Abstract
Understanding how insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) interact with their hosts is crucial to fully explain the molecular bases of Bt specificity and insecticidal activity. Previous studies support ATP binding cassette transporters (ABCC2/3) and one cadherin-like protein are Cry1Ac functional receptors in the beet armyworm (Spodoptera exigua). In this study, a combined one-dimensional gel electrophoresis and immunoblotting approach identified aminopeptidase N (APNs) as putative Cry1Ac binding proteins in the midgut brush border membrane of S. exigua larvae. Functional analyses by gene silencing of six different S. exigua APN genes (SeAPN1, SeAPN2, SeAPN3, SeAPN4, SeAPN5 and SeAPN6) showed that only suppression of SeAPN1 resulted in decreased larval susceptibility to Cry1Ac toxin. These results support that SeAPN1 plays important functional role in Cry1Ac toxicity in S. exigua.
Similar content being viewed by others
Introduction
The crystal (Cry) proteins from the bacterium Bacillus thuringiensis (Bt) are a diverse group of insecticidal proteins employed for the control of numerous pest species from different insect orders1. These Cry proteins are active ingredients in Bt sprayable formulations, and cry genes have been transformed into transgenic plants for resistance to insect attack. Understanding how Cry toxins interact with their insect hosts is crucial to fully explain the molecular bases of specificity and to develop efficient resistance management tools.
The mode of action of Cry toxins in lepidopteran larvae has been thoroughly investigated2. Once the parasporal crystalline bodies containing the Cry proteins are ingested by a susceptible insect, they are solubilized to a protoxin form in the alkaline digestive fluids, and then processed by midgut proteases to an active toxin core. Upon traversing the peritrophic matrix, the activated toxin core binds to specific binding sites on the brush border membrane of the midgut. Binding results in oligomerization and formation of toxin pores that lead to osmotic cell death, compromising the midgut epithelial barrier and allowing resident bacteria to invade the hemocoel to cause septicemia and death of the insect. A number of proteins have been proposed as receptors for the Cry1A family of proteins, including aminopeptidase N (APN), cadherin, ABC transporters and alkaline phosphatase3,4,5,6,7.
The beet armyworm, Spodoptera exigua, has recently become a major economic cotton pest in China8,9,10,11, probably due to the reduced pesticide usage attributed to adoption of Bt cotton producing the Cry1Ac protein. Previous studies reported that knockdown of ABCC transporter 2/3 and one cadherin-like protein in S. exigua larvae decreased their susceptibility to Cry1Ac12,13. In the current work, we used a combined one-dimensional (1D) gel electrophoresis and immunoblotting approach to identify APNs as Cry1Ac binding proteins in the midgut of S. exigua larvae. Using functional assays by RNA interference (RNAi) to individually silence expression of known S. exigua APN genes, we document the identification of APN protein relevant to Cry1Ac intoxication in that insect pest.
Results
Binding proteins of Cry1Ac of S. exigua BBMV
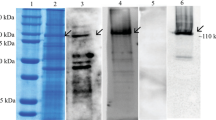
Ligand blots of midgut brush border membrane proteins from S. exigua larvae. Figure 1a identified two prominent Cry1Ac-binding protein bands of about 110- and 130-kDa in size, respectively, which were numbered as bands 1 and 2 (Fig. 1b, panel 2). The specificity of the anti-Cry1Ac antisera used for ligand blotting was confirmed by the lack of cross-reactivity in blots with no Cry1Ac (Fig. 1b, panel 1). The Cry1Ac binding bands were excised and submitted to LC-MS/MS analysis and protein database searching. Parameters used for protein identification included at least two unique peptides detected and molecular weight similar to the Cry1Ac protein bands. The list of detected proteins fulfilling these conditions in each Cry1Ac-binding band is presented in Supplementary Table S1. Among all these proteins, the most abundant in both bands 1 and 2 were N-aminopeptidases (APNs) from S. exigua (Table 1). Consequently, we focused our analyses on testing the functional Cry1Ac-receptor role of S. exigua APNs (SeAPNs).
