Abstract
Hyaluronan is a linear glycosaminoglycan that forms the backbone of perineuronal nets around neurons in the cerebral cortex. However, it remains controversial whether neurons are capable of independent hyaluronan synthesis. Herein, we examined the expression of hyaluronan and hyaluronan synthases (HASs) throughout cortical neuron development in vitro. Enriched cultures of cortical neurons were established from E16 rats. Neurons were collected at days in vitro (DIV) 0 (4 h), 1, 3, 7, 14, and 21 for qPCR or immunocytochemistry. In the relative absence of glia, neurons exhibited HAS1–3 mRNA at all time-points. By immunocytochemistry, puncta of HAS2–3 protein and hyaluronan were located on neuronal cell bodies, neurites, and lamellipodia/growth cones from as early as 4 h in culture. As neurons matured, hyaluronan was also detected on dendrites, filopodia, and axons, and around synapses. Percentages of hyaluronan-positive neurons increased with culture time to ~93% by DIV21, while only half of neurons at DIV21 expressed the perineuronal net marker Wisteria floribunda agglutinin. These data clearly demonstrate that neurons in vitro can independently synthesise hyaluronan throughout all maturational stages, and that hyaluronan production is not limited to neurons expressing perineuronal nets. The specific structural localisation of hyaluronan suggests potential roles in neuronal development and function.
Similar content being viewed by others
Introduction
Hyaluronic acid (hyaluronan) is a linear glycosaminoglycan found in the extracellular matrix of most tissues throughout the body. Hyaluronan is produced by a family of three transmembrane hyaluronan synthases (HAS1–3)1,2,3, and exists most commonly as a high molecular weight molecule, with a size ranging from ≤3 × 106 to ≥6 × 106 Da4. Hyaluronan was originally described as an important structural scaffold for tissues, although there is increasing evidence for widespread roles in cellular signalling, differentiation, proliferation, and migration in peripheral organs and in the central nervous system (CNS)5,6.
Hyaluronan is widely expressed in the developing and adult CNS6,7,8,9,10. However, there are limited studies examining the cellular sources of hyaluronan in the brain11,12,13, and it is controversial whether neurons independently contribute to hyaluronan synthesis. Astrocytes are well established to produce hyaluronan, and express a range of HAS genes11,12,14,15. In vitro, astrocytes were also shown to form pericellular hyaluronan matrices, while cortical neurons in the same cultures did not16. More recently, however, hyaluronan was observed on a subset of mature parvalbumin-positive neurons in cortical cultures devoid of astrocytes17,18, suggesting potential for neuronal hyaluronan production.
In the cerebral cortex, as well as other grey matter regions, hyaluronan can form the backbone of specialised lattice-like extracellular matrix structures termed perineuronal nets, which surround the soma and proximal processes of mature GABAergic interneurons19,20,21,22,23,24. Perineuronal nets appear postnatally in the developing rodent and human brain19,25,26,27, and are associated with synaptic stability28,29 and closure of critical periods of plasticity29,30,31,32. However, the expression and function of hyaluronan on neurons without perineuronal nets is unclear.
In the present study, using enriched primary cultures of cortical neurons, we assessed the specific timing and structural localisation of hyaluronan and HASs throughout neuronal development.
Methods
Neuronal culture
Dissociated cultures of cortical neurons were generated from time-mated embryonic day 16 (E16) rat brains using established protocols33,34. The E16 rat brain is widely used for enriched cultures of cortical neurons, as the main period of gliogenesis in the rat cortex occurs after E17, and neuronal stem cells remain the predominant cell type until E19–2035. In brief, neurons were plated onto poly-D-lysine (80 μg/mL; Thermo Fisher Scientific, Auckland, NZ) and laminin (8 μg/mL; Sigma-Aldrich, Auckland, NZ) coated glass coverslips (Heinz Herenz Medizinalbedarf GmbH, Hamburg, Germany) in 24-well plates (1 × 105 cells/well) for immunocytochemistry or 100 mm dishes (2 × 106cells/dish) for mRNA analyses. Cultures were maintained in Neurobasal media containing B-27 Supplement (1X), Penicillin-Streptomycin (10 μg/mL), and GlutaMAX (1X) (Thermo Fisher Scientific). Neurons were collected at 4 h (day 0), and 1, 3, 7, 14, and 21 days in vitro (DIV) to cover key neuronal maturation stages, including dendrite, axon, and synapse development36,37,38. Neurons collected at DIV21 were treated with β-cytosine arabinofuranoside (AraC, 500 nM; Sigma-Aldrich) at DIV1 to inhibit glial proliferation associated with longer-term cultures17,39, and media was exchanged for non-AraC containing media at DIV3. The percentage of GFAP-positive astrocytes in the neuronal cultures was 0% at DIV0, DIV1, DIV3, and DIV7, <3% at DIV14, and <1% at DIV21. Using qPCR, only seven samples out of 23 showed detectable GFAP expression (at DIV0, DIV14, and DIV21), while when all time-points from DIV0–21 were combined, the average GFAP mRNA expression was >10,000-fold less than that for MAP2. All protocols were approved by the University of Auckland Animal Ethics Committee, and were performed in accordance with the New Zealand Government Animal Welfare Act.
