Abstract
Priming improves an organism's performance upon a future stress. To test whether cold priming supports protection in spring and how it is affected by cold acclimation, we compared seven Arabidopsis accessions with different cold acclimation potentials in the field and in the greenhouse for growth, photosynthetic performance and reproductive fitness in March and May after a 14 day long cold-pretreatment at 4 °C. In the plants transferred to the field in May, the effect of the cold pretreatment on the seed yield correlated with the cold acclimation potential of the accessions. In the March transferred plants, the reproductive fitness was most supported by the cold pretreatment in the accessions with the weakest cold acclimation potential. The fitness effect was linked to long-term effects of the cold pretreatment on photosystem II activity stabilization and leaf blade expansion. The study demonstrated that cold priming stronger impacts on plant fitness than cold acclimation in spring in accessions with intermediate and low cold acclimation potential.
Similar content being viewed by others
Introduction
Arabidopsis thaliana has spread over the northern hemisphere in post-glacial times and colonized habitats from cold continental areas to warm maritime ones1,2. With separation and adaptation, the radiating populations partly lost or modified their cold tolerance mechanisms in warmer areas, while they maintained and specified them in cold ones3,4,5.
Arabidopsis activates cold acclimation in response to persisting or oscillating cold stress6,7. It is quickly lost, when the temperatures increase8,9. As shown recently10, Arabidopsis can nevertheless memorize an earlier (priming) cold stress for several days over a stress-free period.
Plants sense cold e.g. by changes in the membrane fluidity, by membrane proteins, photosynthetic imbalances and changes in metabolic activity11,12,13,14,15. Subsequently, Ca2+, cAMP, hormone and ROS signaling and the CBF (C-REPEAT BINDING FACTOR)-regulon are activated15,16,17,18,19,20. Finally, osmolytes and stress protection proteins accumulate and the concentration of unsaturated fatty acids increases21,22. These responses protect the plants against chilling stress and often harden them also against subzero temperatures (freezing stress)6,23.
The speed and extent of cold acclimation and deacclimation vary between Arabidopsis accessions4,8,24,25,26. Accessions with fast and strong activation of cold acclimation also deacclimate more slowly8. Expression of genes encoding enzymes of the plastid antioxidant system (PAS) is either more decreased or poorly induced by cold in these accessions,. In response to weaker antioxidant protection, more reactive oxygen species (ROS) accumulate and the ratio of free radicals to H2O2 shifts stronger upon deacclimation24. Post-cold regulation of plastid ascorbate peroxidases mitigates chloroplast-to-nucleus ROS signaling and limits cold induction of target genes for ROS signaling for several days10. We hypothesized that cold priming of chloroplast-to-nucleus signaling is especially important in spring. In the first weeks of the new vegetation period, the temperature and the light intensity increase. The environmental parameters strongly fluctuate and differentially influence the signaling hubs, which regulate plant development27.
To evaluate the costs and benefits of a cold pretreatment in a natural environment, we compared cold (4 °C) pretreated and naïve plants of seven Arabidopsis thaliana accessions with different cold acclimation potentials in the field in four experimental series. Two started in the first days of March and two in the begin of May in two subsequent years. For control, plant sets of the same size and composition were cultivated in a temperature and humidity controlled greenhouse. Three of the seven accessions, namely N13, Ms-0 and Kas-1, strongly activate CBF-regulon controlled cold acclimation8,28. The other four accessions, WS, Col-0, Van-0 and Cvi-0, have an intermediate to low cold acclimation potential8,28 and express and regulate PAS genes stronger in the cold24,29. Plant robustness and reproductive fitness (seed production per plant) were determined by analysis of photosystem II activity, growth parameters, induction of flowering and seed yields.
Results
Seven Arabidopsis thaliana accessions with gradually different cold acclimation potential were transferred to the field in the traditional Dahlem agricultural research area and to a close-by greenhouse after a 2 week long cold pretreatment at 4 °C. The plants were grown in randomized patterns with not cold-treated (naïve) 4 week old plants, which were indistinguishable from cold-treated ones in leaf number, weight and pigmentation10.
Weather conditions
Berlin is situated in the North-East of Germany in the Warsaw-Berlin valley between the Barnim and the Teltow plateaus. The climate is widely continental with cold winters, hot summers and short spring periods. Frequently, like in 2014 and 2015, March is already snow-free with day- and night-time temperatures above −4 °C (Fig. 1 and Suppl. 1). From day 6 of the experiment onwards, the mean temperatures increased in both years. As it is typical for the spring season in Berlin, they dropped again to values below 5 °C, mid of March, end of March, in the first half of April and beginning of May in 2015 and mid of April and in early May in 2014 (Suppl. 1).
The global radiation was 400–580 W m−2 on mid-day on the first days of the March experiments (Fig. 1 Middle), which corresponds to a maximum quantum flux of approximately 10 times the light intensity the plants were acclimated to in the growth chamber. In 2014, the maximum and mean radiations (1452 W m−2 and 209 W m−2) were higher than in 2015 (1385 W m−2 and 193 W m−2).
The air humidity was around 95% during the nights and between 50 and 65% during the days in the first week of March 2014 (Fig. 1 Bottom). The vegetation period was in general a bit dryer in 2015 than in 2014. The air humidity differed strongest between the two years in June and July, when the seeds matured (Suppl. 1).
Photosynthetic performance in the field
Temperature and light variation influence photosynthetic electron transport and carbon assimilation18,30. The maximum and effective quantum yields of photosystem II (FV/FM and ΦPS-II) are frequently analyzed indicators for photosystem-II (PS-II) damage and regulation31,32. They can be determined non-destructively by chlorophyll-a fluorescence32. As reported recently under exactly the same pre-cultivation conditions, FV/FM is 0.83 in Arabidopsis plants directly after 2 weeks at 4 °C due to acclimation10. It stays high in the plants, after they are transferred to optimal growth conditions with temperatures around 20 °C10. In the figures, the data were arranged according to the LT50 values of the accessions for freezing tolerance after 2 weeks a 4 °C (as determined by electrolyte leakage from pretreated leaves at sub-zero temperatures8,28) (Table 1).