(a) Total protein silver staining detection of separated S. exigua BBMV proteins. (b) S. exigua BBMV proteins binding Cry1Ac in ligand blots, as detected with Cry1Ac antisera. Panel 1, blotting assay without Cry1Ac, Panel 2, blotting assay with Cry1Ac. Arrows indicate detected Cry1Ac-binding protein bands.
Functional Cry1Ac receptor assays of APNs in S. exigua
Phylogenetic analyses identified six SeAPN genes (SeAPN1 to SeAPN6) belonging to the same number of APN families14, which were selected for further testing. To test their putative Cry1Ac receptor role, we used a gene silencing approach by RNA interference (RNAi) through ingestion of double-stranded RNA (dsRNA) targeting each SeAPN gene. After ingestion of purified dsRNAs specific to SeAPN1, SeAPN2, SeAPN3, SeAPN4, SeAPN5 or SeAPN6 for 48 h, the transcript levels for these genes were significantly reduced by 53%, 62%, 79%, 80.6%, 81% and 53%, respectively, when compared to larvae fed on dsEGFP or water as controls (Fig. 2). Subsequent feeding larvae exposed to dsRNA to a diet overlaid with 3 μg/cm2 of Cry1Ac resulted in 83% and 68% mortality in the water and dsEGFP controls, respectively. In contrast, mortality was 32% (dsAPN1), 67% (dsAPN2), 68% (dsAPN3), 82% (dsAPN4), 96% (dsAPN5), and 62% (dsAPN6) in the experimental treatments (Fig. 3). Statistical analyses (ANOVA, P < 0.05) revealed that the only treatment affecting Cry1Ac susceptibility was feeding on dsSeAPN1 when compared to the water or dsEGFP treatments.
Relative levels of SeAPN transcripts were determined by qRT-PCR of S. exigua larvae fed artificial diet overlaid with either water or dsEGFP as controls, or dsRNA targeting each specific SeAPN. The SeGAPDH and SeRpL10 housekeeping genes were used to normalize transcript levels. Asterisks indicate significant differences (ANOVA followed by Tukey’s HSD posthoc test, P < 0.05).
Larvae were fed on diet overlaid with dsRNA targeting EGFP, SeAPN1, SeAPN2, SeAPN3, SeAPN4, SeAPN5 or SeAPN6, and then they were exposed to diet contaminated with 3 μg/cm2 of Cry1Ac. Bars denotes standard error of the mean calculated from five replicates. Different letters on top of bars indicate significant differences (ANOVA followed by Tukey’s HSD posthoc test, P < 0.05).
Discussion
Aminopeptidases N (APNs) are a class of metalloenzymes widely present in the apical membranes of the insect midgut that remove neutral amino acids from the N-terminus of polypeptides4. A number of studies support APNs as binding proteins for Cry toxins in lepidopteran and dipteran insects15,16,17,18,19,20,21. For instance, expression of a Manduca sexta APN gene in transgenic Drosophila resulted in susceptibility to Cry1Ac toxin22. Silencing of APNs expression results in reduced susceptibility to Cry1C in S. litura23, to Cry1Ac in H. armigera24, or to Cry4Ba in A. aegypti25. Moreover, resistance to Cry1Ac was correlated with down-regulation and deletion mutations in APN1 genes in Trichoplusia ni and H. armigera, respectively26,27. Very recently, APN1 has been identified as a Cry1Ac receptor in H. zea28, while APN1 and APN2 genes were reported as Cry11A receptors in A. aegypti20,29. Two partial APN fragments from Anopheles gambiae had inhibitory effects on the larval susceptibility to Cry11B toxin19.
