Abstract
Intra-guild predation (IGP) is an important phenomenon structuring ecological communities and affects the success of biological control. Here we show that parasitism by the koinobiont wasp Cotesia vestalis is associated with behavioural changes in its larval host (diamondback moth, Plutella xylostella) that reduce risk of IGP. Compared with unparasitised caterpillars, parasitised P. xylostella moved less frequently to new feeding patches on plants and were less likely to fall from the plant. Wolf spiders killed significantly fewer parasitised larvae. Reflecting their reduced movement and capacity to select plant tissue of optimal quality, parasitised caterpillars fed at a lower rate and exhibited delayed development suggesting a trade-off between IGP avoidance and nutrient intake by the host. This change in behaviour to reduce risk may cascade to the first trophic level and help explain the stability of IGP systems.
Similar content being viewed by others
Introduction
There are many reports of parasites manipulating the behaviour of their host1,2,3,4,5,6,7,8. Among the best studied cases of this puppet master phenomenon9 are those where the host is manipulated to perform bizarre, abnormal behaviour10, or where the manipulation of the ‘zombie’11 host culminates in its grotesque death. In such cases, altered host behaviour is associated with facilitating the parasite life cycle12,13. For example, the nematomorph Paragordius tricuspidatus Dufour causes its cricket host (Nemobius sylvestris Bosc D’Antic) to jump suicidally into water4, whilst infection of gypsy moth (Lymantria dispar L.) by baculoviruses result in the larva climbing to the top of trees to die14, resulting in benefit to the parasite by enhanced dissemination or host location. Underling this phenomenon, parasites have been reported to have effects on host development, physiology and immune system15,16,17.
Koinobiont parasitoids such as Cotesia vestalis (Haliday) (Hymenoptera: Braconidae) allow the host to continue feeding and development while feeding upon it; in contrast to “idiobionts” in which host development ceases once parasitised. This is an important life history strategy for the parasitoid to ‘capture’ the host at early stage yet allowing it to feed to compensate for its initially low nutritional value18. If, however, the host is killed before the parasitoid has developed sufficiently, the parasitoid perishes too19. Reflecting these facts, koinobionts can be predicted to avoid disrupting the host’s normal, evolutionarily-optimised feeding behaviour unless there is a sufficiently large gain by reducing risk from mortality.
Intra-guild predation (IGP), where competing species prey on each other as well as on a shared prey20,21, has emerged as a common phenomenon in ecological communities22. A predator and a parasitoid can attack a shared prey/host species23 but the resulting IGP is asymmetrical because, whilst the predator can consume parasitised hosts, the parasitoid cannot exert reciprocal attack on the predator. Parasitoid adults can alter their foraging to reduce the risk of IGP24 and immobile parasitised aphid ‘mummies’ can develop a thickened, protective cuticle to prevent attack25. It is, however, unclear if the capacity of immature koinobiont parasitoids extends to altering the behaviour of their active hosts to minimise IGP risk.
The overarching aim of this study was to test the hypothesis that the larval foraging behaviour of the globally significant brassica pest, diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae), is altered when parasitised by C. vestalis in a manner that reduces the risk of IGP. Movement on a plant is risky for insect herbivores26,27,28. In particular, herbivores that move to detect superior feeding sites may be exposed to elevated risk from predators29. Accordingly, an initial study tested the hypothesis that parasitized hosts exhibit reduced movement on foliage compared with healthy larvae; whether or not caused by the parasitoid manipulating the behaviour of the host. Any such change in foraging could originate as a ‘side effect’ of parasitism that led to greater lethargy in the host but could still be acted upon by natural selection. Evolution is not directed or teleological so any phenotypic variability in the parasitoid that happens to have an effect on the host and leads it to move less often would increase fitness if it reduced mortality by factors such as intra-guild predation. Though Poulin2 proposes multiple criteria to establish whether behavioural modifications may be considered as manipulation by parasites, including the complexity of the behavioural change, these were subsequently considered to be too conservative and that demonstration of fitness benefits provided the strongest evidence of parasite manipulation30. Accordingly, potential parasite manipulation can manifest as a change in host behaviour that is modest compared with the more dramatic examples described above. Subsequent studies explored whether fitness was enhanced in our study system using simulated wind or foraging C. vestalis since both these types of disturbances are faced by P. xylostella in the field; whilst predation was measured in the presence of the wolf spider (Pardosa pseudoannulata Koch (Araneae: Lycosidae). Each of these experiments tested the hypothesis that parasitized larvae were less strongly affected by the respective disturbance factor than were healthy hosts. Finally, we tested the hypothesis that reduced movement on the plant by parasitized larvae results in a reduction in nutrient acquisition by parasitised larvae, consistent with a trade-off from the altered feeding behaviour leading to a poorer quality diet.
