviral interferon responses contribute to the action of schisandrin A against DENV replication. Schisandrin A represents a potential antiviral agent to block DENV replication in vitro and in vivo. In conclusion, stimulation of STAT1/2-mediated antiviral interferon responses is a promising strategy to develop antiviral drug.
Similar content being viewed by others
Introduction
Dengue virus (DENV) is an arthropod-borne pathogen of a human viral disease, which infects 400 million people in the world, and 2.5 billion people are at risk of infection in tropical and subtropical areas1. DENV belongs to the Flavivirus genus of the Flaviviridae family. The genome of DENV is a positive sense of 11-kb single-stranded RNA encoding a single polyprotein2. The polyprotein is processed by viral and host proteases, resulting in the maturation of three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5)3. DENV is classified into four serotypes (DENV 1–4)4, which is of importance with regard to the clinical manifestations ranging from dengue fever (DF) to dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS)5,6. Infection with more than one serotype of DENV could cause high risk of DHF and DSS, leading to tens of thousands of deaths annually7,8. Today, because of lack of approved drugs or effective vaccines against DENV infection, it is of utmost importance to find new therapeutics to treat the disease.
Type I interferon (IFN-I) pathway is an important innate immune response to protect the host from pathogen invasion7,9. The binding of IFN-I cytokines, such as IFN-α and IFN-β, and cell surface type I IFN-alpha receptor (IFNAR) activates phosphorylation of Janus kinases 1 (JAK1) and tyrosine kinase 2 (Tyk2), which leads to activation of the signal transducer and activator of transcription factors 1 and 2 (STAT1 and STAT2) via tyrosine phosphorylation10. Subsequently, phosphorylated STAT1–STAT2 heterodimer binds to IFN-regulated gene 9 and form a transcriptional complex of IFN-stimulated gene factor 3 (ISGF3). ISGF3 then translocates into the nucleus and binds to the IFN- stimulated response element (ISRE) to trigger the expression of several antiviral IFN-stimulated genes (ISGs), including 2′-5′-oligoadenylate synthetase 1 (OAS1), OAS2, OAS3, and protein kinase R (PKR), which ultimately collapses different stages of virus replication9,11,12. In case of DENV infection, NS2B/3 protease subverts IFN activation by targeting MITA protein, NS4B interferes IFN pathway by blocking STAT1 phosphorylation, and NS5 blocks JAK-STAT2 pathway by degradation of STAT2 protein to prevent antiviral gene expression9,13,14. Therefore, IFN-mediated antiviral responses display a critical role in the prevention of DENV infection and DENV-related pathogenesis.
Schisandra chinensis (Turcz.) Baill. (S. chinensis) is a widely used herbal medicine, and its fruit is frequently used as a sedative, analgesic, and antipyretic and to treat hepatitis, myocardial disorders, and hyperlipidemia15,16. In addition, the extract of S. chinensis has also been used to treat neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease17. Nine major bioactive ingredients within S. chinensis were identified, including schisandrol A, schisandrol B, angeloylgomisin H, gomisin G, schisantherin A, schisanhenol, schisandrin A, schisandrin B, and schisandrin C16. Current reports demonstrated that these ingredients of S. chinensis possess several biological activities, such as antioxidant, antitumor, anti-inflammatory, and immunoregulatory activities15,18,19,20,21,22,23, and schisandrin A and schisandrin B exhibit antiviral activity against HIV24. Here, we investigated whether schisandrin A, schisandrin B, or schisandrin C could exhibit anti-DENV activity in vitro and in vivo and further investigated the molecular mechanism by which the effective ingredient inhibits DENV replication.