RNA isolation and quantitative real-time PCR
For each time point, three to four samples from independent cultures were obtained by pooling three 100-mm dishes per culture. Total RNA was extracted from cortical neurons using TRIzol according to the manufacturer’s protocol (Thermo Fisher Scientific), and samples were stored at −80 °C until use. RNA concentration and purity were assessed using the NanoDrop 2000 (Thermo Fisher Scientific).
For quantitative real-time PCR (qPCR), cDNA was synthesised from 1 μg total RNA using the iScript cDNA synthesis kit (Bio-Rad, Auckland, New Zealand) according to the manufacturer’s protocol. Experiments were performed using the QuantStudio 12 K Flex Real-Time PCR System (Thermo Fisher Scientific) with pre-optimised hydrolysis probe assays (Integrated DNA Technologies, Coralville, IA, USA; or Thermo Fisher Scientific). A minimum of three technical replicates were used per sample. Genes examined and details of probes used are shown in Table 1. All probes were exon-spanning and designed to detect no genomic DNA. GFAP and MAP2 probes were used to confirm culture purity (Table 2).
Data analysis was performed using QuantStudio software (Thermo Fisher Scientific), and calculations were performed in Microsoft Excel (Microsoft Co., Redmond, WA, USA). Relative gene expression was normalised to three endogenous controls, including β-actin, TATAA-box binding protein (TBP), and acidic ribosomal phosphoprotein P0 (ARBP) using global normalisation. mRNA expression was quantified by the ΔΔCq (Cq: quantification cycle) method40 using DIV0 neurons as reference samples. Cq values of the β-actin, TBP, and ARBP genes did not differ significantly between developmental time points or biological replicates (data not shown). These genes were previously reported as suitable qPCR reference genes for use in cortical neurons41.
Immunocytochemistry
For optimal hyaluronan staining, neurons on coverslips were fixed in ethanol-acetic acid-formalin (70%, 5%, 4% v/v, respectively) in phosphate buffered saline (PBS) for 5 min at −20 °C42. Coverslips were then washed for 3 × 5 min in PBS, and blocked for 1 h in 5% bovine serum albumin (BSA)/PBS. Cells were incubated with biotinylated hyaluronic acid binding protein (bHABP, 1:500; #385911, Merck Millipore, NZ) and mouse monoclonal anti-microtubule-associated protein 2 (MAP2, 1:500; #M4403, Sigma-Aldrich) in PBS/3% BSA overnight at 4 °C. Cells were then washed for 3 × 5 min in PBS, and incubated in PBS/3% BSA with appropriate secondary antibodies (Thermo Fisher Scientific; 1:500 for each), including streptavidin-conjugated Alexa Fluor 594 and goat anti-mouse Alexa Fluor 488/660, for 2.5 h at room temperature, followed by Hoechst 33258 (1:10,000) to identify cell nuclei. Axons were also identified on neurons stained with bHABP/MAP2, and were classified as thin diameter, non-tapered processes that showed weak or no MAP2 immunoreactivity.
For HAS2–3 immunocytochemistry, neurons were fixed in 2% paraformaldehyde for 20 min. Cells were then washed 3 × 5 min in PBS, permeabilised in 0.1% Triton-X-100 in PBS for 10 min, washed twice in PBS, and then blocked in 5% NGS for 1 h. Neurons were incubated with rabbit polyclonal anti-HAS2 (1:100; #sc-66916, Santa Cruz Biotechnology) or rabbit polyclonal anti-HAS3 (1:200; #NBP1-86328, Novus Biologicals, Littleton, CO, USA), with anti-MAP2 (1:500; #M4403, Sigma-Aldrich) overnight at 4 °C. Phalloidin-488 (1:100; Thermo Fisher Scientific) was added with secondary antibodies for 2.5 h. The commercial HAS2 antibody is a rabbit polyclonal raised against amino acids 121–180 (DGN SED DLY MMD IFS EVM GRD KSA TYI WKN NFH EKG PGE TDE SHK ESS QHV TQL VLS NKS) within an internal region of HAS2 of human origin. The HAS3 antibody is a rabbit polyclonal raised against amino acids 127–186 (RQE DAY MLD IFH EVL GGT EQA GFF VWR SNF HEA GEG ETE ASL QEG MDR VRD VVR AST FSC) within an internal region of HAS3 of human origin. The full rat HAS2 (NCBI accession, AAB63209) and HAS3 (NCBI accession, NP_758822) amino acid sequences have 70% similarity, while the HAS2 and HAS3 sequences used to generate the antibodies have 40% similarity. Further, the HAS2 antibody amino acid sequence has only 42% similarity to rat HAS3, with no overlapping regions spanning more than four amino acids in length, while the HAS3 antibody amino acid sequence has only 41% similarity to rat HAS2, with no overlapping regions spanning more than four amino acids in length. The specificity of the HAS2 and HAS3 antibodies was further confirmed by western blot (see Supplementary Methods), which showed a single band at the expected molecular weight for each antibody (see Supplementary Fig. S1). Finally, to examine for potential cross-reactivity of the HAS2 and HAS3 antibodies, each antibody was pre-incubated with a blocking peptide (10:1 concentration, #NBP1-86328PEP; Novus Biologicals) against the HAS3 antibody; a blocking peptide for the HAS2 was not commercially available. By immunocytochemistry, there was a marked reduction in neuronal HAS3 staining when using the HAS3 antibody that was pre-incubated with the HAS3 blocking peptide, supporting the specificity of the HAS3 antibody (see Supplementary Fig. S2A,B). Further, a normal pattern of neuronal HAS2 staining was observed when using the HAS2 antibody that was pre-incubated with the HAS3 blocking peptide, suggesting no cross-reactivity of the HAS2 antibody with endogenous HAS3 protein (see Supplementary Fig. S2C,D).
