Abstract
Peptoids are an alternative approach to antimicrobial peptides that offer higher stability towards enzymatic degradation. It is essential when developing new types of peptoids, that mimic the function of antimicrobial peptides, to understand their mechanism of action. Few studies on the specific mechanism of action of antimicrobial peptoids have been described in the literature, despite the plethora of studies on the mode of action of antimicrobial peptides. Here, we investigate the mechanism of action of two short cationic peptoids, rich in lysine and tryptophan side chain functionalities. We demonstrate that both peptoids are able to cause loss of viability in E. coli susceptible cells at their MIC (16–32 μg/ml) concentrations. Dye leakage assays demonstrate slow and low membrane permeabilization for peptoid 1, that is still higher for lipid compositions mimicking bacterial membranes than lipid compositions containing Cholesterol. At concentrations of 4 × MIC (64–128 μg/ml), pore formation, leakage of cytoplasmic content and filamentation were the most commonly observed morphological changes seen by SEM in E. coli treated with both peptoids. Flow cytometry data supports the increase of cell size as observed in the quantification analysis from the SEM images and suggests overall decrease of DNA per cell mass over time.
Similar content being viewed by others
Introduction
Traditional antibiotics gradually lose their efficacy against many pathogens due to fast resistance development by the microorganisms1. Moreover, the number of new antibiotics developed and approved is steadily decreasing2. Therefore there is an urgent demand for development of novel antimicrobial drugs that offer different treatment strategies. Antimicrobial peptides (AMPs), are a class of antimicrobial agents that have long served all living organisms in combating infectious diseases by their ability to kill or inhibit the growth of pathogens3,4. There is increasing evidence indicating that the primary direct antimicrobial activity of cationic antimicrobial peptides is through interaction with anionic bacterial surfaces4,5. On the other hand, the non-membrane disruptive mechanisms are often ascribed to the ability of certain AMPs to bind to intracellular targets and act on DNA, RNA and protein synthesis3,4,5. Other AMPs use a combination of membrane and intracellular activity, thus hampering their development as novel drug candidates with a specific target. Despite their effectiveness, one of the limitations of AMPs is their low bioavailability as a result of enzymatic degradation6. Therefore, AMPs have been subjected to various modifications to design novel classes of potent antimicrobials with improved stability and activity profiles, namely peptidomimetics. Peptoids, are oligomers of N-substituted glycines, a class of compounds that mimic the structure of peptides7. For more than 15 years, the research in peptoid synthesis and application, has dramatically increased due to their potential as compounds with broad biological activity profiles. Recently, studies have also demonstrated their antimicrobial properties8,9,10. Peptoids are less prone to enzymatic and proteolytic degradation and are more membrane permeable than peptides6,11. We and others, have previously demonstrated that the antimicrobial activities are retained upon translation of peptides into peptoids, thus suggesting that this class of antimicrobials acts via similar mechanisms8,12. Short cationic peptoids are shown to be able to decrease the number of viable bacterial cells, form pores, and bind to intracellular targets such as DNA13,14. Other have reported that the mechanism behind their bacterial killing is primarily associated with membrane disruption usually by pore forming mechanisms13,15. In an attempt to better understand the mechanism by which the most selective short cationic Peptoids 1 and 2, impair the ability of Gram-negative E. coli to divide, this paper describes their killing kinetics, membrane activity and their effect on E. coli morphology. Data clearly demonstrate that at peptoid MIC concentrations (Peptoid 1 MIC = 16 μg/ml, Peptoid 2 MIC = 32 μg/ml), the bacterial growth is successfully inhibited without any degree of membrane disruption, and a small degree of disruption is only detected at 4 × MIC.
Results and Discussion
In Vitro Antibacterial Activity
We have previously reported the broad spectrum activity of a library of cationic tryptophan rich peptoids against six bacterial strains and compared those activities with the ones of similar antimicrobial peptides12,16. In addition, the hemolytic activity and cytotoxicity against HeLa cells were analysed, altogether demonstrating good selectivity profiles for these peptoids. Two peptoids showed good selectivity index values with respect to their activity against E. coli and their haemolytic values and were therefore chosen for further studies (Table 1). Both peptoids have relatively short sequences (9 residues, Fig. 1) and exhibit MIC ( ≤ 22.2 μM) values that are comparable to that of the antimicrobial peptide indolicidin (Table 1) and are within the range of other antimicrobial peptoids reported in the literature8,17.