Results
Schisandrin A inhibits DENV RNA replication and protein synthesis
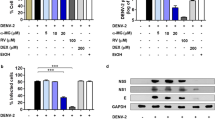
The DENV-infected Huh-7 cells were treated with individual schisandrin derivatives at the indicated concentrations for 3 days. DENV RNA and protein levels were analyzed by RT-qPCR and Western blotting, respectively, in which the amount of NS2B represented the DENV protein synthesis level. As shown in Fig. 1B, schisandrin A effectively reduced DENV RNA and protein levels compared with schisandrin A-untreated cells. In contrast. schisandrin B and schisandrin C exerted lower inhibitory effect on DENV RNA and protein levels than that of schisandrin A-treated cells (Fig. 1C and D). The half-maximal effective concentration (EC50) values of schisandrin A, schisandrin B, and schisandrin C were determined as 28.1 ± 0.42 μM, 34.0 ± 0.95 μM, and 42.6 ± 3.48 μM, respectively. No significant cytotoxicity was observed when the cells were exposed to effective antiviral concentration of each schisandrin derivative (Figure S1). Therefore, we selected schisandrin A as a potential inhibitor for subsequent studies. We first examined whether schisandrin A treatment could suppress viral titer of DENV, and the result showed that schisandrin A treatment decreased DENV titer (Fig. 2A). We further tested the antiviral effect of schisandrin A on the four DENV serotypes. The DENV-infected Huh-7 cells were treated with 30 or 40 μM of schisandrin A for 3 days. The RT-qPCR results revealed that schisandrin A can block the replication of the four serotypes of DENV in a concentration-dependent manner (Fig. 2B).
(A) Structure of schisandrin A, schisandrin B, and schisandrin C. (B–D) Schisandrin A efficiently inhibits DENV RNA replication and protein synthesis. Huh-7 cells were infected with DENV for 2 h, followed by 0.1% of DMSO (dose 0, a negative control), 25, 30, 35, and 40 μM of schisandrin A, schisandrin B, or schisandrin C treatment for 3 days. The DENV RNA and protein levels were analyzed by RT-qPCR and Western blotting, respectively. For RT-qPCR, the DENV RNA level was normalized by the cellular gapdh mRNA level. The relative DENV RNA of each sample was presented as the % change compared to schisandrin A-untreated control (100%). For Western blotting, GAPDH served as an equal loading control. Error bars denote the means ± SD of five independent experiments (N = 5). *P < 0.05.
(A) Schisandrin A inhibits DENV titer. Huh-7 cells were infected by DENV at an MOI of 0.1 for 2 h and treated by 0.1% of DMSO (dose 0, a negative control), 25, 30, 35 and 40 μM schisandrin A. After 3 days treatment, the supernatant was collected, and the DENV titer was determined by plague assay. (B) Huh-7 cells were infected with the four DENV serotypes at an MOI of 0.1 for 2 h, followed by 0 (black bars), 30 (white bars), and 40 μM (gray bars) of schisandrin A treatment. The DENV RNA level was normalized by the cellular gapdh mRNA level in RT-qPCR. The relative DENV RNA of each sample was presented as the % change compared to schisandrin A-untreated control (100%). Error bars denote the means ± SD of five independent experiments (N = 5). *P < 0.05.
Schisandrin A decreases the mortality of DENV-infected ICR suckling mice
To further examine the anti-DENV activity of schisandrin A in vivo, 6-day-old ICR suckling mice were intracerebrally injected with active DENV or heat-inactivated DENV (iDENV), in which injection of iDENV served as a negative control, and injection of 100 U/g IFN served as a positive control. The DENV-infected mice were administered either saline or schisandrin A injection at 1, 3, and 5 days postinfection (dpi). The clinical score, body weight, and the survival rate were daily monitored. The brain tissue was collected to analyze the virus titer using plaque assay at 6 dpi. As shown in Fig. 3A, the DENV-infected mice treated with schisandrin A showed a lower clinical score than that of mice treated with saline and the iDENV-infected control mice. The body weights of DENV-infected mice receiving schisandrin A treatment were recovered, compared to those of iDENV-infected or DENV-infected mice at 6 dpi (Fig. 3B). The survival rate of DENV-infected mice treated with schisandrin A reached 80%, compared to that of DENV-infected mice with saline treatment at 6 dpi (Fig. 3C). An approximate two log decrease in the viral titer in the brain of DENV-infected mice treated with schisandrin A was measured, compared to that of DENV-infected mice without schisandrin A treatment (Fig. 3D).