Results and Discussion
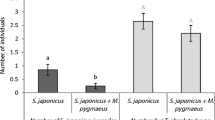
Parasitised larvae exploited significantly (p < 0.005) fewer feeding patches on excised leaves compared with unparasitised larvae in 2, 6 and 24 hour-long experiments (Fig. 1A). Similarly, reduced movement of parasitised larvae was evident in a separate study using whole plants that extended over three successive days and assessed the numbers of separate leaves fed upon by individual larvae (GLM treatment main effect, F = 6.612, p = 0.013; Fig. 1B). Subsequent studies tested whether this reduced host movement might be adaptive for the immature parasitoid by reducing the risk of IGP.
(A) Numbers of feeding patches exploited on a single leaf (±SE) (t-test, 2 h: t = −2.924, df = 47.429, p = 0.005; 6 h: t = −4.225, df = 46.172, p < 0.001; 24 h: t = −4.707, df = 58.854, p < 0.001) (B) Numbers of separate leaves exploited (±SE) (Treatment main effect: F = 6.612, p = 0.013; Time main effect: F = 40.518, p < 0.001; Treatment * Time: F = 4.199, p = 0.028). (C) Proportion of larvae falling from plant under the influence of simulated wind (±SE) (Treatment main effect: F = 26.1, p < 0.001; Time main effect: F = 10.112, p = 0.001; Treatment * Time: F = 0.933, p = 0.410); (D) Proportion of larvae falling from plant in presence of a foraging C. vestalis female (±SE) (Treatment main effect: F = 44.693, p < 0.001; Time main effect: F = 16.818, p < 0.001; Treatment * Time: F = 0.795, p = 0.465); (E) Proportional mortality in presence of a predator (P. pseudoannulata) allowed to forage on the plant (±SE) (Treatment main effect: F = 7.569, p = 0.020; Time main effect: F = 39.160, p < 0.001; Treatment * Time: F = 0.305, p = 0.740); (F) Proportional mortality under the influence of simulated wind and in presence of a predator (P. pseudoannulata) prevented from foraging on the plant (±SE) (Treatment main effect: F = 6.081, p = 0.027; Time main effect: F = 17.144, p < 0.001; Treatment * Time: F = 0.178, p = 0.838).
In a study in which a light breeze was simulated using an electric fan, parasitised larvae had a significantly lower rate of falling from whole plants compared with unparasitised larvae (GLM treatment main effect, F = 26.1, p < 0.001; Fig. 1C). In a similar study in which wind was replaced by a gravid C. vestalis, parasitised larvae were again much less likely to fall from the plant than were unparasitised larvae (GLM treatment main effect: F = 44.693, p < 0.001; Fig. 1D). This study did not partition the potential effect of the foraging parasitoid discriminating against parasitised larvae and focusing greater attention on unparasitised hosts rather than engaging in superparasitism. Any such effect is, however, likely to be minor because once gravid Cotesia detect an infested plant the formerly important orientation by chemical cues associated with herbivory31 is replaced by random searching until antennal contact with the host32. Observations of parasitoid foraging made during our study were consistent with this non-selective host location scenario. Accordingly, the higher rate of dropping from the plant by unparasitised hosts reflects response of these larvae rather than their detectability.