March
On the first day after the transfer of cold-pretreated and naïve plants to the field in early March, FV/FM and ΦPS-II dropped in all naïve plants (Fig. 2 left). FV/FM and ΦPS-II were significantly higher in the cold-pretreated plants. The two accessions with the weakest cold acclimation potential, Van-0 and Cvi-0 had the lowest FV/FM and ΦPS-II values. Over all accessions, ΦPS-II correlated with FV/FM (rP = 0.996) demonstrating that inactivation of photosystem II dominated over regulation of photosynthetic electron transport.
Within the first week in the field, FV/FM increased in the cold-pretreated plants to levels close to those observed in the greenhouse (Fig. 3 left). In previously naïve plants, except Cvi-0, recovery of the PS-II quantum yield efficiency was delayed (Fig. 3 left). This reaction was slowest in the strongest cold acclimating accessions N13, Ms-0 and Kas-1. In these three accessions, FV/FM and ΦPS-II decreased only on day 2 or day 3 in the field. Afterwards PS-II activity recovered, like in the other plants.
In 2014 (Fig. 2 left), FV/FM was less decreased in the not cold-pretreated plants than in 2015. The light intensities and humidity were similar in both years beginning of March, but the outdoor daytime temperatures were higher in 2014 on the first four days of the experiment (Fig. 1), indicating that the PS-II activity decrease mainly correlated with temperature sensitivity.
On day 18 and 20 of the March experiment, the temperatures decreased slightly below 0 °C (−2.4 and −1.8 °C) in 2015, but were 3.8–11 °C in 2014 (Fig. 1). On day 21, all accessions, except Ms-0, showed at least slightly higher maximum quantum yields of PS-II (FV/FM) in the cold-pretreated plants in 2015 (Fig. 4 Top). In the accessions with intermediate ability to acclimate to cold28,8, namely WS, Col-0 and Van-0, ΦPS-II was higher, if the plants were cold-treated before they were transferred to the field (Fig. 4 Top). The cold pretreatment stronger affected ΦPS-II than FV/FM, demonstrating that it mainly supported light-dependent activity regulation of PS-II. In the 2014 plant sets, various cold-pretreated plants showed higher FV/FM and higher ΦPS-II values still after three weeks (Fig. 4 Top).
Top and mid: Regulation of the maximum quantum yield of photosystem II (FV/FM) (left) and effective quantum yield of photosystem II (ΦPS-II) (right) on day 21 of the March experiment in cold-pretreated (dark-blue) and naïve plants (white) of the seven tested accessions in the field in 2015 (top) and 2014 (bottom). The asterisks mark statistical significance between cold-treated and naïve plants (ANOVA, p < 0.05; n = 6–7). Bottom: The relative effect of the cold pretreatment as determined by comparison of the relative differences between the ratios of ΦPS-II and FV/FM in cold-pretreated (C) and naïve plants (N) in the seven accessions on day 21 of the 2015 (right) and 2014 (left) experiment. The three accessions, N13, Ms-0 and Kas-1, which can acquire a higher cold acclimation potential, are labeled in blue. The red line marks 1.00 (=“no regulatory effect”).
For comparison of the cold-priming effect between the accessions, first the ΦPS-II values were normalized on the FV/FM values. Afterwards, the results for cold-pretreated plants (C) were divided by the results for naïve plants (N) ([ΦPS-II/(FV/FM)]C/[ΦPS-II/(FV/FM)]N) (Fig. 4 Bottom). In 2015, the plants faced cold on day 20 of the March experiment after a warmer period (Fig. 1). On the next day, [ΦPS-II/(FV/FM)]C/[ΦPS-II/(FV/FM)]N was lower in the three accessions with higher cold acclimation potential than in the other accessions. In 2014, the temperatures were more stable and higher end of March. The [ΦPS-II/(FV/FM)]C/[ΦPS-II/(FV/FM)]N effect of 2015 was almost perfectly inverted in 2014 (rP = −0.980) (Fig. 4 Bottom), highlighting the reciprocity of costs and benefits of the cold pretreatment relative to the necessity of cold acclimation in the environment.
May
In May, despite approximately more than 15-fold higher radiation than in the growth chamber, FV/FM and ΦPS-II were only slightly decreased 24 h after transfer of the plants into the field (Fig. 2 right), but the FV/FM and ΦPS-II values were significantly (ANOVA, p < 0.01) lower in naïve plants of all accessions, except in N13, Van-0 and Cvi-0, which have highest and lowest cold-acclimation potential (Table 1). In the greenhouse, like in March, at least slightly higher FV/FM were observed in naïve plants of all accessions and higher ΦPS-II values in the accessions WS, Col-0, Van-0 and Cvi-0.
The FV/FM and ΦPS-II values also quickly increased in May in the field (Fig. 3 right). On day 2 and day 3 in the 2015 experiment, when the daytime temperatures had reached 20 °C (Fig. 1), the quantum yields of PS-II transiently decreased in not cold-pretreated Kas-1 (Fig. 3 right). Similar, but slighter transient decreases were observed for the other two accessions with high cold acclimation potential, N13 and Ms-0, on day 3 of the experiment.
Effects of cold pretreatment on the leaf areas
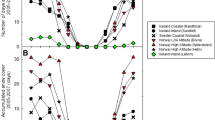
Leaf area expansion is only partly genetically fixed33 and modulated e.g. by the extent of photosynthetic carbon assimilation, which also impacts on the reproductive fitness34,35. In Arabidopsis, the leaves expand strongest between the 8-leaves-stage (stage 1.0836) and bolting (stage 536). Here, we compared the areas of the largest leaves in the rosettes three weeks after transferring the plants to the field and to the greenhouse (Fig. 5 left). These leaves were formed during the pre-cultivation period, but were not fully developed at the time-point of the transfer. By average, the leaf areas of cold-pretreated plants expanded 2.91-fold in the field in the March plant set and 6.04-fold in the greenhouse. In plants transferred to the field in March, the leaf blades of naïve plants, except N13, were at least 50% smaller than in the greenhouse.