For synaptic staining, cells were incubated with a combination of bHABP (1:500) and rabbit polyclonal anti-synaptophysin (pre-synaptic marker, 1:300; #ab68851, Abcam, Cambridge, MA, USA) or mouse monoclonal anti-PSD95 (post-synaptic marker, 1:200; #MA1-046, Affinity BioReagents, Golden, CO, USA).
Cells were washed for 3 × 5 min in PBS, and incubated in appropriate secondary antibodies, including streptavidin-conjugated Alexa Fluor 594 and goat anti-mouse Alexa Fluor 488/660, for 2.5 h at room temperature, followed by Hoechst 33258 to identify cell nuclei. In preliminary experiments using goat polyclonal anti-HAS1 antibody (1:100; #sc-23145, Santa Cruz Biotechnology, Dallas, TX, USA), we were unable to produce optimal HAS1 staining. To detect perineuronal nets, neurons were stained with the established chondroitin sulphate proteoglycan marker biotinylated Wisteria floribunda agglutinin (WFA, 1:500; #L1516, Sigma-Aldrich)19 and MAP2 using the procedures described above. Omission of primary antibodies/binding proteins resulted in no positive staining.
After 3 × 5 min washing in PBS, coverslips were mounted onto glass slides using Prolong Gold Antifade medium (Thermo Fisher Scientific), cured overnight at room temperature, and then sealed. The specificity of hyaluronan staining was confirmed by pre-treatment of fixed neurons with hyaluronidase from Streptomyces hyalurolyticus (4 U/mL, 37 °C for 1 h; Sigma-Aldrich), which eliminated the HABP signal (see Supplementary Fig. S3)43. Neurons were imaged using an Olympus FV1000 confocal microscope (Olympus Co., Center Valley, PA, USA) under a 60X (N.A. 1.35) or 100X (N.A. 1.4) objective, while a Zeiss AxioImager M2 fluorescent microscope (Carl Zeiss, Thornwood, NY, USA) under a 20X (N.A. 0.5) objective was used for cell counts. Combined differential interference contrast (DIC) and fluorescent images were also obtained using the Olympus FV1000 confocal microscope.
Quantitative analysis of hyaluronan expression
Assessment of the percentage of cortical neurons that expressed hyaluronan was performed on neurons double-labelled with MAP2 and bHABP. A circular region of interest was traced 1000 μm inside the outer edge of each coverslip, and 10 random sites were imaged (Zeiss AxioImager M2). MAP2-positive neurons were then classified as either positive or negative for hyaluronan, and counts were obtained for each site using ImageJ44.
Statistical analysis
For qPCR experiments, data are presented as relative expression values (RQ) compared to DIV0 of culture. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test was used to assess differences between the reference group (DIV0) and other time points using ΔCq values. One-way ANOVA with Tukey’s multiple comparisons post-hoc test was used to analyse differences in the percentages of hyaluronan-positive neurons between time points in culture. A value of P < 0.05 was considered statistically significant. Graphs were generated using Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Cultured cortical neurons express the family of hyaluronan synthases throughout development in vitro
mRNA expression by qPCR
Cultured cortical neurons expressed HAS1, HAS2, and HAS3 mRNA at all time points. Using normalised ΔCq data, HAS3 was expressed at the highest levels (~670 fold higher than HAS1), followed by HAS2 (~130 fold greater than HAS1), while HAS1 consistently had the lowest expression (Table 2).
There was a significant overall effect of time in culture on HAS1–3 gene expression (one-way ANOVA, P < 0.03). Thus, we assessed the fold-change in target gene expression over time in culture. Relative to DIV0, HAS1 and HAS3 mRNA expression progressively increased to peak at DIV21 and DIV7, respectively (Fig. 1A,C), while HAS3 expression progressively decreased thereafter. HAS2 expression peaked at DIV3, and decreased thereafter (Fig. 1B).
Protein expression by immunocytochemistry
Next, we assessed the expression of HAS2 and HAS3 proteins on MAP2-positive cortical neurons by immunocytochemistry. At DIV0, immature neurons showed limited somatic HAS2 and HAS3 immunoreactivity, while by DIV3, most neurons exhibited a punctate pattern of HAS2 (Fig. 2) and HAS3 (Fig. 3) on the cell soma and processes. With neuronal maturation (DIV7 and DIV14), more extensive HAS2 and HAS3 protein expression was observed on neuronal processes (e.g., Fig. 2B). At early developmental stages (up until DIV7), there was also evidence of HAS2 and HAS3 expression on actin-positive lamellipodia, growth cones, and filopodia (e.g., Fig. 3A).