A further study used single wolf spiders as intra-guild predators. Pardosa species, also known as ground spiders33, are generally considered ground-foraging, active hunters34 but also forage on low foliage35. Whilst spiders consumed nearly all of the unparasitised larvae in our three-day-long mesocosms studies, significantly fewer of the parasitized larvae were eaten (GLM treatment main effect: F = 7.569, p = 0.020; Fig. 1E). A supplementary study without plants established that spiders did not discriminate between parasitised and unparasitised larvae and that equal numbers of each were consumed (Supplementary Figure 1) thus demonstrating the significance of on-plant behaviour and dislodgement in relative predation risk of parasitised and unparasitised larvae.
An additional study confined wolf spiders to the floor of mesocosms by mounting the plant atop a glass column up which only the larvae were able to climb. By confining the spiders to the floor of the arena, this removed the effect of spiders directly disturbing caterpillars and causing them to fall from the plant. Accordingly, only caterpillars that fell as a result of the simulated light breeze became available as prey. Simulated wind was applied as previously described and dislodged larvae were exposed to predation. Under these conditions, parasitised larvae were significantly less likely to be consumed (GLM treatment main effect, F = 6.081, p = 0.027; Fig. 1F).
Predator avoidance is recognised to constrain Lepidoptera larvae foraging to less than the nutritionally and physiologically ideal diets29,36. For example, herbivory in the field has been shown to be reduced by the presence of predators37. Patch feeding is employed by herbivores, including P. xylostella38, to locate plant tissue of optimal nutritional value and this can involve avoidance of sites in which plant defences have reduced the local quality of the plant tissue. Though P. xylostella is adapted to Brassica spp. allelochemicals such as glucosinolates, reduced movement of larvae may result in consumption of plant tissue that is harder, waxier, and lower in nutritional value27.
Our results showed that parasitised P. xylostella larvae moved less frequently on cabbage foliage than unparasitised larvae and this was associated with reduced risk of falling from the plant and IGP but this appears to carry a trade-off in terms of nutrient acquisition. In the study of patch movement on excised leaves, parasitised larvae consumed less than unparasitised counterparts (GLM treatment main effect: F = 47.421, p < 0.001) (Fig. 2A). This can be attributed to the larvae handling poorer quality plant tissue because parasitism did not affect the total duration of pauses in feeding or the number of feeding interruptions (Supplementary file, Supplementary Figures 2 and 3). Our longer term study using intact plants found that larval development was slower for parasitised compared with unparasitised P. xylostella (t-test, 3rd instars: t = 4.083, df = 78, p < 0.001; 4th instars: t = 4.747, df = 71, p < 0.001) (Fig. 2B,C), an effect in agreement with Shi, et al.39 and Bae and Kim17. Daily leaf consumption on whole plants peaked at 143.84 ± 11.14 mm2 on day 8 for unparasitised larvae but parasitised larvae consumed no more than 99.58 ± 6.66 mm2 per day with this peak not occurring until day 10 (Fig. 2D). The extended developmental time of parasitised larvae meant that total leaf consumption during development was similar to that of unparasitised larvae, 384.38 ± 24.62 mm2 versus 442.99 ± 20.70 mm2, respectively (t-test, t = −1.822, df = 78, p = 0.072). This reduced rate of nutrient acquisition may represent a trade-off for reduced risk of IGP. Such a scenario is consistent with increased fitness of the parasitoid provided that the disadvantages of slowed development are more than offset by a reduced risk of dislodgement and predation, with natural selection acting to favour the trade-off ‘setting’ that optimises fitness.