In S. exigua, a total of six APN genes were identified in a previous study14. Silencing of SeAPN1, SeAPN3 or SeAPN6 expression was shown to reduced susceptibility to Cry1Ca14. Moreover, S. exigua resistance to Cry1Ca was associated with lack of expression of a SeAPN1 gene30. In the present work, we present the identification of SeAPN1 as a Cry1Ac receptor, and data supporting that other SeAPNs do not serve as receptors for this toxin. Together with previous reports, these data support that SeAPN1 is a common functional receptor for Cry1Ac and Cry1Ca toxins30. This observation would suggest that cross-resistance between Cry1Ac and Cry1Ca in S. exigua is likely. In agreement with this hypothesis, cross-resistance was observed between Cry1Ab and Cry1Ca in S. exigua larvae after selection with toxin31. The possible reason of this cross-resistance might be that the two toxins share same binding site of SeAPN1, while further work would be needed to test this hypothesis, sharing of binding proteins between Cry1 and Cry1Ca proteins would have important consequences for resistance management tactics, as these proteins have been proposed as candidates for gene pyramiding in transgenic crops32,33.
Our data also clearly support that SeAPN1 is the only SeAPN functioning as a Cry1Ac receptor. Other reports also support APN1 proteins as receptors for Cry1Ac in T. ni27, M. sexta34 and H. armigera26. However, there is evidence for alternative APNs interacting with Cry1Ac in other lepidopteran insects. For instance, APN2 binds Cry1Ac in H. armigera35, but not in Lymantria dispar36, M. sexta, P. xylostella or B. mori16,37,38. Both APN3 and APN5 can bind Cry1Ac in H. armigera39 and P. xylostella40, but their involvement in Cry1Ac resistance remains to be confirmed. In contrast, there is no evidence supporting a Cry1Ac receptor role for APN4, APN6, APN7 or APN8 proteins. Specific glycosylation or sequence attributes may explain this specificity of Cry1Ac for some APN proteins.
In our combined ligand blotting and MS/MS analyses we identified two protein bands as SeAPN1 and SeAPN3. In addition to SeAPNs, we also detected other proteins in the Cry1Ac-binding bands that may specifically interact with Cry1Ac. For example, S. exigua cadherin peptide was detected in band 1, albeit with low probability, and cadherins have been demonstrated to act as Cry1Ac receptors in previous studies12,41, The functional role for these alternative proteins needs to be examined. Based on relative abundance and the results from gene silencing, the present work identifies SeAPN1 as the only SeAPN acting as a functional Cry1Ac receptor in S. exigua larvae. The potential sharing of this receptor needs to be further explored to evaluate risks of resistance evolution for pyramided Cry1A and Cry1Ca genes in transgenic crops.
Materials and Methods
Insect rearing, midgut dissection and BBMV preparation
S. exigua larvae were collected at the campus greenhouse of the Huazhong Agricultural University in June 2012 and reared without exposure to Cry toxins. Insects were maintained at 28 ± 1 °C, a 14 L:10D photoperiod, and 70–80% relative humidity. Larvae were reared on an artificial diet42 and adults fed on 10% sucrose. Actively feeding fourth-instar larvae were chilled for 5 minutes on ice and dissected, the midgut tissue was cleaned from trachea, Malpighian tubules, peritrophic membrane and food bolus and rinsed briefly in ice-cold MET buffer (300 mM Mannitol, 17 mM Tris-HCl, 5 mM EGTA, pH 7.5). Dissected midguts were stored frozen at −80 °C until used.
BBMV were prepared from the dissected midguts by the differential magnesium precipitation method43. Briefly, midguts were homogenized in nine volume of midgut weight MET buffer containing 1 mM Phenylmethanesulfonyl fluoride (PMSF) using a tissue homogenizer, an equal volume of 24 mM MgCl2 was added and samples were incubated on ice for 15 min before centrifugation at 2,500 g for 15 min. This step was repeated three times, and the combined supernatant was collected and centrifuged at 30,000 g for 30 min. The final BBMV pellet was resuspended in ice-cold buffer (10 mM HEPES, 130 mM KCl, 10% glycerol, pH 7.5) containing 1 mM PMSF43. Protein concentration was determined by the method of Bradford44 with bovine serum albumin (BSA) as a standard.