(A–D) Six-day-old ICR suckling mice were intracerebrally injected with heat-inactivated DENV (iDENV, blue line) or active DENV. DENV-infected mice received saline (DENV, blue line), 100 U/g IFN (DENV+IFN, green line), 5 (DENV+schisandrin A 5 mg/kg, yellow line) or 10 mg/kg (DENV+Schisandrin A 10 mg/kg, purple line) schisandrin A treatment every 2 days. Mice were sacrificed at 6 days postinfection, and the (A) clinical scores, (B) body weight, and (C) survival rates were measured daily. Disease severity was scored as follows: 0: no symptom, 1: slight losing weight and ruffled hair, 2: slowing of activity, 3: asthenia, 4: paralysis and mortally ill, and 5: death. (D) The brain tissue was collected to analyze the virus titer by plaque-forming assay. Each group included ten mice. Error bars denote the means ± SD. *P < 0.05.
Schisandrin A induces antiviral IFN-I gene expression
IFN responses have been demonstrated to play an important role in resistance to viral infections. To evaluate whether antiviral IFN responses are involved in the anti-DENV activity of schisandrin A, the DENV-infected Huh-7 cells were treated with schisandrin A for 3 days, and the mRNA levels of IFN-alpha-2 (IFN-α-2), IFN-alpha-5 (IFN-α-5), and IFN-alpha-17 (IFN-α-17) were analyzed by RT-qPCR. As shown in Fig. 4A–C, schisandrin A significantly elevated DENV-reduced IFN-α gene expression in a dose-dependent manner. As expected, schisandrin A increased the amount of IFN-α protein production from DENV-infected Huh-7 cells in a concentration-dependent manner by ELISA analysis (Fig. 4D). Additionally, we also characterized that schisandrin A increased IFN-α-2, IFN-α-5 and IFN-α-17 expression in naïve Huh-7 cells (Figure S2), which indicated that schisandrin A exerts the inductive activity of antiviral IFN-I gene expression.
(A–D) The DENV-infected Huh-7 cells were treated with 0.1% of DMSO (dose 0, a negative control), 25, 30, 35, and 40 μM of schisandrin A for 3 days. Total RNA was collected and the cellular (A) IFN-α-2, (B) IFN-α-5, and (C) IFN-α-17 mRNA levels were determined by RT-qPCR. The gene expression was normalized by the cellular gapdh mRNA level. (D) The supernatant was collected and the IFN-α protein level was analyzed using ELISA kit. Error bars denote the means ± SD of five independent experiments (N = 5). *P < 0.05.
Schisandrin A increased STAT1/2 phosphorylation for induction of antiviral IFN responses
Phosphorylation of STAT1 and STAT2 is required for activation of IFN-mediated antiviral responses9. To further identify whether schisandrin A could mediate STAT activity for anti-DENV action, we examined the phosphorylation status of STAT1 and 2 in DENV-infected Huh-7 cells in the presence or absence of schisandrin A. The Western blotting results indicated that the amount of phospho-STAT1/2 level was time-dependently accumulated upon schisandrin A treatment (Fig. 5A, lanes 2, 4, and 6), with a 2- and 2.5-fold change relative to the schisandrin A-untreated control mice (Fig. 5A, lanes 1, 3, and 5), following quantification by densitometric analysis. To further clarify the role of STAT1/2-mediated signaling pathway in the anti-DENV activity of schisandrin A, we silenced STAT1/2 expression using specific shRNAs against STAT1 and STAT2, respectively, and DENV protein synthesis was measured by Western blotting. As shown in Fig. 5B, downregulation of STAT1/2 expression significantly restored DENV replication (upper panel, lanes 3–5) compared to nonspecific eGFP shRNA-transfected Huh-7 cells with or without schisandrin A treatment (lanes 1 and 2). To investigate the STAT1/2-dependent stimulation on antiviral IFN responses, Huh-7 cells were transiently transfected with pISRE-Luc reporter plasmid carrying IFN-stimulated response element (ISRE)-driven firefly luciferase in the presence of DENV infection and schisandrin A treatment for 3 days. As shown in Fig. 6A, the ISRE promoter activity was significantly increased with schisandrin A treatment. We subsequently examined the expression of IFN-based antiviral genes, including OAS1, OAS2, OAS3, and PKR, in DENV-infected Huh-7 cells with schisandrin A treatment. The RT-qPCR results showed that schisandrin A significantly stimulated the antiviral gene expression in a concentration-dependent manner (Fig. 6B–E).