(A) Short-term leaf consumption (±SE) of parasitised and unparasitised larvae on cabbage (t-test, 2 h: t = −7.412, df = 61.743, p < 0.001; 6 h: t = −6.639, df = 65.755, p < 0.001; 24 h: t = −7.858, df = 78, p < 0.001); (B) Duration (±SE) of parasitised and unparasitised 3rd instar P. xylostella larvae; (C) Duration (±SE) of parasitised and unparasitised 4th instar P. xylostella larvae; (D) Daily leaf consumption (±SE) of parasitised and unparasitised larvae (t-test, day5: t = −6.044, df = 65.155, p < 0.001; day 6: t = −1.067, df = 46.047, p = 0.291; day 7: t = −6.992, df = 48.237, p < 0.001;. day 8: t = −6.858, df = 53.856, p < 0.001; day 9: t = 0.292, df = 67, p = 0.771; day 10: t = 2.568, df = 29.627, p = 0.016.
Overall these results are consistent with, rather than conclusive evidence for, parasitoid manipulation of host behaviour in this system. If confirmed in future work, this provides a formerly unrecognised form of parasite manipulation that has potential cascading effects to the first trophic level. Though parasitised larvae fed on similar volumes of plant tissue, their diet is likely to be poorer quality and less valuable to the plant than that consumed by more mobile unparasitised larvae. Further, the diet of parasitised larvae is consumed over a longer time period, better allowing the plant to undergo compensatory growth. The ecological significance of cascading effects on the first trophic level from this form of parasite manipulation may be important, especially in agroecosystems, but remains to be explored. Clearly, field studies to test for the strength of this phenomenon under more realistic and complex conditions are necessary.
Cotesis vestalis has been reported to be an effective biological control agent of P. xylostella in multiple countries40,41,42. If changes in parasitized host behaviour are confirmed in field studies, it may help explain the success of this species and signal the need for equivalent work in other host-parasitoid systems including those of biological control significance.
Fire ants (Solenopsis invitica (Boisduval)) parasitised by the decapitating fly (Pseudacteon tricuspis Borgmeier) have been reported to remain in the nest rather than engaging in foraging11 and this may reflect manipulation of host behaviour to reduce predation risk to the parasitoid. In that study, however, there was no assessment of IGP and no trade-off in nutrient acquisition because the parasitoid consumes only the head capsule contents rather than the entire host as is the case with Cotesisa. Because IGP exerts such negative effects on parasitoids, any available trait that reduces risk is likely to be selected24. Accordingly, even a minor change in host behaviour, such as reduced movement that may originate as a ‘side effect’ of parasitism, potentially provides a fitness advantage to the parasitoid. Our studies appear to be the first to test for effects consistent with the behaviour of a parasitised host being manipulated by its parasitoid in order to reduce IGP. If this phenomenon is found to occur in the field and within multiple host-parasite systems it would help reconcile the theoretical instability of these tri-partite systems20 with the fact that they are ubiquitous in nature22. Complexity appears key to developing robust models of IGP43 and parasitoid puppet masters might play a formerly-unrecognised stabilising role.
Materials and Methods
Insect and plant preparation
Founders for the P. xylostella and C. vestalis cultures were sourced as pupae/cocoons from a cabbage field in the town of Nantong, Fujian Province, in May 2014. The spider, P. pseudoannulata, was from a colony at the Institute of Plant Protection, Fujian Academy of Agricultural Sciences. All arthropods were maintained and tested in an environmental chamber at 25 ± 2 °C, 50% ± 10% RH, with a photoperiod of L14:D10. P. xylostella was reared on radish sprouts (Raphanus sativus L.) and C. vestalis was reared on larvae of P. xylostella. To prepare parasitised P. xylostella, neonate larvae were placed on cabbage for five days then third instar larvae were randomly chosen. Each larva was exposed to a mated female C. vestalis individually in a glass tube and monitored until parasitism occurred, a protocol that has been shown to lead to a parasitism rate of over 93%44.
Cabbage (Brassica oleracea var. capitata (L.), cv. Jing-feng No.1) was sown in boxes (40 cm × 30 cm × 14 cm) and placed in an outdoor mesh cage. About 20–30 days after seeding (2–3 leaf stage), individual plants were transplanted in larger pots (9 cm diameter). The cabbage plants were at the 4–6 leaf stage when used in the experiments.