Ligand blotting and mass spectrometry
Proteins of S. exigua BBMV (10 μg) were separated by 8% SDS-PAGE and transferred 25 minutes to PVDF filters at 15 V constant voltage. After blocking in PBST buffer (135 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1.7 mM KH2PO4, pH 7.5, 0.1% Tween-20) containing 5% (w/v) skim milk for 2 h, filters were incubated with 0.3 μg/ml of activated Cry1Ac for 2 h at room temperature. A control experiment was performed without incubation with Cy1Ac toxin. The filters were washed in PBST buffer three times followed by probing with a 1:3,500 dilution of polyclonal antibody to Cry1Ac for 2 h. After washing as above, the membranes were incubated in 1:5,000 diluted goat anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody. The filters were developed with an ECL kit (Fermentas/Thermo Fisher Scientific, Waltham, MA USA) following manufacturer’s recommendations.
After ligand blotting, the gel bands observed to bind Cry1Ac were excised and rinsed in destaining solution (30% acetonitrile/100 mM NH4HCO3). The gel bands were then incubated with 100 mM Dithiothreitol (DTT) at 56 °C for 30 minutes, treated with 200 mM indole-3-acetic acid (IAA) after abandon supernatant, and then incubated with 100 mM NH4HCO3 then remove liquid. As a last step, samples were treated with 100% acetonitrile for 5 minutes and freeze dried before digestion with 2.5–10 ng/μg trypsin for 24 hours at 37 °C and analysis by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) at the Shanghai Life Science Research Institute (China Academy of Sciences, Shanghai, China). The mass spectrometry results were queried to the uniprot database using the Mascot2.2 software.
RNA interference of SeAPNs
The pET-2P plasmid was used to produce double-stranded RNAs targeting SeAPNs (dsSeRNA) and EGFP (dsEGFP), as described by Ren et al.14. The primers used in cloning the dsRNA fragments are listed in Table 2. Amplicons were purified and digested with restriction enzymes (Table 2), and then ligated into the previously digested pET-2P, to generate pET-2P/SeAPN and pET-2P/EGFP dsRNAs plasmids. Correct inserts were confirmed by sequencing at Genscript Biology Company, Nanjing, China. For dsRNA expression, 200 ng of plasmid DNA was transformed into Escherichia coli HT115 (DE3) competent cells, positive clones were cultured in 500 ml LB medium and induced to express dsRNA by adding 0.4 mM isopropyl-D-thiogalactoside (IPTG). The same methods described by Timmons et al.45 and Dong et al.46 were used to extract dsRNA from aliquots of bacteria, and the size of dsRNA was confirmed by electrophoresis on a 1% agarose gel.
Bioassay
Newly hatched S. exigua larvae were fed artificial diet overlaid with 50 μg/cm2 of dsSeAPNs, dsEGFP or water for 48 h at 27 °C. Larvae were then transferred to wells of a 6-well plate where they were allowed to feed for 7 days on artificial diet contaminated with 3 μg/cm2 of activated Cry1Ac toxin (equivalent to the LC70 value according to preliminary experiment), or on diet contaminated with water as a control. A total of 120 larvae were used for five replicated bioassays for each treatment. To monitor the silencing efficiency for each target gene, 15 larvae whole body from each replicate, 3 replicates for each dsRNA treatment were used to extract total RNA. The relative differences of target gene expression level were detected by qRT-PCR with the primers presented in Table 2, which were designed in the NCBI profile server (http://www.ncbi.nlm.nih.gov/tools/primer-blast). The SeGAPDH and SeRpL1047,48 genes were used as reference for normalization. The qPCR protocol followed was described elsewhere41.