Cortical neurons produce hyaluronan throughout development in vitro
To determine whether the neuronal HASs were functional, we assessed the expression of hyaluronan on cortical neurons using biotinylated hyaluronic acid binding protein (bHABP), which selectively labels hyaluronan ≥10 monosaccharides in size45. The percentages of hyaluronan-positive neurons over time in culture are shown in Table 3.
At 4 h after culture, ~16% of cortical neurons showed a pattern of small hyaluronan puncta on cell bodies (Fig. 4A). At DIV1 and DIV3, neurons showed a more extensive pattern of pericellular hyaluronan surrounding the cell body and extending along the length of developing neurites (Fig. 4B,C). By DIV7, DIV14, and DIV21, almost all neurons expressed hyaluronan (Table 3), with larger puncta localised to the cell bodies, and smaller puncta along processes (Fig. 4D,E). Additionally, from DIV14, more diffuse hyaluronan was present in the pericellular and extracellular regions. Pre-treatment of cultured neurons with hyaluronidase from Streptomyces hyalurolyticus abolished hyaluronan staining (see Supplementary Fig. S3).
Hyaluronan is localised to specific neuronal structures in vitro
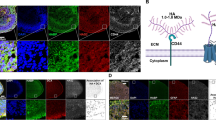
To confirm our findings of HAS expression on specific neuronal structures, we examined the detailed expression patterns of neuronal hyaluronan. Hyaluronan was frequently localised to neuronal lamellipodia (Fig. 5A), immature neurites (Fig. 5B,C), and growth cones (Fig. 5C) in developing neurons from DIV1–7 (only DIV3 images are shown), with occasional expression on filopodia (Fig. 5B,C). In more mature neurons, hyaluronan was also localised to dendrites and axons (Fig. 5D). In addition, we examined the spatial association of neuronal hyaluronan with the mature synapse by comparing hyaluronan labelling with immunoreactivity for presynaptic (synaptophysin) and postsynaptic (PSD-95) markers on mature neurons. Both presynaptic boutons and postsynaptic sites were closely associated with hyaluronan matrix along neuronal processes, although we did not observe any direct overlap of hyaluronan with synaptic markers (Fig. 6).
Subcellular structures were visualised under differential interference contrast (DIC) optics. bHABP localisation (red) was observed on neuronal lamellipodia (arrowheads; A), immature neurites (between arrowheads; B), growth cones (arrowheads; C), filopodia (arrows; B,C), dendrites (arrows; D), and axons (defined as a thin, MAP2-negative process, arrowheads; D). MAP2, green. Scale bars: 10 μm (D), 30 μm (A–C).
Representative examples of neurons labelled with hyaluronan (red) and synaptophysin (A, green) or PSD-95 (B, green) at DIV21 are shown. High magnification images indicate small spatial separation, but no colocalisation, between hyaluronan (C,D) and synaptophysin (SYN; E,G) or PSD-95 (F,H) immunoreactive puncta on neuronal processes. Scale bars: 5 μm (A,B), 20 μm (C–H).
Development of mature perineuronal net components on cortical neurons in vitro
Finally, we assessed the timing of formation of perineuronal net-like structures in our culture system using WFA, which labels the N-acetylgalactosamine component of chondroitin sulphate chains and is commonly used to identify mature perineuronal nets19. The percentages of WFA-positive neurons over time in culture are shown in Table 3. There was no evidence of WFA expression on cortical neurons at DIV0–3 (Table 3). However, at DIV7, DIV14, and DIV21, ~7%, ~17%, and 53%, respectively, of all cortical neurons expressed pericellular WFA. This contrasts with our finding that almost all neurons (>90%) showed hyaluronan labelling from DIV7–DIV21. At DIV7, only a few small WFA-positive puncta were visible on neuronal cell bodies, with no obvious process staining (Fig. 7A). By DIV14 there were larger WFA-positive puncta localised to neuronal cell bodies, and expression of smaller puncta along processes (Fig. 7B). By DIV21, there was a more extensive pattern of pericellular WFA (Fig. 7C), in a pattern closely resembling a classical mesh-like perineuronal net structure18,22.
Discussion
There is now strong evidence that perineuronal nets can control CNS plasticity, particularly in the cerebral cortex and hippocampus29. Hyaluronan is a major component of the extracellular matrix in the brain, and contributes to perineuronal net-mediated regulation of neuronal signalling and synaptic plasticity in developmentally mature neurons46,47,48. Herein, we provide new in vitro evidence that cortical neurons express the entire family of HAS enzymes, and produce hyaluronan on multiple structures important for neuronal development and synaptic function.