Larval feeding patch assessment and short-term leaf consumption
To investigate the movement of larvae from patch to patch on a leaf, parasitised and unparasitised 3rd instar larvae were individually reared on the cabbage until they reached the 4th instar. Larvae were starved for 6 hours before being introduced individually to a fresh cabbage plant. The larvae were then allowed to feed for 2 hours and the numbers of feeding patches the larvae exploited were recorded for forty replicates. This was repeated for periods of 6 and 24 hours feeding with different larvae and plants. The leaf area consumed was measured using a calibrated stereomicroscope (Olympus-SZX2) and analysed using the software “cellSens dimension” (Olympus Ltd, Tokyo, Janpan) to determine leaf consumption by each larva. Student’s t-test was used to compare unparasitised and parasitised larvae wihin each of the three experiments.
Larval movement from leaf to leaf
Thirty parasitised and thirty unparasitised 3rd instar larvae were individually placed on the third leaf of intact cabbage plants. The numbers of cabbage leaves fed upon by each larva were recorded every day for three days. A General Linear Model (GLM) with repeated measures was used to determine the effect of treatments (between-subject variation) and time (within-subject variation). The measures were the numbers of leaves fed upon, with data being collected on successive time periods.
Effect of simulated wind on larval dislodgement
To determine the effect of wind on dislodgement from the plant of parasitised versus unparasitised larvae, a system was designed to simulate wind of a nature likely to be encountered in the field. Two potted cabbage plants were placed on a bench equidistant from an oscillating, electric fan with parasitised larvae on one plant and unparasitised larvae on the second. These constituted one block and replication was achieved by use of six such systems. Air speed was measured using a wind meter (testo405-V1 (Testo Ltd, Stuttgart, Germany)) to peak at 1.2 m/s (a ‘light breeze’ in the Beaufort scale) with a periodicity between oscillations of 3 sec. Prior to the fan being turned on, 10 parasitised or unparasitised 3rd instar larvae were placed on the plants to settle for 30 min and during this period, fallen larvae were placed back onto the plant and all individuals were observed to have established feeding. The fan was then turned on and ran continuously for three days. Each day, numbers of larvae trapped on a sticky base beneath each plant were counted. A General Linear Model (GLM) with repeated measures was used to compare the effect of parasitism, time and interaction. The measures were the numbers of fallen larvae, with data being collected for each of these on successive time periods.
Effect of foraging C. vestalis on larval dislodgement
Individual cabbage plants were placed in cuboid plastic arenas (15 cm × 15 cm × 25 cm) with a ventilated mesh lid. Prior to use, plants were removed from soil, the roots wrapped with wet cotton wool (adding water every day) and then covered with silver paper. The base of the arena was covered by a layer of water 8–10 mm deep to trap larvae that fell from the plants. Ten 3rd instar larvae were used per plant with six replicates for each of parasitised and unparasitised larvae. After larvae settled for 30 min, a single mated C. vestalis female was introduced to the arena, and a cotton wool swab soaked in 10% honey solution was put on the mesh lid (adding honey solution every day). Fallen larvae were removed and counted daily for three days. The falling rate (number of larvae falling from the plant) was analysed as described for the simulated wind experiment.
Effect of foraging spider on larval mortality
This study used plastic arenas (long: 24 cm, wide: 15 cm, tall: 9 cm) with one 4th instar female spider (P. pseudoannulata) in each. The spider was previously starved for 48 hours and a wet cotton wool swab was placed in the arena to supply it water. Plants were prepared as described for the C. vestalis study with 10 parasitised or unparasitised larvae placed on the plant and allowed to settle for 30 minutes. The spider was placed on the floor of the arena and was free to forage there and over the plant. Larvae remaining on the plant were recorded every 24 hours for three days whilst all larvae that fell from the plant were consumed by the spider. A ring of tacky adhesive around the arena’s wall prevented larvae from leaving but without entrapping them. Both unparasitised and parasitised treatments were repeated eight times. Data were analysed as described for the simulated wind experiment.