(A) Accumulation of phosphorylated STAT1 and STAT2 levels upon schisandrin A. DENV-infected Huh-7 cells were treated with 0 or 40 μM of schisandrin A for 0, 24, or 36 h. STAT1 and STAT 2 phosphorylation (P-STAT1 and P-STAT2) was analyzed by Western blotting with anti-phosphorylated STAT1 and STAT2 antibodies. The total STAT1 and STAT2 level (T-STAT1 and T-STAT2) were determined by anti-STAT1 and STAT2 antibodies. The band intensities were quantified by densitometry, and the fold-change that was relative to schisandrin A-untreated control (defined as 1) at different time points was normalized by GAPDH protein level. (B) STAT1 and STAT2 silencing restored the inhibitory effect of schisandrin A on DENV replication. Huh-7 cells were transfected with different amounts of shRNA against STAT1, STAT2 (0.5 μg), or eGFP (0.5 μg), followed by DENV infection and schisandrin A treatment. DENV, STAT1, and STAT 2 protein levels were analyzed by Western blotting.
(A) Induction of ISRE activity by schisandrin A. Huh-7 cells transiently expressing pISRE-Luc cells were infected with DENV, followed by treatment with 0.1% of DMSO (dose 0, a negative control), 25, 30, 35, and 40 μM of schisandrin A for 3 days. The luciferase activity was analyzed by Steady-GloLuciferase Assay System (Promega). (B–E) Induction of antiviral IFN responses by schisandrin A. DENV-infected Huh-7 cells were treated with schisandrin A at the indicated concentrations. After 3 days, the antiviral gene expression was analyzed by RT-qPCR using specific primers. The expression of genes was normalized by the cellular gapdh mRNA level. Error bars denote the means ± SD of five independent experiments (N = 5). *P < 0.05.
Schisandrin A inhibits DENV replication and stimulates IFN-mediated antiviral responses in vivo
To confirm the mechanism by which schisandrin A inhibits DENV replication through the stimulation of IFN-mediated antiviral responses in vivo, we performed a DENV-infected ICR suckling mice model to measure IFN-α-2, IFN-α-5, and IFN-based antiviral gene expression in brain tissues by RT-qPCR analysis. As shown in Fig. 7A, both IFN-α-2 and IFN-α-5 RNA levels were induced by schisandrin A. Consistently, the RNA levels of IFN-based antiviral genes were also stimulated by schisandrin A due to IFN production (Fig. 7B).
(A,B) DENV-infected suckling mice were sacrificed at 6 dpi, and then the brain tissue was collected to determine (A) IFN-α-2, (B) IFN-α-5, (C) OAS1, (D) OAS2, (E) OAS3, and (F) PKR expression levels by RT-qPCR using specific primers. Gene expression was normalized by the cellular gapdh mRNA level. Filled circles, open circles, filled triangles and filled triangles indicate schisandrin A-untreated, IFN-treated, 5 or 10 mg/kg of schisandrin A-treated mice, respectively. Each group included ten mice. *P < 0.05.
Discussion
In the present study, we observed that schisandrin A treatment significantly induced IFN-α and its downstream antiviral ISG expression against DENV replication in vitro and in vivo (Figs 4, 6, 7 and S2). Furthermore, we verified that schisandrin A-mediated STAT1/2 phosphorylation is involved in IFN-based inhibition of DENV replication (Fig. 5) and proposed the action mechanism of schisandrin A against DENV replication (Fig. 8). These observations are consistent with the conclusion of Michael S. Diamond et al. that stimulation of antiviral IFN pathway is a promising strategy to disrupt DENV replication25. There are two signaling pathways to activate the host innate response against viral infection through production IFN-α and IFN-β; one is toll-like receptor (TLR)-mediated NF-κB, IFN regulatory factor-3 (IRF3), and IRF7 expression, and the other is RNA helicase retinoic acid-inducible gene I (RIG-I)-mediated mitochondrial antiviral signaling protein (MAVS) activation for activation of NF-κB and IRF3/726,27. Further experiments will be carried out to investigate the correlation between schisandrin A and the upstream mediators of IFNs. To escape host antiviral immune responses and facilitate virus replication, viral proteins would interfere with antiviral IFN responses for an efficient virus production, such as human papilloma virus (HPV), human parainfluenza virus 2 (HPIV2), and West Nile virus (WNV)28,29,30. Current studies have demonstrated that enhanced ISG expression sufficiently suppresses DENV replication through IFN-induced transmembrane protein 2 and 3 (IFITM2 and 3), PKR, and OAS genes7,12. It would be noteworthy to further clarify how schisandrin A interrupts DENV protein-suppressed antiviral IFN pathway.