Astrocytes are a major source of hyaluronan in the brain14,15, and contribute to the formation of perineuronal nets in vitro and in vivo13,24. Indeed, in co-cultures with cortical neurons, astrocytes produced a pericellular hyaluronan matrix by DIV7, while the neurons did not16. However, in longer recovery experiments in purified neuronal cultures, a small subset of cortical neurons expressed pericellular hyaluronan in a classical perineuronal net-like pattern at DIV12 and DIV2117,18. Further, cultured hippocampal neurons expressed hyaluronan at DIV24, but not at DIV1046. By contrast, in our enriched neuronal cultures largely devoid of astrocytes, we found punctate hyaluronan expression on ~16% of immature cortical neurons as early as 4 h in vitro, while ~85% of neurons expressed hyaluronan by DIV3, and >90% at DIV7–21. Further, only ~17% of DIV14 neurons and 53% of DIV21 neurons were positive for WFA, a marker of perineuronal net-like structures present on mature cortical neurons19. Overall, these findings suggest that hyaluronan expression is not limited to neurons with perineuronal nets, and that hyaluronan expression appears much earlier in the maturation of post-mitotic neurons than previously reported.
We observed abundant hyaluronan puncta on multiple structures on immature neurons, including cell bodies and developing neurites. The function of this hyaluronan is unclear. However, growth of dorsal root ganglion neurons on a hyaluronan substrate reduces neurite extension49. Further, the major hyaluronan receptor CD44 is expressed on neuronal dendrites, and blockade of CD44 increases dendritic arborisation of cortical and hippocampal neurons both in vitro and in vivo50. Conversely, blockade of RHAMM, another hyaluronan receptor, inhibits neurite extension in cultures of primary neurons and other neural cell lines51. Thus, hyaluronan located on developing neurites may be important for neurite outgrowth, potentially through receptor-mediated signalling pathways. Indeed, hyaluronan signalling through the CD44 receptor regulates various cytoskeleton-mediated processes, including motility in astrocytes and adhesion and metastasis in cancer cells52,53.
We also found evidence of hyaluronan expression on lamellipodia and filopodia of immature cortical neurons in vitro. Although there are no comparable studies in the brain, hyaluronan is found on lamellipodia and filopodia of oesophageal carcinoma cells and epidermal keratinocytes, where it plays a key role in outgrowth and adhesion54,55. Further, addition of hyaluronan induces lamellipodia outgrowth in mouse mammary epithelial cells56. Interestingly, growth of chick dorsal root ganglion neurons on a substrate of synthetic hyaluronan reduces growth cone extension49. In addition, CD44 expression enhances filopodia growth of neuroblastoma cells57. Thus, hyaluronan may play an important role in controlling cytoskeletal adhesion and outgrowth in early stage cortical neurons.
Hyaluronan was also located along axons of more mature neurons (i.e., from DIV7–DIV21 of development). Although axonal localisation of hyaluronan in cortical neurons has not been reported, hyaluronan is expressed in axons of granule cells in the rat cerebellum45 and surrounds axons in the white matter and spinal cord8. Other perineuronal net components such as brevican are expressed at particularly high levels on the axon initial segment of mature hippocampal neurons58 and motor neurons in the superior colliculus59. ‘Axonal coats’ of aggrecan are also present in various human basal ganglia regions60, in the human lateral geniculate body61, and in the rat thalamus62, often independently of perineuronal nets. Interestingly, there is evidence that hyaluronan can control axonal outgrowth of optic tract and locus ceruleus neurons63,64,65. Further, removal of hyaluronan promotes regeneration of severed axons in the rat brain66, although addition of low molecular weight hyaluronan oligosaccharides enhances axonal regrowth following spinal cord injury67. Overall, these data suggest potential roles of hyaluronan in axon development and function.
In mature cortical neurons (DIV21), we observed a close spatial association of hyaluronan with synaptic contact sites. In mature hippocampal neuronal cultures, hyaluronan enwraps spine necks46, while synapses are also embedded in brevican and neurocan, both chondroitin sulphate proteoglycan components of mature perineuronal nets58. In the present study, we found no obvious overlap of hyaluronan with synaptophysin or PSD-95, which is supported by evidence that perineuronal net components enclose synapses, but are absent from the sites of synaptic contact46,58. This synaptic association of hyaluronan on cortical neurons supports its role as part of the perisynaptic matrix, which may stabilise synaptic architecture and assist in maintaining the synaptic microenvironment by regulating receptor trafficking46, ion diffusion68, and neurotransmitter spillover69. Indeed, removal of hyaluronan using hyaluronidase impairs voltage-dependent calcium currents and long-term potentiation in brain slices48, and fear memory in vivo48,70. Similarly, disruption of the chondroitin sulphate proteoglycan components of perineuronal nets using chondroitinase impairs fear memory71, synaptic stability, and postsynaptic currents72, prevents critical period closure31, and enhances recognition memory and long-term depression73. Together, these studies implicate hyaluronan, both alone and as a major perineuronal net scaffold, in the control of synaptic plasticity.