In May, the differences between greenhouse and field grown plants were less pronounced (Fig. 5 left). The leaves expanded 6–7-fold within the first three weeks in both growth regimes in 2015. In 2014, when the temperatures were lower than in 2015 in the first days of the May experiment (Fig. 1), leaf expansion was as restricted as in the March transferred plants. However, in general, cold-primed plants had at least slightly larger leaf areas in the field and smaller ones in the greenhouse (Fig. 5 left).
The quotient of the value in cold treated plants and the value in naïve plants [C-value/N-value] shows the priming effect. It was similar in all field-cultivated accessions in the May experiment (Fig. 6 top). In the March experiment, stronger priming effects were observed in WS, Ms-0, Col-0, Van-0 and Cvi-0 than in N13 and Kas-1, which are the accessions with the highest and third highest cold acclimation potential (Table 1). In N13 and Kas-1 only slightly higher priming effects on the leaf blade size were observed showing that strong cold acclimation counteracts the priming effect.
Effects of cold pretreatment on the leaf and petiole lengths
The length of the longest leaf was determined as an additional morphogenetic parameter summarizing light, metabolite and hormonal regulation37 (Fig. 5 right). In the field, the leaves of cold-pretreated Ms-0, WS, Van-0 and Cvi-0 were longer than the leaves of naïve plants in the March experiments. In the greenhouse, no effect was observed in 2014 and longer leaves for WS, Van-0 and Cvi-0 in 2015. The petioles were also slightly longer in cold-treated plants in the field, but the effect was not significant due to high variation between individual leaves (data not shown).
In the May data set, also a trend towards longer leaves was observed after cold pretreatment, but the data were also not significant due to higher variability. In the greenhouse, N13 and WS showed trends to longer leaves in naïve plants.
Consequences of cold pretreatment on bolting
In regions with cold- and/or drought-restricted vegetation periods, the speed of bolting induction can be a critical factor for plant fitness38,39. Bolting visibly marks the transition from vegetative growth to regenerative growth. It affects leaf expansion, root growth, nutrient uptake and the seed number40,41. As a measure for bolting induction, we determined the time until the plants had formed at least 1 cm long inflorescences (Fig. 7).
In the March experiments, cold-pretreated plants bolted 3–4 weeks earlier in the greenhouse than in the field. Cold-pretreatment at least slightly accelerated bolting in the field. In the greenhouse, it promoted bolting in the accessions with higher cold acclimation potential (N13, Ms-0 and Kas-1 in both years and in 2015 also in WS and Col-0) (Fig. 7), while Van-0 and Can-0 did not show a priming response on bolting.
In the May experiment (Fig. 7), cold-pretreated Ms-0 and N13 bolted faster than naïve plants in the greenhouse. In the field, these accessions did not show significant differences in 2014. On the contrary, Van-0 and Cvi-0, which have a low cold-acclimation potential (Table 1), bolted earlier if they were cold-pretreated. In 2015, when the temperatures and the humidity were lower than in 2014 (Suppl. 1), N13, Ms-0, Kas-1 and WS bolted earlier in the field if they were cold-primed (Fig. 7 bottom).
Consequences of cold pretreatment on plant fitness
Arabidopsis thaliana is a typical r-selected organism42: It generates high numbers of small seeds to improve its fitness in habitats with variable environmental conditions. Alternatively, plants can improve their fitness by producing larger and/or heavier seeds, which support the embryo and the seedling with more energy resources43. Natural variation of seed size and weight in Arabidopsis is well-known in literature43,44. The total seed mass is negatively correlated with the number of seeds produced and positively correlated with seedling survival45. Here, we quantified the seed mass of individual seeds and, as a mixed parameter for fecundity and quality, the total seed number.
Kas-1 and Cvi-0, which originate from higher altitudes, formed generally slightly heavier seeds, but the cold pretreatment did not affect the individual seed weight in any accession (Fig. 8). However, cold-primed plants of all accessions formed more seeds in the field in the March and in the May experiments. In the greenhouse, only the strongest cold-adapted accessions N13, Ms-0 and Kas-1 (Table 1) produced more seeds (Fig. 8).
To differentiate pretreatment specific and adaptation dependent acclimation effects, the overall fitness effects were compared by normalizing the total seed weight in cold-pretreated plants on the total seed weight in naïve plants (seed weight of C-plants/ seed weight of N-plants) (Fig. 6 bottom). In the March planted series, the two accessions with the lowest cold acclimation potential, Van-0 and Cvi-0, showed the strongest cold effect (Fig. 6 bottom). The strongest absolute response was observed in N13, which was the accession with the highest cold acclimation potential in this study. This result excludes a general correlation of the fitness effects with the cold acclimation potential. On the contrary, the effects of the cold pretreatment on the total seed mass almost gradually decreased from the accessions with high cold acclimation potentials to those with low ones in the May experiment (Fig. 6 bottom).
Correlation between seed mass and PS-II activity
To test whether the priming effect on the reproductive fitness (Fig. 8) depends on PS-II regulation, the C-values of both parameters were normalized on the N-values [C-value/N-value]. The 2015 data, which show stronger effect variation than the 2014 data (Figs 2–5), were analyzed for cluster phenomenons and linear correlations. Squared Euclidean distance analysis of the data pairs for seed yield and ΦPS-II on day 1 and day 14 separated N13 and Ms-0 from the accessions with lower cold acclimation potential in the May data set (Fig. 9). The third strongly cold acclimating accession, Kas-1, was placed closest to these clusters (Fig. 9). In the four accessions with intermediate or weak cold acclimation potential, namely WS, Col-0, Van-0 and Cvi-0, the seed yield linearly correlated with the ΦPS-II on day 1 (rP = 0.731) and vaguely with ΦPS-II on day 14 (rP = 0.304) (Fig. 9).
Clusters identified by squared Euclidean distance analysis and linear correlations are depicted by circles and lines, respectively. The Pearson correlation coefficient (rP) is provided as a measure for the intensity of the linear correlations. The three accessions with high cold acclimation potential are depicted in blue, the others in orange. Bottom: Cluster and correlation analysis of the effect of cold pretreatment on the total seed weight per plant in seven accessions relative to the total seed weight in naïve plants with the effect of cold pretreatment on the relative leaf area in March (left) and May 2015 (right). Correlations are depicted by lines, clusters by circles.