Effect of ground-foraging spider and simulated wind on larval mortality
This study used a mesocosm design similar to the preceding spider study except simulated wind was applied to the plants (as described previously) and spiders were confined to the floor of the arena by placing the plant roots in a glass cylinder (diameter: 2.5 cm, height: 7 cm and filled with water) up which larvae, but not the spider, were able to climb back onto the plant. Ten larvae (parasitised or unparasitised) were used on each plant and numbers remaining on the plant were recorded every 24 hours for three days. All larvae that fell from the plant were consumed by the assessment time each day. Eight replicates were used and data were analysed using GLM as describe above.
Larval development and long-term leaf consumption
To measure the duration of 3rd and 4th (final) instar stages, 40 parasitised and 40 unparasitised 3rd instars were placed individually on cabbage plants. The leaf bearing the larva was sealed in a punctured plastic bag (10 cm × 7 cm) to prevent escape. After 1 day, each larva was transferred onto a fresh plant, until death or pupation. Damaged leaves were removed from plants to measure leaf consumption as described for the short term consumption study (above). Student’s t-test was used to compare the total leaf consumption and development time of unparasitised and parasitised larvae. Since the number of individuals varied from day to day due to pupation and death, to explore where there were significant differences between the two treatments, a t-test were performed to compare each day separately instead of a repeated measures analysis of variance.
Additional Information
How to cite this article: Chen, W.- et al. Parasitised caterpillars suffer reduced predation: potential implications for intra-guild predation. Sci. Rep. 7, 42636; doi: 10.1038/srep42636 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Thomas, F., Adamo, S. & Moore, J. Parasitic manipulation: where are we and where should we go? Behavioural Processes 68, 185–199, doi: 10.1016/j.beproc.2004.06.010 (2005).
Poulin, R. “Adaptive” changes in the behaviour of parasitized animals: A critical review. Int. J. Parasit. 25, 1371–1383, doi: 10.1016/0020-7519(95)00100-X (1995).
Moore, J. Parasites and the Behavior of Animals. (Oxford University Press, 2002).
Thomas, F. et al. Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts? J. Evol. Biol. 15, 356–361, doi: 10.1046/j.1420-9101.2002.00410.x (2002).
Eberhard, W. G. Spider manipulation by a wasp larva. Nature 406, 255–256, doi: 10.1038/35018636 (2000).
Vyas, A., Kim, S.-K., Giacomini, N., Boothroyd, J. C. & Sapolsky, R. M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. USA 104, 6442–6447, doi: 10.1073/pnas.0608310104 (2007).
Adamo, S. A. Modulating the modulators: Parasites, neuromodulators and host behavioral change. Brain, Behavior and Evolution 60, 370–377, doi: 10.1159/000067790 (2002).
Roy, H. E., Steinkraus, D. C., Eilenberg, J., Hajek, A. E. & Pell, J. K. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu. Rev. Entomol. 51, 331–357, doi: 10.1146/annurev.ento.51.110104.150941 (2006).
Hughes, D., Thomas, F. & Adamo, S. A. In Parasitic Manipulation (eds D. Hughes & F. Thomas ) 36–51 (Oxford University Press, 2012).
Grosman, A. H. et al. Parasitoid increases survival of its pupae by inducing hosts to fight predators. PLOS ONE 3, e2276, doi: 10.1371/journal.pone.0002276 (2008).
Henne, D. C. & Johnson, S. J. Zombie fire ant workers: behavior controlled by decapitating fly parasitoids. Insectes Soc. 54, 150–153, doi: 10.1007/s00040-007-0924-y (2007).
van Houte, S., Ros, V. I. D. & van Oers, M. M. Walking with insects: molecular mechanisms behind parasitic manipulation of host behaviour. Mol. Ecol. 22, 3458–3475, doi: 10.1111/mec.12307 (2013).