The capacity of cultured cortical neurons to independently produce hyaluronan was confirmed by robust expression of HAS2 and HAS3 mRNA. Further, HAS2 and HAS3 protein expression coincided with the widespread distribution of hyaluronan throughout various neuronal structures. By contrast, expression of HAS1 mRNA was very low, and we were unable to detect HAS1 protein by immunocytochemistry. Although there are limited studies of HAS expression in the CNS, HAS2 and HAS3 mRNA (but not HAS1) are expressed in the rat embryonic brain and in selected neurons in the rat visual cortex and cerebellum13,74, while HAS1 and HAS3 mRNA are found in developing spinal cord neurons75, and HAS3 mRNA in cultured cortical neurons at DIV817. HAS1 and HAS2 proteins are also expressed in neurons following grey matter stroke in humans76. The specific contributions of HAS1–3 to neuronal hyaluronan production are unknown. However, in cultured HEK cells, HAS2 or HAS3 expression results in a larger pericellular hyaluronan matrix compared with HAS177,78. Interestingly, HAS1 and HAS2 synthesise higher molecular weight hyaluronan (2 × 105–2 × 106 Da) compared with HAS3 (1 × 105 Da–1 × 106 Da)79. Further, the biological effects of hyaluronan are dependent on its size, through activation of different receptor-mediated signalling pathways80,81. Thus, differing patterns of HAS expression and molecular weights of hyaluronan on neurons may serve a range of biological functions depending on the timing of their expression.
The delayed (DIV7) but progressive increase in punctate WFA labelling observed on neuronal cell bodies and processes in the present study is consistent with that previously reported in vitro18, and with the delayed formation of perineuronal nets in the rat cerebral cortex in vivo25; in that study, weak perineuronal nets were first observed at postnatal day 14, and then progressively increased until postnatal day 4025. WFA labels the N-acetylgalactosamine component of chondroitin sulphate chains on mature perineuronal nets19, including neurocan, versican, brevican, aggrecan, and phosphacan. These components are linked to the hyaluronan backbone via link proteins to form the lattice-like perineuronal net structure. Although we did not examine the individual expression of these perineuronal net components in the present study, their specific development on neurons has been previously characterised in vitro and in vivo17,18,26,74,82,83, with evidence for both neuronal- and glial-dependent synthesis13,17,18,58,75.
In conclusion, these data suggest that cortical neurons have the capacity to independently synthesise hyaluronan on multiple neuronal structures throughout their development. The specific cellular functions of this neuronal hyaluronan may depend on the stage of neuronal maturation, as well as the co-expression of perineuronal net components. Further studies are required to determine the specific roles of hyaluronan in neuronal development and function.
Additional Information
How to cite this article: Fowke, T. M. et al. Hyaluronan synthesis by developing cortical neurons in vitro. Sci. Rep. 7, 44135; doi: 10.1038/srep44135 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Spicer, A. P., Augustine, M. L. & McDonald, J. A. Molecular cloning and characterization of a putative mouse hyaluronan synthase. J. Biol. Chem. 271, 23400–23406 (1996).
Spicer, A. P., Olson, J. S. & McDonald, J. A. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J. Biol. Chem. 272, 8957–8961 (1997).
Watanabe, K. & Yamaguchi, Y. Molecular identification of a putative human hyaluronan synthase. J. Biol. Chem. 271, 22945–22948 (1996).
Laurent, T. C., Laurent, U. B. & Fraser, J. R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 74, A1–A7 (1996).
Toole, B. P. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. 4, 528–539 (2004).
Preston, M. & Sherman, L. S. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front. Biosci. 3, 1165–1179 (2011).
Morriss-Kay, G. M., Tuckett, F. & Solursh, M. The effects of Streptomyces hyaluronidase on tissue organization and cell cycle time in rat embryos. J. Embryol. Exp. Morphol. 98, 59–70 (1986).
Bignami, A. & Asher, R. Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int. J. Dev. Neurosci. 10, 45–57 (1992).
Margolis, R. U., Margolis, R. K., Chang, L. B. & Preti, C. Glycosaminoglycans of brain during development. Biochemistry 14, 85–88 (1975).
Yasuhara, O., Akiyama, H., McGeer, E. G. & McGeer, P. L. Immunohistochemical localization of hyaluronic acid in rat and human brain. Brain Res. 635, 269–282 (1994).
Cargill, R. et al. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol. Aging 33, 830.e13–830.e24 (2012).
Bugiani, M. et al. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain 136, 209–222 (2013).
Carulli, D. et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 494, 559–577 (2006).
Marret, S. et al. Expression and effects of hyaluronan and of the hyaluronan-binding protein hyaluronectin in newborn rat brain glial cell cultures. J. Neurochem. 62, 1285–1295 (1994).
Asher, R. & Bignami, A. Localization of hyaluronate in primary glial cell cultures derived from newborn rat brain. Exp. Cell Res. 195, 401–411 (1991).
Maleski, M. & Hockfield, S. Glial cells assemble hyaluronan-based pericellular matrices in vitro . Glia 20, 193–202 (1997).
Giamanco, K. A. & Matthews, R. T. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience 218, 367–384 (2012).
Miyata, S., Nishimura, Y., Hayashi, N. & Oohira, A. Construction of perineuronal net-like structure by cortical neurons in culture. Neuroscience 136, 95–104 (2005).
Hartig, W., Brauer, K. & Bruckner, G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport 3, 869–872 (1992).
Hartig, W., Brauer, K., Bigl, V. & Bruckner, G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 635, 307–311 (1994).