E. coli killing kinetics
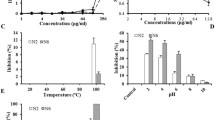
In order to differentiate between bacteriostatic and bactericidal mode of action of peptoids 1 and 2, E. coli cultures were challenged with different concentrations in a period of 6 hours and growth inhibition patterns were compared. Data supports the inhibition capacity of both peptoids which show clear concentration dependent inhibition of E. coli. Both Peptoids 1 and 2 at 1 × MIC concentrations exhibit bacteriostatic mode of action on E. coli growth (Fig. 2A,B). The slow killing for Peptoids 1 and 2 may indicate that at MIC concentration these peptoids target intracellular metabolic processes that cause bacterial growth inhibition. With respect to this observation, delayed killing kinetics have been observed for AMPs that target intracellular compartments18,19. These could be inhibition of DNA synthesis or cell filamentation as observed for bacteriostatic antimicrobial compounds20. At 2 × MIC concentrations different killing kinetics are observed, where Peptoid 2 acts via bactericidal mechanism (Fig. 2A). The observed difference may be partly due to the different hydrophobicity profiles of these peptoids measured by retention times on HPLC. Here, Peptoid 2 is globally more hydrophobic and this property could enhance its insertion into bacterial membranes. Similar killing kinetic profiles as Peptoid 2 have been previously observed for other antimicrobial peptidomimetics13,21,22,23. At 4 × MIC concentrations, both peptoids show significant bactericidal behaviour which is marginally higher than that shown for ciprofloxacin, an antibiotic that prevents DNA replication, recombination and repair (Fig. 2B)24. The fast killing kinetics is still not higher than that reported in the literature for polymyxin B, a fast permeabilizing antimicrobial peptide, where upon reaching a threshold concentration, the accumulated peptide molecules of polymyxin B can rapidly cause bacterial death25.
(A) Optical density measurements at 600 nm of exponentially growing E. coli, with and without Peptoid 1 at 1 × MIC (16 μg/ml), 2 × MIC (32 μg/ml) and 4 × MIC (64 μg/ml) and Peptoid 2 at 1 × MIC (32 μg/ml), 2 × MIC (64 μg/ml) and 4 × MIC (128 μg/ml) concentrations. (B) Time-kill study of E. coli ATCC 25922 challenged with 1 × MIC (16 μg/ml, 32 μg/ml and 0.05 μg/ml) and 4 × MIC (64 μg/ml, 128 μg/ml and 0.2 μg/ml) concentrations of Peptoids 1 and 2 and ciprofloxacin, respectively. For both experiments E. coli cultures are in log phase at 2–4 × 107 CFU/ml. Data represents mean and SEM of 3 independent experiments.
Quantification of membrane permeabilization
The mechanism of action of antimicrobial peptides and related mimetics that carry net positive charge and moderate hydrophobicity on bacteria is generally associated with fast killing kinetics as a result of membrane permeabilization18,23,26,27,28. To investigate whether the bactericidal effect observed from the killing kinetics experiments is related to the ability of the peptoids to disrupt bacterial membranes, we calculated the percentile of viable and non-viable cells using Live/Dead quantification assay. In this assay two nucleic acid dyes, syto 9 green and propidium iodide (PI) red, that have different membrane permeabilities were used as indicators of live bacteria with intact membranes versus dead bacteria with compromised membranes, respectively. Peptoid 1 caused gradual decrease of the number of PI stained cells at 1 × MIC concentration. In response to Peptoid 2 treatment, 90 percent of E. coli showed high membrane permeabilization within one hour of treatment, that supports the observation for the higher potency exhibited by this peptoid from the killing kinetics experiments (Fig. 3). Importantly, the activity of both peptoids remains stable after 5 hours of incubation. This is in contrast to the activity observed for the peptide from which these peptoids are derived, where re-established growth is observed within 2 hours of peptide administration16.
Percentile live bacteria treated with Peptoid 1 at 1 × MIC (16 μg/ml), 2 × MIC (32 μg/ml) and 4 × MIC (64 μg/ml) and Peptoid 2 at 1 × MIC (32 μg/ml), 2 × MIC (64 μg/ml) and 4 × MIC (128 μg/ml) (Eq. 1 see Experimental Section). 100% is set as the value from untreated samples at time zero. Results are from 3 independent experiments with SEM error bars.