Infection with more than one serotype of DENV leads to high risk of DHF and DSS, which is the major cause of death among DENV-infected patients5. Previous studies reported that the plasma viremia in DHF/DSS patients is higher than that in DF patients, which indicates that reducing DENV viral level has the possibility to prevent or lessen the chances of patients progressing to DHF/DSS7,8. Our study demonstrated that schisandrin A can inhibit the four serotypes of DENV (Fig. 2), indicating that schisandrin A not only is a potential anti-DENV agent but also provides a capable therapy to prevent DHF/DSS. Previously, the extract of S. chinensis has been used in clinical trials to treat HCV infection, and no obvious side effects were observed31. In the present study, we identified that schisandrin A could protect ICR suckling mice from death due to DENV infection through the upregulation of IFN antiviral responses in vitro and in vivo (Figs 4 and 7), which offers the possibility of advancing the plant-derived natural product to clinical use against DENV infection and severe DENV-induced diseases.
In conclusion, we identified schisandrin A as a potential antiviral agent against DENV in vitro and in vivo. We further clarified the mechanism by which schisandrin A increased IFN expression and activated JAK-STAT pathway to trigger antiviral innate responses against DENV replication.
Materials and Methods
Ethics statement and experimental animals
Six-days-old ICR suckling mice were used in this study and beeder mice of the ICR strain were obtained from BioLasco Taiwan Co. Ltd. All animal studies were conducted in specific pathogen-free conditions and methods were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. The experimental protocol were approved by the Animal Research Committee of Kaohsiung Medical University of Taiwan (IACUC, 102177) under the guidance of the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. All mice received humane care and were fed with standard rodent chew and water ad libitum. Mice were acclimatized under a standard laboratory condition following the Animal Use Protocol of Kaohsiung Medical University for a week before experiment.
Chemicals
Schisandrin A, schisandrin B, and schisandrin C (Fig. 1A) were purchased from Fusol-Material, Tainan, Taiwan. All these compounds were dissolved in dimethyl sulfoxide (DMSO) to establish stock solution of 50 mM and stored at −20 °C. All reactions were carried out in a final concentration of DMSO at 0.1%.
Cells and virus
Huh-7 cells and BHK-21 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids, and 1% antibiotic–antimycotic in 5% CO2 supplement at 37 °C. The C6/36 cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 1% nonessential amino acids, 1% L-glutamine, 1% sodium pyruvate, and 1% antibiotic–antimycotic in 5% CO2 supplement at 37 °C. DENV-2 strain 16681 was kindly provided by Dr. Huey-Nan Wu, and the other types of DENV (DENV-1:DN8700828; DENV-3: DN8700829; DENV-4: S9201818) were obtained from the Centers for Disease Control, Department of Health, Taiwan. DENV was generated in the C6/36 mosquito cells2,32. Virus titer was determined by plaque assay.
Plasmid construction
pISRE-Luc containing firefly luciferase under the control of an IFN-stimulated response element (ISRE) was used to measure IFN response-dependent transcriptional activity (Stratagene, Agilent Technologies, Palo Alto, CA, USA). STAT1 (NM_007315), STAT2 (NM_005419), and eGFP short hairpin RNA (shRNA) were purchased from the National RNAi Core Facility, Institute of Molecular Biology/Genomic Research Center, Academia Sinica, Taipei, Taiwan.