Celio, M. R., Spreafico, R., De Biasi, S. & Vitellaro-Zuccarello, L. Perineuronal nets: past and present. Trends Neurosci. 21, 510–515 (1998).
Celio, M. R. & Blumcke, I. Perineuronal nets - a specialized form of extracellular matrix in the adult nervous system. Brain Res. Res. Rev. 19, 128–145 (1994).
Celio, M. R. & Chiquet-Ehrismann, R. ‘Perineuronal nets’ around cortical interneurons expressing parvalbumin are rich in tenascin. Neurosci. Lett. 162, 137–140 (1993).
Bruckner, G. et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8, 183–200 (1993).
Bruckner, G. et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J. Comp. Neurol. 428, 616–629 (2000).
Koppe, G., Bruckner, G., Brauer, K., Hartig, W. & Bigl, V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 288, 33–41 (1997).
Mauney, S. A. et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol. Psychiatry 74, 427–435 (2013).
Hockfield, S., Kalb, R. G., Zaremba, S. & Fryer, H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb. Symp. Quant. Biol. 55, 505–514 (1990).
Wang, D. & Fawcett, J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 349, 147–160 (2012).
Sur, M., Frost, D. O. & Hockfield, S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J. Neurosci. 8, 874–882 (1988).
Carulli, D. et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331–2347 (2010).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002).
Beaudoin, G. M. 3rd et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 7, 1741–1754 (2012).
Polleux, F. & Ghosh, A. The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal development. Sci. Signal. 2002, pl9 (2002).
Bayer, S. A. & Altman, J. In The Rat Nervous System, doi: 10.1016/B978-012547638-6/50003-1, 27–73 (2004).
Polleux, F. & Snider, W. Initiating and growing an axon. Cold Spring Harb. Perspect. Biol. 2, a001925 (2010).
Dotti, C. G., Sullivan, C. A. & Banker, G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 (1988).
de Lima, A. D., Merten, M. D. & Voigt, T. Neuritic differentiation and synaptogenesis in serum-free neuronal cultures of the rat cerebral cortex. J. Comp. Neurol. 382, 230–246 (1997).
Robert, F., Cloix, J. F. & Hevor, T. Ultrastructural characterization of rat neurons in primary culture. Neuroscience 200, 248–260 (2012).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Zhang, L. et al. Real-time qPCR identifies suitable reference genes for Borna disease virus-infected rat cortical neurons. Int. J. Mol. Sci. 15, 21825–21839 (2014).
de la Motte, C. A. & Drazba, J. A. Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer. J. Histochem. Cytochem. 59, 252–257 (2011).
Ohya, T. & Kaneko, Y. Novel hyaluronidase from streptomyces. Biochim. Biophys. Acta 198, 607–609 (1970).
Schindelin, J., Rueden, C. T., Hiner, M. C. & Eliceiri, K. W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529 (2015).
Ripellino, J. A., Klinger, M. M., Margolis, R. U. & Margolis, R. K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J. Histochem. Cytochem. 33, 1060–1066 (1985).
Frischknecht, R. et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 12, 897–904 (2009).
Kul’chitskii, S. V. et al. Changes in neuropil ultrastructure in hippocampal field CA1 in rat pups after application of hyaluronidase. Neurosci. Behav. Physiol. 39, 517–521 (2009).
Kochlamazashvili, G. et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron 67, 116–128 (2010).
Saxod, R. & Bizet, M. C. Substrate effects on the dynamics of neurite growth in vitro: a quantitative multi-parametric analysis. Int. J. Dev. Neurosci. 6, 177–191 (1988).
Skupien, A. et al. CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J. Cell Sci. 127, 5038–5051 (2014).
Nagy, J. I., Hacking, J., Frankenstein, U. N. & Turley, E. A. Requirement of the hyaluronan receptor RHAMM in neurite extension and motility as demonstrated in primary neurons and neuronal cell lines. J. Neurosci. 15, 241–252 (1995).
Bourguignon, L. Y. Hyaluronan-CD44 interaction promotes microRNA signaling and RhoGTPase activation leading to tumor progression. Small GTPases 3, 53–59 (2012).
Martin, T. A., Harrison, G., Mansel, R. E. & Jiang, W. G. The role of the CD44/ezrin complex in cancer metastasis. Crit. Rev. Oncol. Hematol. 46, 165–186 (2003).
Rilla, K. et al. Changed lamellipodial extension, adhesion plaques and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J. Cell Sci. 115, 3633–3643 (2002).
Twarock, S., Tammi, M. I., Savani, R. C. & Fischer, J. W. Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J. Biol. Chem. 285, 23276–23284 (2010).
Oliferenko, S., Kaverina, I., Small, J. V. & Huber, L. A. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J. Cell Biol. 148, 1159–1164 (2000).
Pusch, A. et al. CD44 and hyaluronan promote invasive growth of B35 neuroblastoma cells into the brain. Incl. Spec. Sect. Formins 1803, 261–274 (2010).
John, N. et al. Brevican-containing perineuronal nets of extracellular matrix in dissociated hippocampal primary cultures. Mol. Cell. Neurosci. 31, 774–784 (2006).