Release of fluorescent dyes from different liposome compositions
Two fluorescent dye leakage assays were used to assess the disruptive ability of peptoids. The two assays use similar fluorescent dyes, carboxyfluorescein (CF) and calcein to measure the leakage from the liposomes. These two assays offer information on the electrostatic interaction with membranes mimicking bacterial surfaces and interaction with membranes with different fluidities. Bacterial membranes have a highly negative transmembrane potential (approximately −120 mV) in contrast to the weak membrane potential maintained by mammalian cells29. Thus they attract positively charged compounds such as cationic peptoids. Therefore, to test the existence of a specific interaction between Peptoid 1 and the glycerol polar headgroup of palmitoyl-2-oleoyl-sn-glycero−3-phospho-(1′-rac-glycerol) (POPG) or, if the interactions are purely electrostatic, carboxyfluorescein (CF) leakage assays from liposomes of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) containing 20 mol % of 1-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphate (POPA) or POPG were performed initially. Both POPA and POPG are negatively charged phospholipids but with different polar headgroup structures; while PA phospholipid only has a phosphate group linked to C3 of the glycerol moiety, PG has a phosphoglycerol group at this same position. Liposomes containing 20 mol % of negatively charged POPA showed to be more susceptible to Peptoid 1 when compared to liposomes with 20 mol % of POPG (Fig. 4). This indicates that there is no specificity in peptoid binding to PG. Again, the greater extent of dye release from liposomes with 40 mol % of POPG compared to 20 mol % of POPG shows the importance of electrostatic interactions between the peptoid and liposomes and that its activity increases with the increase of overall net surface charge (Fig. 4).
Furthermore, besides electrostatic interactions, hydrophobic side chain functionalities contribute greatly to the peptoid–membrane interactions. Tryptophan-like residues have strong membrane disruptive activities due to their ability to interact with the membrane interface. This activity is assigned partly due to the flat and rigid structure of the indole ring that may favour alignment of the side-chain in the membrane interface region4. To evaluate the permeability of the peptoids to membranes, we used the calcein leakage assay where calcein was encapsulated in two different combinations of selected lipids POPC:POPG (7:3) and POPC, POPG and Cholesterol (5:2:3). The membranes of mammalian cells have sterol molecules such as Cholesterol in their membrane compositions and this property can prevent peptoid insertion in the lipid bilayers. We used membrane composition containing POPC, POPG and Cholesterol (5:2:3), as reported previously, to mimic membrane environment with decreased fluidity while retaining negative charge to ensure peptoid binding16,30. In addition, the liposomes composed of POPC, POPG and Cholesterol also mimic membrane compositions of some of the membranes of tumour cells and neurons, even though POPG is not found as a part of the composition of mammalian membranes31. The total amount of released calcein was monitored and the degree of membrane permeabilization of the peptoids was calculated using Equation 2. Distinct tendency to lyse liposomes mimicking bacterial membranes (POPC:POPG) over liposomes mimicking less fluidic membranes, was observed for Peptoid 1, in contrast to the indiscriminative membrane permeabilization activity of the control peptide melittin that acts via the toroidal pore formation mechanism (Fig. 5A,B)32. Membrane permeabilzation is expected for the peptoids in this study because of their structural compositions. Short tryptophan rich cationic peptides have been reported to exhibit high membrane activity on E. coli where the peptide with the highest tryptophan content (2 W) and charge (+9) showed highest percentage of membrane permeabilization (approximately 60%)33. Similarly, the peptoids in this study are rich in charged (+4) and tryptophan-like residues (4 W) which are expected to contribute significantly to the observed membrane permeabilizing properties. The permeability of peptoid 1 was greater for liposome compositions that mimic bacterial membranes (Fig. 5). Maximum calcein leakage (<30%) was observed from sub MIC concentrations of Peptoid 1 and an increase in the peptoid concentration from 6.25 to 25 μg/ml did not contribute to an increase in the calcein release from these liposomes (Fig. 5B), thus indicating that Peptoid 1 only has a weak membrane activity. The membrane activity is also much lower than the one observed for structurally similar GN-4 antimicrobial peptide we have published previously16. The unique physiochemical properties of peptoids such as loss of hydrogen donation and backbone chirality may be expected to impact the membrane interactive properties when compared with peptides. However, not all antimicrobial peptides insert and disrupt the membranes or form pores. Studies on mechanism of action of cationic tryptophan rich peptides have shown that they either primarily lyse the membrane of Gram-negative bacteria by pore formation or they translocate and act on internal targets34. The overall total thickness of the membrane of Gram-negative bacteria varies from 30–50 nm. In order for a peptide or peptidomimetic to form a pore in the membrane, it has to be long enough to span the membrane e.g. barrel-stave model. Some peptides are too short to span the membranes35,36. They usually induce pore formation via a toroidal model by stabilizing the lipid pore and forming a peptide-lipid complex37. Indolicidin, a short (13 residue), tryptophan rich, extended structure cationic peptide, is able to cause leakage of calcein and carboxyfluorescein from negatively charged liposomes via non-membrane pore formation mechanism, but rather via translocation of dye molecules across the membrane in the form of dye-peptide complexes35.