In the March data sets squared Euclidean distance analysis did not support separation of stronger and weaker cold acclimatible accessions in distinct clusters. Correlation analysis for the stronger cold acclimating accessions N13, Ms-0 and Kas-1 and for the four other accessions showed high negative linear correlation (rP = −0.950 for Cvi-0, Van-0, Col-0 and WS and rP = −0.925 for N13, Kas-1 and Ms-0) between the priming effect on the seed yields and the priming effect on ΦPS-II on the first day in the field (Fig. 9). For N13, Ms-0 and Kas-1 a similar difference in ΦPS-II between cold-pretreated and naïve plants resulted in lower seed yields. The correlation lines of the two groups of accession was almost parallel shifted and separated the accessions into two regulatory units.
The leaf area after 3 weeks in the field almost perfectly correlated (rP = 0.999) with the total seed mass of naïve plants in the March experiments. The correlation was lost in the cold-adapted accessions (N13, Ms-0, Kas-1) and almost fully inverted (rP = −0.841) in the other accessions for the priming effects [C-value/N-value] on the leaf areas and the seed yields (Fig. 9 bottom left). In the May experiment, variation of the leaf areas was very low (Fig. 9 Bottom right). Ms-0 and N13 clustered separately from the other accessions due to slightly higher seed yields (Fig. 9 Bottom right).
Discussion
Costs and benefits of cold pretreatment under stable environmental conditions
According to the allocation cost theories, attribution of energy and metabolites into defense and acclimation limit growth and reproduction46,47,48. The costs of the cold pretreatment can be observed if acclimated plants are transferred to non-stress conditions. Here, cold pretreatment resulted in smaller leaf blades and (except in March 2014) in shorter leaves in the greenhouse in various accessions (Fig. 5) indicating resource restriction49. The seed numbers or seed weights were not decreased (Fig. 8) demonstrating that the cold pretreatment impacted not on the reproductive fitness.
The accessions N13, Ms-0 and Kas-1 hardly produced seeds in the greenhouse without cold pretreatment (Fig. 8; greenhouse data). These accessions originate from areas with shorter vegetation periods and long cold winters (Table 1) and have stronger vernalization dependent alleles of FLC (FLOWERING LOCUS C) and FRI (FRIGIDA)5,50. FLC and FRI control meristem transition51 and support cold acclimation5,38,52. The release of osmolytes, e.g. trehalose53,54, also supports meristem transition and induces bolting. N13, Ms-0 and Kas-1 accumulate more osmolytes in the cold than the other accessions8. The fast release of higher amounts of osmolytes upon early deacclimation8 provides a stronger metabolite flux in the cold-pretreated plants in the greenhouse. In the field, the accessions also flowered efficiently without cold-pretreatment. N-plants of the accessions N13, Ms-0 and Kas-1 formed similar or even more seeds in the field than cold-pretreated plants in the greenhouse did (Fig. 8).
Effect of cold pretreatment on bolting in the field
In May, the plants were exposed to long-day conditions in the field similar to the greenhouse illumination regime, but the temperatures were lower, especially during the nights (Fig. 1). While photoperiod sensing should have supported meristem transition55,56,57, bolting was only slightly (May 2015) or not promoted (May 2014) in the accessions with high cold acclimation potential (N13, Ms-0 and Kas-1). Low night temperatures in combination with diurnal temperature variations activate cold acclimation stronger than continuous cold stress does7. Decreased conveyance of osmolytes into cold acclimation can explain the delay in bolting based on the allocation cost theories46,47,48.
As shown in the Arabidopsis mutant gigantea and by application of ascorbate, stronger antioxidant protection delays flowering58,59,60. At low temperatures, ascorbate accumulates and the genes for ascorbate and glutathione regenerating enzymes are expressed stronger10,24,61. Consistent with the bolting effect (Fig. 7), the more cold adapted accessions decrease the ascorbate pool size more slowly after cold acclimation than the accessions with lower cold acclimation potential24.
Benefits of cold adaptation on the reproductive fitness
Cold-pretreated plants produced more seeds than naïve ones in the field in the May experiment (Fig. 8). The effect was widely independent from changes in the leaf area and the photosynthetic activity (Figs 2 and 5). The total seed mass in cold-pretreated plants relative to that in naïve plants (C/N-ratio) gradually decreased from the accessions with higher cold acclimation potentials to the accessions with the lower ones (Fig. 6 bottom). The best cold adapted accessions N13, Ms-0 and Kas-1 activate the CBF-regulon stronger in the cold8. Either directly or via its upstream regulator ICE1, cold impacts on the circadian clock, FLC-regulation and hormone, redox and phytochrome signaling62,63,64 and controls stress protection, which supports the reproductive fitness65,66. The trend observed here is consistent with a large body of literature demonstrating that cold acclimation supports the reproductive fitness in the field.
Costs of a high cold acclimation potential
Harsher weather conditions (Fig. 1) caused stronger effects in the field in March than in May (Figs 2–6). As frequently reported in literature (for review: refs 11 and 18), cold-pretreated plants benefit in the cold from previous cold acclimation by better PS-II protection (Fig. 2 left). In our study, FV/FM decreased on the first and/or second day after transfer of the plants to the field in naïve N13, Ms-0 and Kas-1 (Fig. 2 left) demonstrating weaker stabilization of PS-II activity against natural environmental conditions with e.g. light and temperature fluctuations. The other accessions did not show the effect. Lower PS-II activity in the best cold adapted accessions demonstrated costs of cold acclimation. The difference in the dynamic of PS-II activity recovery (Fig. 3 left) is not linked to regulation of photosynthetic electron transport as cold acclimation results in more efficient QA oxidation67. Weaker expression of plastid antioxidant enzymes in the cold24 increases the risk for photodamage68,69 and inhibits PS-II69,70,71. Here, PS-II was inhibited stronger in naïve plants, if the plants were exposed to the field conditions (Fig. 2 left). Recovery often includes proteolytic digestion, protein de novo synthesis and photosystem re-assembly72. These processes take time and are temperature sensitive73. The weaker chloroplast antioxidant protection in accessions with a higher cold acclimation potential24 delays the recovery of PS-II activity and can explain the slower response in N13, Ms-0 and Kas-1 (Fig. 3 left).