Bruckner, G., Szeoke, S., Pavlica, S., Grosche, J. & Kacza, J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience 138, 365–375 (2006).
Brückner, G., Morawski, M. & Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 151, 489–504 (2008).
Lendvai, D. et al. Perisynaptic aggrecan-based extracellular matrix coats in the human lateral geniculate body devoid of perineuronal nets. J. Neurosci. Res. 90, 376–387 (2012).
Gáti, G. et al. Distribution and classification of aggrecan-based extracellular matrix in the thalamus of the rat. J. Neurosci. Res. 88, 3257–3266 (2010).
Chan, C. K., Wang, J., Lin, L., Hao, Y. & Chan, S. O. Enzymatic removal of hyaluronan affects routing of axons in the mouse optic chiasm. Neuroreport 18, 1533–1538 (2007).
Lin, L., Wang, J., Chan, C. K. & Chan, S. O. Effects of exogenous hyaluronan on midline crossing and axon divergence in the optic chiasm of mouse embryos. Eur. J. Neurosci. 26, 1–11 (2007).
Nagy, J. I., Price, M. L., Staines, W. A., Lynn, B. D. & Granholm, A. C. The hyaluronan receptor RHAMM in noradrenergic fibers contributes to axon growth capacity of locus coeruleus neurons in an intraocular transplant model. Neuroscience 86, 241–255 (1998).
Moon, L. D., Asher, R. A. & Fawcett, J. W. Limited growth of severed CNS axons after treatment of adult rat brain with hyaluronidase. J. Neurosci. Res. 71, 23–37 (2003).
Wakao, N. et al. Hyaluronan oligosaccharides promote functional recovery after spinal cord injury in rats. Neurosci. Lett. 488, 299–304 (2011).
Bekku, Y. et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30, 3113–3123 (2010).
Murakami, T. & Ohtsuka, A. Perisynaptic barrier of proteoglycans in the mature brain and spinal cord. Arch. Histol. Cytol. 66, 195–207 (2003).
Hylin, M. J., Orsi, S. A., Moore, A. N. & Dash, P. K. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn. Mem. 20, 267–273 (2013).
Gogolla, N., Caroni, P., Luthi, A. & Herry, C. Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261 (2009).
Geissler, M. et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J. Neurosci. 33, 7742–7755 (2013).
Romberg, C. et al. Depletion of perineuronalnets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 33, 7057–7065 (2013).
Carulli, D., Rhodes, K. E. & Fawcett, J. W. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J. Comp. Neurol. 501, 83–94 (2007).
Galtrey, C. M., Kwok, J. C., Carulli, D., Rhodes, K. E. & Fawcett, J. W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 27, 1373–1390 (2008).
Al’Qteishat, A. et al. Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain 129, 2158–2176 (2006).
Kwok, J. C., Carulli, D. & Fawcett, J. W. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J. Neurochem. 114, 1447–1459 (2010).
Spicer, A. P. & McDonald, J. A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J. Biol. Chem. 273, 1923–1932 (1998).
Itano, N. et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 (1999).
Stern, R. Devising a pathway for hyaluronan catabolism: Are we there yet? Glycobiology 13, (2003).
Cyphert, J. M., Trempus, C. S. & Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. International Journal of Cell Biology 2015 (2015).
Deepa, S. S. et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 281, 17789–17800 (2006).
Lander, C., Zhang, H. & Hockfield, S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J. Neurosci. 18, 174–83 (1998).
Acknowledgements
The authors thank Jacqui Ross (Biomedical Imaging Research Unit, University of Auckland) for imaging support, and Chantelle Fourie (Centre for Brain Research, University of Auckland) and Meagan Barclay (Department of Physiology and Centre for Brain Research, University of Auckland) for providing synaptic antibodies. This work was supported by the Marsden Fund, the Auckland Medical Research Foundation, the Neurological Foundation of New Zealand, and the University of Auckland. The sponsors had no role in in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
T.F. and J.D. contributed to study design, experimental studies, and manuscript preparation and revision. R.K. contributed to experimental studies and manuscript revision. J.B. and S.J. contributed to experimental studies. A.G. contributed to manuscript preparation and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fowke, T., Karunasinghe, R., Bai, JZ. et al. Hyaluronan synthesis by developing cortical neurons in vitro. Sci Rep 7, 44135 (2017). https://doi.org/10.1038/srep44135
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44135
- Springer Nature Limited
This article is cited by
-
Generation of nanoscopic membrane curvature for membrane trafficking
Nature Reviews Molecular Cell Biology (2023)
-
Ischemic Preconditioning Alleviates Cerebral Ischemia–Reperfusion Injury by Interfering With Glycocalyx
Translational Stroke Research (2023)
-
Enzymatic Degradation of Cortical Perineuronal Nets Reverses GABAergic Interneuron Maturation
Molecular Neurobiology (2022)
-
Microglia as hackers of the matrix: sculpting synapses and the extracellular space
Cellular & Molecular Immunology (2021)
-
Coherence and cognition in the cortex: the fundamental role of parvalbumin, myelin, and the perineuronal net
Brain Structure and Function (2021)