Data analysis
Abbott’s formula was used to calculate the larval corrected mortalities49, means and variances of treatments were analyzed by one-way ANOVA using SPSS for Windows (SPSS 18.0, Chicago, IL, USA). Quantitative expression data were analyzed by the 2−∆∆Ct method50.
Additional Information
How to cite this article: Qiu, L. et al. Aminopeptidase N1 is involved in Bacillus thuringiensis Cry1Ac toxicity in the beet armyworm, Spodoptera exigua. Sci. Rep. 7, 45007; doi: 10.1038/srep45007 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bravo, A., Likitvivatanavong, S., Gill, S. S. & Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem Mol Biol. 41, 423–431 (2011).
Adang, M., Crickmore, N. & Jurat-Fuentes, J. L. InAdvances in Insect Physiology, Vol. 47 (eds Dhadialla, T. S. & Gill, S. ), 39–87 (Academic Press, 2014).
Bravo, A., Gill. S. S. & Soberon, M. Bacillus thuringiensis mechanisms and use. Comprehensive molecular insect science. 6, 175–205 (2005).
Pigott, C. R. & Ellar, D. J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 71, 255–281 (2007).
Soberon, M., Gill, S. S. & Bravo, A. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci. 66, 1337–1349 (2009).
Pardo-Lopez, L., Soberon, M. & Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 37, 3–22 (2013).
Guo, Z. et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 11, e1005124 (2015).
Adamczyk, J. J. et al. Evaluations of bollgardR, bollgard IIR, and widestrike (R) technologies against beet and fall armyworm larvae (lepidoptera: noctuidae). Fla Entomol. 91, 531–536 (2008).
Avisar, D. et al. The Bacillus thuringiensis delta-endotoxin Cry1C as a potential bioinsecticide in plants. Plant Sci. 176, 315–324 (2009).
Stewart, S., Adamczyk, J., Knighten, K. & Davis, F. Impact of Bt cottons expressing one or two insecticidal proteins of Bacillus thuringiensis berliner on growth and survival of noctuid (Lepidoptera) larvae. J Econ Entomol. 94, 752–760 (2001).
Zheng, X. L., Wang, P., Wang, X. P. & Lei, C. L. Damage, occurrence and control of Spodoptera exigua on transgenic cottons. Plant Prot. 3, 009 (2010).
Chen, R. R. et al. A cadherin-like protein from the beet armyworm Spodoptera exigua (Lepidoptera: noctuidae) is a putative Cry1Ac receptor. Arch Insect Biochem Physiol. 86, 58–71 (2014).
Park, Y. et al. ABCC transporters mediate insect resistance to multiple Bt toxins revealed by bulk segregant analysis. BMC Biol. 12, 15 (2014).
Ren, X. L., Ma, Y., Cui, J. J. & Li, G. Q. RNA interference-mediated knockdown of three putative aminopeptidases N affects susceptibility of Spodoptera exigua larvae to Bacillus thuringiensis Cry1Ca. J Insect Physiol. 67, 28–36 (2014).
Gill, S. S., Cowles, E. A. & Francis, V. Identification, isolation, and cloning of a Bacillus thuringiensis Cry1Ac toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens . J Biol Chem. 270, 27277–27282 (1995).
Masson, L., Lu, Y. J., Mazza, A., Brousseau, R. & Adang, M. J. The Cry1A(c) receptor purified from Manduca sexta displays multiple specificities. J Biol Chem. 270, 20309–20315 (1995).
Burton, S. L., Ellar, D. J., Li, J. & Derbyshire, D. J. N-acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J Mol Biol. 287, 1011–1022 (1999).
Jenkins, J. L., Lee, M. K., Valaitis, A. P., Curtiss, A. & Dean, D. H. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J Biol Chem. 275, 14423–14431 (2000).