Differentiating cold acclimation and cold priming responses
The benefit of the cold-pretreatment on the seed yield was higher in the best and also in the worst cold-adapting accessions, after the plants were transferred to the field in March (Fig. 6 bottom). The two-directional effect indicates an overlay of two parallel acting seed yield supporting mechanisms. As shown recently under growth chamber conditions in the Arabidopsis accession Col-010, protection by cold acclimation is accompanied by independently regulated priming effects, such as mitigating chloroplast ROS signaling and strengthening pleiotropic stress responses. In contrast to acclimation, priming is not coupled to the persistence of a stressful situation. Priming is defined as a mechanism transmitting information on a previous stress over a stress-free period74. Comparison of 24 h and 14 day long cold pretreated plants demonstrated that the response to cold priming decreases if the first stress stimulus is long enough to activate cold acclimation10. We concluded that cold priming is antagonized by cold acclimation10. Cold-pretreated accessions showed better PS-II activity stabilization than naïve plants after 3 weeks (Fig. 4) demonstrating positive cold priming effects in the field (Fig. 4). PS-II activity was indistinguishable from that in naïve plants already 1 week after the transfer (Fig. 3 left). One day before the chlorophyll-a fluorescence analysis (day 20), the plants faced a triggering stimulus after a cold-free period (Fig. 1; Suppl. 1). Consistent with the definition of priming74 and with the previous hypothesis that priming acts independently from cold acclimation and is of importance in nature in spring10, the cold pretreatment resulted in higher ΦPS-II in the intermediate cold-adapted accessions WS, Col-0 and Van-0 than in the most cold-adapted accessions N13, Ms-0 and Kas-1 (Fig. 4). The priming memory depends on post-stress regulation of the chloroplast antioxidant system, which stabilizes PS-II activity75,76. It antagonizes chloroplast-to-nucleus ROS signaling, while chloroplast-independent ROS signaling is unaffected10. A triggering stress induces pleiotropic stress protection stronger in primed plants10. Here, the priming effect on the total seed mass correlated negatively with the priming effect on ΦPS-II at day 1 after the transfer into the field (Fig. 9; March) demonstrating that the protective effect is independent from the direct effect of cold acclimation, but controlled by priming-induced regulation.
Conclusion
Cold pretreatment supported the reproductive fitness in all tested accessions in the field, regardless whether the plants were transferred in March or in May. In March, the effect on the total seed mass was linked to stabilization of ΦPS-II. In the plants transferred in May, it negatively correlated with the cold acclimation potential independent of effects on PS-II activity, which excludes metabolite allocation and metabolite signaling effects. The March transferred plants experienced cold in the field. In this plant group, the cold-pretreatment supported the seed yield the most in the two accessions with the lowest cold acclimation potential, Van-0 and Cvi-0. Higher ΦPS-II after 3 weeks in the field furthermore demonstrated that accessions with weaker cold acclimation potential benefited more from cold acclimation than accessions with high, adapted cold tolerance.
Methods
Plant material and growth conditions
The Arabidopsis thaliana accessions Col-0 (INRA Accession ID 186AV), Cvi-0 (166AV), Kas-1 (434AV), Ms-0 (93AV), N13 (266AV), Van-0 (161AV) and WS (84 AV) were propagated in the greenhouse. The seeds of parallelly grown plant sets of 60 (2014) and 160 plants per accession were stratified in darkness for three days at 4 °C on Arabidopsis soil (70 volumes “Topferde” (Einheitserde, Germany), 70 volumes “Pikiererde” (Einheitserde, Germany), 25 volumes Perligran Classic (Knauf, Germany) supplemented with 0.5 g l−1 dolomite lime (Deutsche Raiffeisen-Warenzentrale, Germany)) and watered with 0.5 g l−1 Axoris Insekten-frei (COMPO, Germany) prior to transfer to a climate-controlled chamber with a day/night rhythm of 10 h at 20 ± 2 °C and 120 μmol photons m−2 s−1 light (L36W/840 Lumilux Cool White fluorescent tubes) and 14 h at 18 ± 2 °C in darkness at 60 ± 5% relative humidity. At an age of 28 days, half of the plants were transferred to 4 °C and cultivated for 14 days in the 10 h light/14 h dark rhythm with the same illumination, aeration settings and humidity as in the growth chamber. Up to this point of the experiment, the cultivation conditions were identical to those used in the LTC (long term cold) data set in the previous study10.
15 (2014) and 35 (2015) cold-pretreated plants (C) and the same numbers of untreated (naïve) plants (N) per accession were transferred to the field (Fig. 10). For optimal soil contact, the pots were removed. The field areas were covered with a natural weed vegetation prior to the experiment. They have not been treated with any fertilizer or herbicide for at least 5 years and were prepared by 20–25 cm deep ploughing one week before the experiments were started. Slug interference was minimized by application of ‘Mesurol Schneckenkorn’ pellets (Bayer, Germany). The same numbers of the cold-treated and naïve plants were transferred to a temperature (20 °C during the 14 h long day period and 18 °C during the night), light intensity (illumination with 55 klux with Philips SON-T AGRO, if the external light intensity was <50 klux during the day phase, and activation of shading, if the external light intensity was >55 klux, which corresponds to 100–120 μmol photons m−2 s−1 on plant rosette level) and humidity controlled (45% relative humidity) greenhouse. The position of accessions and treatments were randomized between and inside the trays and baskets. The plants were cultivated in the field and in the greenhouse until the end of the seed harvest (Fig. 8). Data from plants, which were damaged during the experiment, were removed from the data set. For the final data analysis, only data obtained with the same plants were used.
(A + B) Field-experiment: Cold-pretreated and naïve plants were planted in an open basket system in randomized patterns. (C) Field experiment: At the time the May experiment was started in the outer lanes, most plants of the March-experiment started to set seeds, which were collected in cellophane bags. (D) Greenhouse experiment: the plants were grown in randomized patterns in trays. Like in the field, the seeds were collected on the plants.