Calcein release from two liposome compositions, POPC:POPG (7:3) and POPC:POPG:Cholesterol (5:2:3) for (A) Melittin and (B) Peptoid 1. Calcein release as a result of different concentrations and concentration dependent release over time is shown. Results are from minimum of 3 independent experiments with SEM error bars.
Concentration dependent calcein release was observed from liposomes containing Cholesterol, but with a significantly slower release rate and a lower threshold, which is in agreement with the low toxicity reported for Peptoid 1 at concentration as high as 4 × MIC. Calcein leakage in a concentration dependent manner is also observed for antimicrobial peptides38. This concentration dependent activity of antimicrobial peptides has been explained by a two-state model37. This model illustrates initial binding of peptide monomers parallel to the plane of the membrane. The peptides are then dispersed all across the membrane without causing pore formation. As the concentration of the peptide increases, thinning and formation of transmembrane pores takes place37. Similar membrane thinning has been reported for indolicidin35,39. Our findings suggest that the release of calcein upon addition of Peptoid 1 is concentration dependent and significantly lower than the total calcein release observed for melittin (Fig. 5A,B)40. The total dye leakage together with the results on the bacterial viability quantification at concentrations around 1× and 2 × MIC of Peptoid 1, are in agreement with the killing kinetics experiments indicating that the growth inhibition is partially exerted by some degree of membrane damage that does not include toroidal pore formation.
Changes in Membrane Morphology of bacteria upon peptoid treatment
E. coli were inspected using scanning electron microscopy to visualize any morphological changes (membrane disruption, cell elongation, etc.) after peptoid treatment. Untreated bacterial cells appear with smooth surfaces whereas considerable roughening and leakage of cytoplasmic content is observed for peptoid treated bacteria (Figs 6 and 7). The SEM micrographs show membrane damage upon treatment with both peptoids at 1× and 4 × MIC, with a more pronounced effect at 4 hours (Figs 6 and 7). These cellular changes are related to the growth inhibitory activities observed at 1× and 4 × MIC concentrations. Membrane disintegration is expected for these peptoids due to the presence of four tryptophan like side chains which are strongly associated with high affinity for the interfacial region of bilayers41. Similar changes in bacterial surfaces, such as membrane damage and blebs have been observed both for antimicrobial peptides and for metal oxide nanoparticles with negative surface potential as a result of their strong interaction with P. aeruginosa and E. coli membranes, respectively5,42. Bleb morphology in E. coli has also been reported for the human antimicrobial peptide defensin 5, where the cause of bleb formation was localization of this peptide at the site of cell division and site poles indicating that the antibacterial activity is exerted in the cytoplasm43. Additionally, E. coli treated with the cationic antimicrobial peptide, gramicidin, illustrated a high degree of blister and dimple formation44. Filamentation, a continuous cell elongation which does not result in cell division, is often observed in bacteria as a result of various stress responses such as those to beta lactams and fluoroquinolones antibiotics45,46. Bacterial filamentation has been reported as a consequence of induction of SOS response mechanism which is responsible for regulation of DNA damage repair47,48. Elongated bacteria were observed among the affected bacterial population indicative of inhibition of bacterial cell division (Figs 6E and 7B). In addition, from the quantitative data, the majority of the E. coli with cell length of 3 μm were those treated with 1 × MIC concentration of peptoid 2 (Fig. 8). Antimicrobial compounds that act via bacteriostatic mechanisms (e.g. inhibition of protein and RNA synthesis) have been shown not to induce filamentation or increase cell size49. Therefore the increase in cell size in bacteria treated with peptoids could be due to possible inhibition of DNA processes inside the cell. These results indicate that peptoids can destabilize the membrane of E. coli causing pronounced damage that does not always lead to complete lysis and cell death, but to cell elongation.