Zhang, R., Hua, G., Urbauer, J. L. & Adang, M. J. Synergistic and inhibitory effects of aminopeptidase peptides on Bacillus thuringiensis Cry11Ba toxicity in the mosquito Anopheles gambiae . Biochemistry. 49, 8512–8519 (2010).
Chen, J., Likitvivatanavong, S., Aimanova, K. G. & Gill, S. S. A 104 kDa Aedes aegypti aminopeptidase N is a putative receptor for the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis . Insect Biochem Mol Biol. 43, 1201–1208 (2013).
Crava, C. M., Bel, Y., Jakubowska, A. K., Ferre, J. & Escriche, B. Midgut aminopeptidase N isoforms from Ostrinia nubilalis: activity characterization and differential binding to Cry1Ab and Cry1Fa proteins from Bacillus thuringiensis . Insect Biochem Mol Biol. 43, 924–935 (2013).
Gill, M. & Ellar, D. Transgenic Drosophila reveals a functional in vivo receptor for the Bacillus thuringiensis toxin Cry1Ac1. Insect Mol Biol. 11, 619–625 (2002).
Rajagopal, R., Sivakumar, S., Agrawal, N., Malhotra, P. & Bhatnagar, R. K. Silencing of midgut aminopeptidase N of Spodoptera litura by double-stranded RNA establishes its role as Bacillus thuringiensis toxin receptor. J Biol Chem. 277, 46849–46851 (2002).
Sivakumar, S., Rajagopal, R., Venkatesh, G. R., Srivastava, A. & Bhatnagar, R. K. Knockdown of aminopeptidase-N from Helicoverpa armigera larvae and in transfected Sf21 cells by RNA interference reveals its functional interaction with Bacillus thuringiensis insecticidal protein Cry1Ac. J Biol Chem. 282, 7312–7319 (2007).
Saengwiman, S. et al. In vivo identification of Bacillus thuringiensis Cry4Ba toxin receptors by RNA interference knockdown of glycosylphosphatidylinositol-linked aminopeptidase N transcripts in Aedes aegypti larvae. Biochem Biophys Res Commun. 407, 708–713 (2011).
Zhang, S. et al. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol. 39, 421–429 (2009).
Tiewsiri, K. & Wang, P. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc Natl Acad Sci USA. 108, 14037–14042 (2011).
Wei, J. et al. APN1 is a functional receptor of Cry1Ac but not Cry2Ab in Helicoverpa zea . Sci Rep. 6, 19179 (2016).
Chen, J., Aimanova, K. G., Pan, S. & Gill, S. S. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem Mol Biol. 39, 688–696 (2009).
Herrero, S., Gechev, T., Bakker, P. L., Moar, W. J. & de Maagd, R. A. Bacillus thuringiensis Cry1Ca-resistant Spodoptera exigua lacks expression of one of four aminopeptidase N genes. BMC Genomics. 6, 96 (2005).
Hernandez-Martinez, P., Ferre, J. & Escriche, B. Broad-spectrum cross-resistance in Spodoptera exigua from selection with a marginally toxic Cry protein. Pest Manag Sci. 65, 645–650 (2009).
Zheng, S. J. et al. Two different Bacillus thuringiensis toxin genes confer resistance to beet armyworm (Spodoptera exigua Hübner) in transgenic Bt-shallots (Allium cepa L.). Transgenic Res. 14, 261–272 (2005).
Jiao, Y., Yang, Y., Meissle, M., Peng, Y. & Li, Y. Comparison of susceptibility of Chilo suppressalis and Bombyx mori to five Bacillus thuringiensis proteins. J Invertebr Pathol. 136 (2016).
Flores-Escobar, B., Rodriguez-Magadan, H., Bravo, A., Soberon, M. & Gomez, I. Differential role of Manduca sexta aminopeptidase-N and alkaline phosphatase in the mode of action of Cry1Aa, Cry1Ab, and Cry1Ac toxins from Bacillus thuringiensis . Appl Environ Microbiol. 79, 4543–4550 (2013).