Chlorophyll-a fluorescence analysis
The maximum quantum efficiency of photosystem II (FV/FM = (FM − F0)/FM) and the effective quantum yield of photosystem II (ΦPSII = = (FM′ − F0)/FM′), were determined by chlorophyll-a fluorescence analysis using a MINI-PAM fluorimeter (Walz, Effeltrich, Germany)32,77 and “Dark leaf clips DLC-8” (Walz, Effeltrich, Germany). The measurement was started with open leaf clips at 10:30 a.m. by determination of ΦPSII in the center of one leaf half of the largest leaf with a 0.8 s long saturating light flash (>3000 μmol photons m−2 s−1). The leaf clips were placed in 25 sec intervals on the various plants. The measurements were started with determination of ΦPSII. 20 min after closing the leaf clips for dark acclimatization, FV/FM was determined in the various plants in the same rhythm as determination of ΦPSII on the same leaf and the same position. Afterwards, the leaf clips were removed.
Plant imaging analysis
Top views of the plants were taken weekly with a digital camera in the field and greenhouse. The pictures were analyzed with the ImageJ software package78 for the leaf length and petiole length in mm and the leaf area in mm2.
Determination of seed numbers and seed weight
The inflorescences were bagged in cellophane before the first siliques opened (Fig. 10C and D). The seeds were air-dried in the bags for 4 weeks at room temperature. After cleaning them from attached petals, sepals and silique valves, the seeds were incubated in the dark and at room temperature for 6 more weeks, before the total seed mass per plant (n = 15) and the average individual seed weight were determined by weighting all seeds and three aliquots of 100–200 seeds on a digital balance (ABT320-4B, Kern & Sohn GmbH, Germany). The exact seed number was determined for each aliquot of seeds originating from 6–7 plants used in the chlorophyll-a fluorescence analysis on digital images using the ‘Analyze particles’ function of the software package ImageJ78. The parameter ‘Size’ was set to 6–150 and the ‘Circularity’ to 0.7–1.0 for counting. The individual seed weights were calculated by dividing the seed weight of the aliquots by the number of seeds counted in the respective aliquot. The total number of seeds per plant was calculated from the total seed weight and the mean individual seed weight in the same batch.
Weather data
The air temperature and the relative air humidity were determined 2 m above the ground and the global radiation was obtained at the meteorological station ‘Dahlem’ of the Free University of Berlin (Institut für Meteorologie der Freien Universität Berlin, Germany) 610 m east and 750 m southeast of the site. All parameters were recorded at one-minute intervals from the beginning of March to the time of the last harvest. For the figures, one-hour-mean values were calculated. Additionally, in the field, the quantum flux (quantum flux [μmol quanta m−2 s−1] = radiation [Wm−2]∙λ [nm]∙0.836∙10−2) was recorded with a MQ-200 quantum flux meter (Apogee Electronics, Santa Monica CA, U.S.A.) and the air temperature and humidity controlled with a Voltcraft DL-121TH data logger (Voltcraft, Switzerland) several times during the experiment for comparison.
Statistical analyses
The ANOVA (Turkey Post-hoc test), cluster analysis (squared Euclidean distance; cluster number set to 2–5 clusters) and Pearson correlation analyses were carried out at α < 0.05 using R (version 3.2.2, Vienna, Austria), SPSS 22 and 23 (IBM Armonk U.S.A.) and Excel (Microsoft, Redmond, U.S.A.).
Additional Information
How to cite this article: Cvetkovic, J. et al. The effect of cold priming on the fitness of Arabidopsis thaliana accessions under natural and controlled conditions. Sci. Rep. 7, 44055; doi: 10.1038/srep44055 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hoffmann, M. H. Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). J. Biogeogr. 29, 125–134 (2002).
Al-Shehbaz, I. A., Beilstein, M. A. & Kellogg, E. A. Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Plant Systematics and Evolution 259, 89–120 (2006).
Gehan, M. A. et al. Natural variation in the C-repeat binding factor cold response pathway correlates with local adaptation of Arabidopsis ecotypes. Plant Journal 84, 682–693 (2015).
Hannah, M. A. et al. Natural genetic variation of freezing tolerance in arabidopsis. Plant Physiology 142, 98–112 (2006).
Johanson, U. et al. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290, 344–347, doi: 10.1126/science.290.5490.344 (2000).
Thomashow, M. F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology 50, 571–599, doi: 10.1146/annurev.arplant.50.1.571 (1999).
Markovskaya, E. F., Sysoeva, M. I. & Sherudilo, E. G. The effect of daily exposure to low hardening temperature on plant vital activity. Russ. J. Dev. Biol. 39, 261–268 (2008).
Zuther, E., Juszczak, I., Lee, Y. P., Baier, M. & Hincha, D. K. Time-dependent deacclimation after cold acclimation in Arabidopsis thaliana accessions. Scientific Reports(2015).
Pagter, M. & Arora, R. Winter survival and deacclimation of perennials under warming climate: physiological perspectives. Physiologia Plantarum 147, 75–87, doi: 10.1111/j.1399-3054.2012.01650.x (2013).
van Buer, J., Cvetkovic, J. & Baier, M. Cold regulation of plastid ascorbate peroxidases serves as a priming hub controlling ROS signaling in Arabidopsis thaliana . BMC Plant Biology 16, 163, doi: 10.1186/s12870-016-0856-7 (2016).
Huner, N. P. A. et al. Photosynthesis, photoinhibition and low-temperature acclimation in cold tolerant plants. Photosynthesis Research 37, 19–39 (1993).
Tahtiharju, S., Sangwan, V., Monroy, A. F., Dhindsa, R. S. & Borg, M. The induction of kin genes in cold-acclimating Arabidopsis thaliana. Evidence of a role for calcium. Planta 203, 442–447 (1997).
Knight, H. & Knight, M. R. Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci. 6, 262–267 (2001).
Seo, P. J. et al. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant Journal 61, 661–671 (2010).