Quantification of RNA level
Total cellular RNA was extracted by RNA Purification Kit (GMbiolab Co, Ltd., Taichung, Taiwan) following the manufacturer’s instructions. DENV RNA or cellular mRNA level was analyzed by quantitative real-time reverse-transcription polymerase chain reaction (RT-qPCR) with specific primers2. The gene expression level was normalized by the cellular glyceraldehyde-3-phosphate dehydrogenase (gapdh) mRNA level. Primers used in the study are listed in Table 1.
Western blotting
The cells were lysed using RIPA lysis buffer and collected for Western blotting assay. The antibodies used in this study were anti-DENV NS2B antibody (1:3000; GeneTex, Inc, Irvine, CA), STAT1 antibody (1:3000; GeneTex), STAT2 antibody (GeneTex), phosphorylated STAT1 (Tyr701) antibody (1:1000; Cell Signaling Technology, Inc. Beverly, MA), phosphorylated STAT2 (Tyr690) antibody (1:1000; Cell Signaling), and anti-GAPDH antibody (1:10000; GeneTex), in which detection of GAPDH protein level served as an internal control33. DENV proteins are generated from proteolysis of single polypeptide translated from viral RNA, therefore each viral protein level can represent the DENV protein synthesis level. In our study, the NS2B served as an indicator of viral protein synthesis.
Transfection
Huh-7 cells were seeded in 24-well plates and then transfected with the indicated plasmids by T-Pro™ transfection reagent (Ji-Feng Biotechnology Co., Ltd, Taipei, Taiwan) according to the manufacturer’s instructions.
Measurement of luciferase activity
Luciferase reporter expression was analyzed by Steady-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions.
Analysis of extracellular IFN-α protein level
Huh-7 cells were seeded in 24-well plates and then infected with DENV at an MOI of 0.2 for 2 h, followed by schisandrin A treatment. After 3 days, the supernatant was collected and IFN-α concentration was measured by human IFN-α ELISA kit (Life Science) according to the manufacturer’s protocol. Absorbance was detected at 450 nm using an Epoch microplate spectrophotometer (BioTek Instruments Inc., USA).
Anti-DENV activity in vivo
Six-day-old ICR suckling mice were randomly divided into three groups (5 mice each group); Group 1 received 60 °C heat-inactivated DENV and saline treatment (iDENV); Group 2 received 2.5 × 105 plaque-forming unit (PFU) of DENV and saline treatment (DENV); Group 3 receive 2.5 × 105 PFU of DENV and 100 U/g of interferon treatment at 1, 3, and 5 days post infection (DENV+IFN); Group 4 received 2.5 × 105 PFU of DENV and 5 mg/kg of schisandrin A treatment at 1, 3, and 5 days post infection (DENV+schisandrin A 5 mg/kg); and Group 5 received 2.5 × 105 PFU of DENV and 10 mg/kg of schisandrin A treatment at 1, 3, and 5 days post infection (DENV+schisandrin A 10 mg/kg). The tested doses were chosen according to the study of Hu et al.23. After 6 days, the suckling mice were sacrificed by carbon dioxide euthanasia for determining the virus titer and virus RNA levels in brain tissues. To determine the virus titer, the brain tissues were weighed and homogenized in 0.5 ml RPMI 1640 medium supplemented with 2% FBS and then centrifuged at 8000 rpm for 15 min at 4 °C. Finally, the supernatants were collected and stored at −80 °C. To determine the DENV RNA levels, the brain tissues were weighed and homogenized in 0.5 ml TRIzol reagent (Invitrogen, Carlsbad, CA) and stored at −80 °C. The RNA samples were extracted according to the manufacturer’s instructions.
Plaque assay
BHK-21 cells were seeded in 12-well plates and infected by serially diluted virus. After 2 h of incubation, the virus inoculum was refreshed with complete DMEM containing 0.8% methyl cellulose (Sigma-Aldrich, St. Louis, MO, USA) for 5 days. Then, the virus-infected cells were fixed and stained with crystal violet solution (1% crystal violet, 0.64% NaCl, and 2% formalin) for 1 h. The crystal violet solution was washed away and the virus titer was calculated.