Libersat, F., Delago, A. & Gal, R. Manipulation of host behavior by parasitic insects and insect parasites. Annu. Rev. Entomol. 54, 189–207, doi: 10.1146/annurev.ento.54.110807.090556 (2009).
Hoover, K. et al. A gene for an extended phenotype. Science 333, 1401, doi: 10.1126/science.1209199 (2011).
Gad, W. & Kim, Y. N-terminal tail of a viral histone H4 encoded in Cotesia plutellae bracovirus is essential to suppress gene expression of host histone H4. Insect Mol. Biol. 18, 111–118, doi: 10.1111/j.1365-2583.2009.00860.x (2009).
Beckage, N. E. & Gelman, D. B. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 49, 299–330, doi: 10.1146/annurev.ento.49.061802.123324 (2004).
Bae, S. & Kim, Y. Host physiological changes due to parasitism of a braconid wasp, Cotesia plutellae, on diamondback moth, Plutella xylostella . Comp. Biochem. Physiol., A: Mol. Integr. Physiol. 138, 39–44, doi: 10.1016/j.cbpb.2004.02.018 (2004).
Godfray, H. C. J. Parasitoids: behavioral and evolutionary ecology. (Princeton University Press, 1994).
Fritz, R. S. Selection for host modification by insect parasitoids. Evolution 36, 283–288, doi: 10.2307/2408046 (1982).
Holt, R. D. & Polis, G. A. A theoretical framework for intraguild predation. The American Naturalist 149, 745–764 (1997).
Polis, G. A., Myers, C. A. & Holt, R. D. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330 (1989).
Arim, M. & Marquet, P. A. Intraguild predation: a widespread interaction related to species biology. Ecol. Lett. 7, 557–564, doi: 10.1111/j.1461-0248.2004.00613.x (2004).
Nakazawa, T. & Yamamura, N. Community structure and stability analysis for intraguild interactions among host, parasitoid, and predator. Popul. Ecol. 48, 139–149, doi: 10.1007/s10144-005-0249-5 (2006).
Meyhöfer, R. & Klug, T. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae): mortality risks and behavioral decisions made under the threats of predation. Biol. Control 25, 239–248, doi: 10.1016/S1049-9644(02)00104-4 (2002).
Bilu, E. & Coll, M. Parasitized aphids are inferior prey for a coccinellid predator: implications for intraguild predation. Environ. Entomol. 38, 153–158 (2009).
Losey, J. E. & Denno, R. F. Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology 79, 2143–2152 (1998).
Ang, G. C. K., Silva, R., Maxwell, S. L., Zalucki, M. P. & Furlong, M. J. Contrary effects of leaf position and identity on oviposition and larval feeding patterns of the diamondback moth. Entomol. Exp. Appl. 151, 86–96, doi: 10.1111/eea.12172 (2014).
Perovic, D. J., Johnson, M.-L., Scholz, B. & Zalucki, M. P. The mortality of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) neonate larvae in relation to drop-off and soil surface temperature: the dangers of bungy jumping. Aust. J. Entomol. 47, 289–296, doi: 10.1111/j.1440-6055.2008.00660.x (2008).
Zalucki, M. P., Clarke, A. R. & Malcolm, S. B. Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol. 47, 361–393 (2002).
Poulin, R. In Advances in the study of behaviour (ed H. J. Brockmann ) 151–186 (Academic Press, 2010).
Potting, R. P. J., Poppy, G. M. & Schuler, T. H. The role of volatiles from cruciferous plants and pre-flight experience in the foraging behaviour of the specialist parasitoid Cotesia plutellae . Entomol. Exp. Appl. 93, 87–95 (1999).
Roux, O., van Baaren, J., Gers, C., Arvanitakis, L. & Legal, L. Antennal structure and oviposition behavior of the Plutella xylostella specialist parasitoid: Cotesia plutellae . Microscopy research and technique 68, 36–44 (2005).