Thomas, L., Marondedze, C., Ederli, L., Pasqualini, S. & Gehring, C. Proteomic signatures implicate cAMP in light and temperature responses in Arabidopsis thaliana . J. Proteomics 83, 47–59 (2013).
Christmann, A. et al. Integration of abscisic acid signalling into plant responses. Plant Biology 8, 314–325 (2006).
Huang, X. Z., Li, Y. S., Zhang, X. Y., Zuo, J. R. & Yang, S. H. The Arabidopsis LSD1 gene plays an important role in the regulation of low temperature-dependent cell death. New Phytologist 187, 301–312, doi: 10.1111/j.1469-8137.2010.03275.x (2010).
Ensminger, I., Busch, F. & Huner, N. P. A. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiologia Plantarum 126, 28–44 (2006).
Dai, F., Huang, Y., Zhou, M. & Zhang, G. The influence of cold acclimation on antioxidative enzymes and antioxidants in sensitive and tolerant barley cultivars. Biologia Plantarum 53, 257–262 (2009).
Knight, H., Zarka, D. G., Okamoto, H., Thomashow, M. E. & Knight, M. R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiology 135, 1710–1717 (2004).
Yamaguchi-Shinozaki, K., Urao, T. & Shinozaki, K. Regulation of genes that are induced by drought stress in Arabidopsis thaliana . J. Plant. Res. 108, 127–136 (1998).
Zhou, M. Q., Shen, C., Wu, L. H., Tang, K. X. & Lin, J. CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Critical Reviews in Biotechnology 31, 186–192, doi: 10.3109/07388551.2010.505910 (2011).
Gray, G. R., Chauvin, L. P., Sarhan, F. & Huner, N. P. A. Cold acclimation and freezing tolerance - A complex interaction of light and temperature. Plant Physiology 114, 467–474 (1997).
Juszczak, I., Cvetkovic, J., Zuther, E., Hincha, D. K. & Baier, M. Natural variation of cold deacclimation correlates with variation of cold-acclimation of th plastid antioxidant system in Arabidopsis thaliana accessions. Frontiers in Plant Science 7, 305, doi: 10.3389/fpls.2016.00305 (2016).
Degenkolbe, T. et al. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana . Plant Journal 72, 972–982, doi: 10.1111/Tpj.12007 (2012).
McKhann, H. I. et al. Natural variation in CBF gene sequence, gene expression and freezing tolerance in the Versailles core collection of Arabidopsis thaliana . Bmc Plant Biology 8, 105, doi: 10.1186/1471-2229-8-105 (2008).
Lorenzo, C. D., Sanchez-Lamas, M., Antonietti, M. S. & Cerdan, P. D. Emerging hubs in plant light and temperature signaling. Photochemistry and Photobiology 92, 3–13 (2016).
Zuther, E., Schulz, E., Childs, L. H. & Hincha, D. K. Clinal variation in the non-acclimated and cold-acclimated freezing tolerance of Arabidopsis thaliana accessions. Plant Cell and Environment 35, 1860–1878, doi: 10.1111/j.1365-3040.2012.02522.x (2012).
Juszczak, I., Rudnik, R., Pietzenuk, B. & Baier, M. Natural genetic variation in the expression regulation of the chloroplast antioxidant system among Arabidopsis thaliana accessions. Physiologia Plantarum 146, 53–70, doi: 10.1111/j.1399-3054.2012.01602.x (2012).
Strånd, A., Hurry, V., Gustafsson, P. & Gardestrom, P. Development of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J. 12, 605–614 (1997).
Genty, B., Briantais, J.-M. & Baker, N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92 (1989).
Schreiber, U., Hormann, H., Neubauer, C. & Klughammer, C. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust. J. Plant Physiol. 22, 209–220 (1995).
Van Volkenburgh, E. Leaf expansion - an integrating plant behaviour. Plant Cell and Environment 22, 1463–1473 (1999).
Andersson, J. et al. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II - effects on photosynthesis, grana stacking and fitness. Plant J. 35, 350–361 (2003).
Krah, N. M. & Logan, B. A. Loss of psbS expression reduces vegetative growth, reproductive output, and light-limited, but not light-saturated, photosynthesis in Arabidopsis thaliana (Brassicaceae) grown in temperate light environments. American Journal of Botany 97, 644–649 (2010).
Boyes, D. C. et al. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13, 1499–1510 (2001).
Tsukaya, H., Kozuka, T. & Kim, G. T. Genetic control of petiole length in Arabidopsis thaliana . Plant and Cell Physiology 43, 1221–1228 (2002).
Korves, T. M. et al. Fitness effects associated with the major flowering time gene FRIGIDA in Arabidopsis thaliana in the field. American Naturalist 169, E141–E157 (2007).
Gimenez-Benavides, L., Garcia-Camacho, R., Iriondo, J. M. & Escudero, A. Selection on flowering time in Mediterranean high-mountain plants under global warming. Evol. Ecol. 25, 777–794 (2011).
Fitter, A. H. & Fitter, R. S. R. Rapid changes in flowering time in British plants. Science 296, 1689–1691 (2002).
Craufurd, P. Q. & Wheeler, T. R. Climate change and the flowering time of annual crops. Journal of Experimental Botany 60, 2529–2539 (2009).
Pianka, E. R. On r- and K-Selection. American Naturalist 104, 592–597 (1970).
Gnan, S., Priest, A. & Kover, P. X. The genetic basis of natural variation in seed size and seed number and their trade-off using Arabidopsis thaliana MAGIC lines. Genetics 198, 1751–1758 (2014).
Alonso-Blanco, C., Blankestijn-de Vries, H., Hanhart, C. J. & Koornneef, M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA 96, 4710–4717 (1999).
Coomes, D. A. & Grubb, P. J. Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol. Evol. 18, 283–291 (2003).
Bazzaz, F. A., Chiariello, N. R., Coley, P. D. & Pitelka, L. F. Allocating resources to reproduction and defense. Bioscience 37, 58–67 (1987).
Herms, D. A. & Mattson, W. J. The dilemma of plants - to grow or defend. Q. Rev. Biol. 67, 283–335 (1992).