Statistical analysis
Data are expressed as mean ± standard deviations of at least five independent experiments (n ≥ 5). Statistical significance were determined using Student’s t test for differences between 2 data groups (drug-treated and -untreated cells). The n value indicates the number of experiments used. *P < 0.05 was considered to be significant.
Additional Information
How to cite this article: Yu, J.-S. et al. Schisandrin A inhibits dengue viral replication via upregulating antiviral interferon responses through STAT signaling pathway. Sci. Rep. 7, 45171; doi: 10.1038/srep45171 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ng, J. K. et al. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS pathogens 10, e1004031, doi: 10.1371/journal.ppat.1004031 (2014).
Lee, J. C. et al. Characterization of the activity of 2′-C-methylcytidine against dengue virus replication. Antiviral research 116, 1–9, doi: 10.1016/j.antiviral.2015.01.002 (2015).
Rice, C. M. et al. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science 229, 726–733 (1985).
Lee, Y. R. et al. Dengue virus infection induces autophagy: an in vivo study. Journal of biomedical science 20, 65, doi: 10.1186/1423-0127-20-65 (2013).
Dalrymple, N. A., Cimica, V. & Mackow, E. R. Dengue Virus NS Proteins Inhibit RIG-I/MAVS Signaling by Blocking TBK1/IRF3 Phosphorylation: Dengue Virus Serotype 1 NS4A Is a Unique Interferon-Regulating Virulence Determinant. mBio 6, e00553–00515, doi: 10.1128/mBio.00553-15 (2015).
Chareonsirisuthigul, T., Kalayanarooj, S. & Ubol, S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. The Journal of general virology 88, 365–375, doi: 10.1099/vir.0.82537-0 (2007).
Jones, M. et al. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. Journal of virology 79, 5414–5420, doi: 10.1128/JVI.79.9.5414-5420.2005 (2005).
Ubol, S. et al. Differences in global gene expression in peripheral blood mononuclear cells indicate a significant role of the innate responses in progression of dengue fever but not dengue hemorrhagic fever. The Journal of infectious diseases 197, 1459–1467, doi: 10.1086/587699 (2008).
Morrison, J. et al. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS pathogens 9, e1003265, doi: 10.1371/journal.ppat.1003265 (2013).
Fink, K. & Grandvaux, N. STAT2 and IRF9: Beyond ISGF3. Jak-Stat 2, e27521, doi: 10.4161/jkst.27521 (2013).
Jiang, D. et al. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. Journal of virology 84, 8332–8341, doi: 10.1128/JVI.02199-09 (2010).
Simon-Loriere, E. et al. High Anti-Dengue Virus Activity of the OAS Gene Family Is Associated With Increased Severity of Dengue. The Journal of infectious diseases 212, 2011–2020, doi: 10.1093/infdis/jiv321 (2015).
Yu, C. Y. et al. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS pathogens 8, e1002780, doi: 10.1371/journal.ppat.1002780 (2012).
Munoz-Jordan, J. L. et al. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. Journal of virology 79, 8004–8013, doi: 10.1128/JVI.79.13.8004-8013.2005 (2005).
Checker, R. et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-kappaB. Free radical biology & medicine 53, 1421–1430, doi: 10.1016/j.freeradbiomed.2012.08.006 (2012).
Liu, H. et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomedicine: international journal of phytotherapy and phytopharmacology 20, 1135–1143, doi: 10.1016/j.phymed.2013.05.001 (2013).
Park, S. Y. et al. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. International immunopharmacology 17, 415–426, doi: 10.1016/j.intimp.2013.06.032 (2013).
Guo, L. Y. et al. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. European journal of pharmacology 591, 293–299, doi: 10.1016/j.ejphar.2008.06.074 (2008).
Panossian, A. & Wikman, G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. Journal of ethnopharmacology 118, 183–212, doi: 10.1016/j.jep.2008.04.020 (2008).
Yuan, G. et al. Simultaneous and rapid determination of main lignans in different parts of Schisandra sphenanthera by micellar electrokinetic capillary chromatography. Molecules 16, 3713–3722, doi: 10.3390/molecules16053713 (2011).