Ruby, T., Hussain, T., Sial, N., Maalik, S. & Mushtaq, S. Survivorship of ground spider Pardosa oakleyi (Gravely) under laboratory conditions on natural and artificial diets. Pakistan Journal of Agricultural Science 49, 451–454 (2012).
Denno, R. F., Gratton, C., Döbel, H. & Finke, D. L. Predation risk affects relative strength of top-down and bottom-up impacts on insect herbivores. Ecology 84, 1032–1044, doi: 10.1890/0012-9658(2003)084[1032:PRARSO]2.0.CO;2 (2003).
Persons, M. H., Walker, S. E. & Rypstra, A. L. Fitness costs and benefits of antipredator behavior mediated by chemotactile cues in the wolf spider Pardosa milvina (Araneae: Lycosidae). Behav. Ecol. 13, 386–392 (2002).
Montllor, C. B. & Bernays, E. A. In Caterpillars: Ecological and Evolutionary Constraints on Foraging (eds N. E. Stamp & T. M. Casey ) 170–202 (Routledge, Chapman and Hall, 1993).
Stamp, N. E. & Bowers, M. D. Foraging behaviour of caterpillars given a choice of plant genotypes in the presence of insect predators. Ecol. Entomol. 25, 486–492 (2000).
Vogel, H., Kroymann, J. & Mitchell-Olds, T. Different transcript patterns in response to specialist and generalist herbivores in the wild Arabidopsis relative Boechera divaricarpa . PLOS One 2, doi: 10.1371/journal.pone.0001081 (2007).
Shi, Z. H., Liu, S. S. & Li, Y. X. Cotesia plutellae parasitizing Plutella xylostella: host-age dependent parasitism and its effect on host development and food consumption. BioControl 47, 499–511, doi: 10.1023/A:1016577406820 (2002).
Grzywacz, D. et al. Current control methods for diamondback moth and other brassica insect pests and the prospects for improved management with lepidopteran-resistant Bt vegetable brassicas in Asia and Africa. Crop Protect. 29, 68–79 (2010).
Li, Z., Feng, X., Liu, S.-S., You, M. & Furlong, M. J. Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 61, 277–296, doi: 10.1146/annurev-ento-010715-023622 (2016).
Talekar, N. S. & Shelton, A. M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 38, 275–301 (1993).
Rosenheim, J. A. Intraguild predation: new theoretical and empirical perspectives. Ecology 88, 2679–2680, doi: 10.1890/07-0790.1 (2007).
Li, Y., Liu, Y. & Liu, S. Effect of superparasitism on bionomics of Cotesia plutellae . Chinese Journal of Biological Control 17, 151–154 (2001).
Acknowledgements
The work was supported by National Natural Science Foundation of China (No. 31230061 and No. 31320103922). LV is supported by the Minjiang Scholar Program in Fujian Province (PRC) and the Advanced Talents of SAFEA, and GMG by the National Thousand Talents Program in China and the Advanced Talents of SAEFA. We thank Professor Jian-quan Yang from Fujian Agriculture and Forestry University and Associate Professor Dong-bao Song from Hunan Agriculture University for confirming identity of C. vestalis. We thank Associate Professor Qi-quan Liu from Fujian Academy of Agricultural Sciences for supplying P. pseudoannulata. Mrs A Johnson of Charles Sturt University formatted the manuscript.
Author information
Authors and Affiliations
Contributions
W.-B.C., G.M.G., M.-S.Y. and L.V. designed the research; W.-B.C., J.-Y.L., C.-X.W. and R.-X.M. performed the research; W.-B.C., G.M.G., M.-S.Y. and L.V. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, Wb., Vasseur, L., You, Ms. et al. Parasitised caterpillars suffer reduced predation: potential implications for intra-guild predation. Sci Rep 7, 42636 (2017). https://doi.org/10.1038/srep42636
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42636
- Springer Nature Limited