Rajagopal, R. et al. Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (American cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. Biochem J. 370, 971–978 (2003).
Valaitis, A. P., Mazza, A., Brousseau, R. & Masson, L. Interaction analyses of Bacillus thuringiensis Cry1A toxins with two aminopeptidases from gypsy moth midgut brush border membranes. Insect Biochem Mol Biol. 27, 529–539 (1997).
Denolf, P. et al. Cloning and characterization of Manduca sexta and Plutella xylostella midgut aminopeptidase N enzymes related to Bacillus thuringiensis toxin-binding proteins. Eur J Biochem. 248, 748–761 (1997).
Nakanishi, K. et al. Aminopeptidase N isoforms from the midgut of Bombyx mori and Plutella xylostella - their classification and the factors that determine their binding specificity to Bacillus thuringiensis Cry1A toxin. FEBS Lett. 519, 215–220 (2002).
Wang, G., Liang, G., Wu, K. & Guo, Y. Gene cloning and sequencing of aminopeptidase N3, a putative receptor for Bacillus thuringiensis insecticidal Cry1Ac toxin in Helicoverpa armigera (Lepidoptera: Noctuidae). Eur. J. Entomol. 102, 13–19 (2005).
Chang, X. et al. Determining the involvement of two aminopeptidase Ns in the resistance of Plutella xylostella to the Bt toxin Cry1Ac: cloning and study of in vitro function. J Biochem Mol Toxicol. 26, 60–70 (2012).
Qiu, L. et al. Cadherin is involved in the action of Bacillus thuringiensis toxins Cry1Ac and Cry2Aa in the beet armyworm, Spodoptera exigua . J Invertebr Pathol. 127, 47–53 (2015).
Goh, H., Lee, S., Lee, B., Choi, K. & Kim, J. Simple mass rearing of beet armyworm Spodoptera exigua (Hübner)(Lepidoptera: Noctuidae), on an artificial diet. Kor J Appl Entomol. 29, 180–183 (1990).
Wolfersberger, M. et al. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp Biochem Physiol A Mol Integr Physiol. 86, 301–308 (1987).
Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Ana1 Biochem. 72, 248–253 (1976).
Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans . Gene. 263, 103–112 (2001).
Dong, X., Li, Q. & Zhang, H. The noa gene is functionally linked to the activation of the Toll/Imd signaling pathways in Bactrocera dorsalis (Hendel). Dev Comp Immunol. 55, 233–240 (2016).
Ren, X. L. et al. A Spodoptera exigua cadherin serves as a putative receptor for Bacillus thuringiensis Cry1Ca toxin and shows differential enhancement of Cry1Ca and Cry1Ac toxicity. Appl Environ Microbiol. 79, 5576–5583 (2013).
Zhu, X. et al. Selection and evaluation of reference genes for expression analysis using qRT-PCR in the beet armyworm Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). PloS one. 9, e84730 (2014).
Abbott, W. S. A method of computing the effectiveness of an insecticide. 1925. J Am Mosq Control Assoc. 3, 302–303 (1987).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Acknowledgements
We thank Dr. Juan Luis Jurat-Fuentes from The University of Tennessee for comments on an earlier version of the manuscript. This work was funded by grants from Ministry of Agriculture of China (Grant No. 2016ZX08011002) and the National Natural Science Foundation of China (grant no. 31101445).
Author information
Authors and Affiliations
Contributions
L.Q., S.C., B.Z. and L.L. performed the experiments, W.M., C.L., X.W. and L.C. conceived and designed the experiments, L.Q. and L.C. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Qiu, L., Cui, S., Liu, L. et al. Aminopeptidase N1 is involved in Bacillus thuringiensis Cry1Ac toxicity in the beet armyworm, Spodoptera exigua. Sci Rep 7, 45007 (2017). https://doi.org/10.1038/srep45007
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45007
- Springer Nature Limited