Kim, H., Ahn, Y. T., Kim, Y. S., Cho, S. I. & An, W. G. Antiasthmatic effects of schizandrae fructus extract in mice with asthma. Pharmacognosy magazine 10, S80–85, doi: 10.4103/0973-1296.127348 (2014).
Li, J. et al. Effect of Schisandra chinensis on interleukins, glucose metabolism, and pituitary-adrenal and gonadal axis in rats under strenuous swimming exercise. Chinese journal of integrative medicine 21, 43–48, doi: 10.1007/s11655-014-1765-y (2015).
Hu, D. et al. Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Abeta(1)(-)(4)(2)-induced memory impairment in mice. Planta medica 78, 1332–1336, doi: 10.1055/s-0032-1315019 (2012).
Xu, L. et al. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. Journal of microbiology 53, 288–293, doi: 10.1007/s12275-015-4652-0 (2015).
Diamond, M. S. et al. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. Journal of virology 74, 4957–4966 (2000).
Chen, H. W. et al. The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus. Journal of immunology 191, 4194–4201, doi: 10.4049/jimmunol.1300799 (2013).
Nasirudeen, A. M. et al. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS neglected tropical diseases 5, e926, doi: 10.1371/journal.pntd.0000926 (2011).
Li, S. et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene 18, 5727–5737, doi: 10.1038/sj.onc.1202960 (1999).
Parisien, J. P. et al. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283, 230–239, doi: 10.1006/viro.2001.0856 (2001).
Mackenzie, J. M., Khromykh, A. A. & Parton, R. G. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell host & microbe 2, 229–239, doi: 10.1016/j.chom.2007.09.003 (2007).
Melhem, A. et al. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. Journal of clinical gastroenterology 39, 737–742 (2005).
Hsu, Y. C. et al. Identification of a small-molecule inhibitor of dengue virus using a replicon system. Archives of virology 157, 681–688, doi: 10.1007/s00705-012-1224-z (2012).
Lee, J. C. et al. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. British journal of pharmacology 171, 237–252, doi: 10.1111/bph.12440 (2014).
Acknowledgements
We are grateful to Dr. Charles Rice (Rockefeller University) and Apath, LLC, NY, USA, for kindly supporting human hepatoma cell line Huh-7 and Centers for Disease Control, Department of Health, Taiwan, for kindly supporting 4 serotypes of dengue virus (DENV-1: DN8700828; DENV-2: DN454009A; DENV-3: DN8700829A; DENV-4: S9201818). This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST105-3111-Y-043-006, MOST104-2320-B-037-025-MY3 and MOST103-2314-B-037-039-MY3), the Kaohsiung Medical University (KMU-TP104H03), and the Chi-Mei Medical Center and Kaohsiung Medical University Research Foundation (103CM-KMU-11 and 104CM-KMU-05).
Author information
Authors and Affiliations
Contributions
Jin-Ching Lee, Jung-Sheng Yu and Yen-Hsu Chen performed the experimental design. Yu-Hsuan Wu, Chin-Kai Tseng and Chun-Kuang Lin performed the research as described in the Material and Method. Yao-Chin Hsu and Jung-Sheng Yu contributed essential reagents. Yu-Hsuan Wu, Chin-Kai Tseng and Chun-Kuang Lin analyzed the data. Jin-Ching Lee and Yen-Hsu Chen wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, JS., Wu, YH., Tseng, CK. et al. Schisandrin A inhibits dengue viral replication via upregulating antiviral interferon responses through STAT signaling pathway. Sci Rep 7, 45171 (2017). https://doi.org/10.1038/srep45171
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45171
- Springer Nature Limited
This article is cited by
-
Specialized metabolites from plants as a source of new multi-target antiviral drugs: a systematic review
Phytochemistry Reviews (2023)
-
Avocado (Persea americana) fruit extract (2R,4R)-1,2,4-trihydroxyheptadec-16-yne inhibits dengue virus replication via upregulation of NF-κB–dependent induction of antiviral interferon responses
Scientific Reports (2019)
-
Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate
Journal of Biomedical Science (2018)
-
Dengue virus and the host innate immune response
Emerging Microbes & Infections (2018)