Walters, D. & Heil, M. Costs and trade-offs associated with induced resistance. Physiological and Molecular Plant Pathology 71, 3–17 (2007).
Kulheim, C. & Jansson, S. What leads to reduced fitness in non-photochemical quenching mutants? Physiologia Plantarum 125, 202–211 (2005).
Berry, S. & Dean, C. Environmental perception and epigenetic memory: mechanistic insight through FLC. Plant Journal 83, 133–148, doi: 10.1111/tpj.12869 (2015).
Henderson, I. R., Shindo, C. & Dean, C. The need for winter in the switch to flowering. Annual Review of Genetics 37, 371–392, doi: 10.1146/annurev.genet.37.110801.142640 (2003).
Edwards, K. D. et al. FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell 18, 639–650 (2006).
Iturriaga, G., Suarez, R. & Nova-Franco, B. Trehalose metabolism: from osmoprotection to signaling. Int. J. Mol. Sci. 10, 3793–3810 (2009).
Francis, D. & Halford, N. G. Nutrient sensing in plant meristems. Plant Molecular Biology 60, 981–993 (2006).
Hayama, R. & Coupland, G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr. Opin. Plant Biol. 6, 13–19 (2003).
Jackson, S. & Thomas, B. Photoreceptors and signals in the photoperiodic control of development. Plant Cell and Environment 20, 790–795 (1997).
Onouchi, H. & Coupland, G. The regulation of flowering time of Arabidopsis in response to daylength. Journal of Plant Research 111, 271–275 (1998).
Attolico, A. D. & De Tullio, M. C. Increased ascorbate content delays flowering in long-day grown Arabidopsis thaliana (L.) Heynh. Plant Physiology and Biochemistry 44, 462–466 (2006).
Kurepa, J., Smalle, J., Van Montagu, M. & Inze, D. Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant Journal 14, 759–764 (1998).
Cao, S. Q., Jiang, S. T. & Zhang, R. X. The role of GIGANTEA gene in mediating the oxidative stress response and in Arabidopsis. Plant Growth Regulation 48, 261–270 (2006).
Karpinski, S., Escobar, C., Karpinska, B., Creissen, G. & Mullineaux, P. M. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis excess light stress. Plant Cell 9, 627–640 (1997).
Fowler, S. G., Cook, D. & Thomashow, M. E. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiology 137, 961–968 (2005).
Kurepin, L. V. et al. Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int. J. Mol. Sci. 14, 12729–12763, doi: 10.3390/ijms140612729 (2013).
Lee, J. H., Jung, J. H. & Park, C. M. INDUCER OF CBF EXPRESSION1 integrates cold signals into FLOWERING LOCUS C-mediated flowering pathways in Arabidopsis. Plant Journal 84, 29–40 (2015).
Hsieh, T. H., Lee, J. T., Charng, Y. Y. & Chan, M. T. Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiology 130, 618–626 (2002).
Budhagatapalli, N., Narasimhan, R., Rajaraman, J., Viswanathan, C. & Nataraja, K. N. Ectopic expression of AtICE1 and OsICE1 transcription factor delays stress-induced senescence and improves tolerance to abiotic stresses in tobacco. J. Plant Biochem. Biot. 25, 285–293 (2016).
Öquist, G., Chow, W. S. & Anderson, J. M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186, 450–460 (1992).
Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1419–1431 (2000).
Foyer, C. H., Lelandais, M. & Kunert, K. J. Photooxidative stress in plants. Physiol. Plant. 92, 696–717 (1994).
Powles, S. B. Photoinhibition of photosynthesis induced by visible light. Annual Review of Plant Physiology and Plant Molecular Biology 35, 15–44 (1984).
Hideg, E., Kalai, T., Hideg, K. & Vass, I. Do oxidative stress conditions impairing photosynthesis in the light manifest as photoinhibition? Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 355, 1511–1516 (2000).
Aro, E. M., Tyystjarvi, E. & Nurmi, A. Temperature-dependent changes in photosystem-II heterogeneity of attached leaves under high light. Physiologia Plantarum 79, 585–592 (1990).
Hurry, V. M. & Huner, N. P. A. Effect of cold hardening on sensitivity of winter and spring wheat leaves to short-term photoinhibition and recovery of photosynthesis. Plant Physiology 100, 1283–1290 (1992).
Hilker, M. et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 12215, doi: 10.1111/brv.12215 (2015).
Baier, M. & Dietz, K. J. Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis. Plant Physiol 119, 1407–1414 (1999).
Kangasjarvi, S. et al. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochemical Journal 412, 275–285 (2008).
van Kooten, O. & Snel, J. F. H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25, 147–150 (1990).
Sheffield, J. B. ImageJ, a useful tool for biological image processing and analysis. Microscopy and Microanalysis 13, 200–201 (2007).
Acknowledgements
We thank the German research foundation (CRC973-C4) and the FU Berlin for funding, Beau Dronsella, Sarah Hollis, Ari Satanowski, Britt Schaffranietz und Elena Reifschneider for technical support, Dr. Christiane Hedtmann, Dr. Rainer Bode and Dr. Jörn van Buer for critical reading and Prof. Dr. Britta Tietjen for helpful discussion on the data analysis.
Author information
Authors and Affiliations
Contributions
J.C. performed the experiments and drafted the figures and parts of the manuscript. K.M. provided the weather data. M.B. supervised the project and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cvetkovic, J., Müller, K. & Baier, M. The effect of cold priming on the fitness of Arabidopsis thaliana accessions under natural and controlled conditions. Sci Rep 7, 44055 (2017). https://doi.org/10.1038/srep44055
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44055
- Springer Nature Limited
This article is cited by
-
Osmotic priming-induced cryotolerance uncovers rejuvenation of grapevine cell cultures: morphogenetic changes and gene expression pattern highlighting enhanced embryogenic potential
Protoplasma (2024)
-
Cold stress and freezing tolerance negatively affect the fitness of Arabidopsis thaliana accessions under field and controlled conditions
Planta (2022)
-
How Do Different Temperature Fluctuations Affect Alcea rosea ‘nigra’ Survival?
Iranian Journal of Science and Technology, Transactions A: Science (2022)