Cultures in log phase at 2–4 × 107 CFU/ml. (A) Untreated E. coli at 1 hour. (B) E. coli treated with 1x MIC (16 μg/ml) at 1 hour. (C) E. coli treated with 4x MIC (64 μg/ml) at 1 hour. (D) Untreated E. coli at 4 hours. (E) E. coli treated with 1 × MIC (16 μg/ml) at 4 hours, and (F) E. coli treated with 4 × MIC (64 μg/ml) at 4 hours.
Changes in size and DNA content
We used flow cytometry to measure the changes in cell size and nucleic acid content reflected by increased light scatter and fluorescence, respectively. Exponentially growing cells were challenged with both peptoids and ciprofloxacin and stained with propidium iodide (PI) cell impermeant dye. PI stains nucleic acids in cells with damaged membranes. Untreated cells retain constant DNA per cell size (Fig. 9). Bacteria treated with ciprofloxacin appear significantly bigger with overall decrease of DNA per cell size. Certain quinolone antibiotics such as ciprofloxacin or norfloxacin interact with DNA gyrase and topoisomerase IV causing DNA replication fork arrest and induction of SOS response in E. coli. This leads to inhibition of cell division and filamentation50,51,52. Ciprofloxacin is also shown not to affect the membrane integrity of E. coli even at high concentrations46,53. The bactericidal effect of ciprofloxacin demonstrated in the killing kinetics experiments is in agreement with the decrease of DNA per cell mass obtained from the flow cytometry measurements. This effect is thought to be related to the release of free DNA ends from DNA gyrase-ciprofloxacin complex that ultimately leads to chromosomal DNA fragmentation20,54. The fluorescence signal from PI binding to nucleic acids in the cells may therefore indicate a different pathway of membrane disturbance to be involved in making the bacteria permeable to the dye55. Investigation into the effect of the peptoids on the cell size and DNA content demonstrated that cell size increased considerably with an increase in concentration for both peptoids. The overall effect of the peptoids was a substantial decrease in DNA per cell mass, which was concentration dependent and it occurred at faster rate for peptoid 2 when compared to that of ciprofloxacin. A similar dose dependent interaction between peptoid and bacterial plasmid DNA was confirmed using a gel retardation assay (Fig. 2, see Supplementary Material). At 1 × MIC the inhibition pattern was somewhat less pronounced for the peptoids in regards to ciprofloxacin demonstrating a possible distinctive antibacterial effect.
Conclusions
Many antimicrobial peptides have been referred to as “dirty drugs” due to their ability to act via multiple mechanisms which involve membrane permeabilization and inhibition of intracellular processes36,56. While dissecting the mode of action of lysine and tryptophan rich peptoids that mimic the structure of antimicrobial peptides, we have demonstrated that the growth inhibition ability of peptoids that show different killing kinetics is most likely based on the different charge density distribution and overall hydrophobicities. We show that the killing mechanism for peptoid 1 is not fully attributed to its ability to cause leakage from the membranes as observed in the dye leakage assays. However, the killing mechanism is definitely supported by some degree of membrane damage exerted by this peptoid. Higher degree of bacterial lysis through membrane damage is observed for peptoid 2. The peptoids exhibited potent killing kinetics which are not explained solely by their membrane permeabilization ability, measured by calcein leakage from lipid vesicles mimicking bacterial membranes. However, the high degree of membrane disturbance measured by Live/Dead assay is not directly translated into a decrease of viable cells. The membrane disruptive ability is also supported by scanning electron micrographs of E. coli treated with both peptoids. Although the mechanism behind the observed increase in size is not clear, it can be reasonably assumed that besides the peptoids effect on the outer membrane they enter the cytoplasm and bind to internal targets thus inhibiting DNA, RNA or protein synthesis. Taken together we propose that these peptoids act via combined mechanism of action involving membrane disruption with probable intracellular targets. Further investigations are needed to support their effects via intracellular targets. The peptoids in this study have low toxicity, high stability and low cost of synthesis, properties which contribute to their high potential as new peptidomimetics with therapeutic applications.
Experimental Section
Materials and Bacterial strains
The strains used in the present study were from our laboratory strain collection. Escherichia coli (ATCC 25922) was obtained from the American Type Culture Collection (ATCC, Rockville, Md.). Other strains include Pseudomonas aeruginosa PAO1 H103 strain and Liverpool epidemic clinical strain H1027, and Escherichia coli clinical isolate expressing extended spectrum β–lactamases (ESBL)57. All tested bacterial strains are categorized as biohazard level 2 pathogens.
Peptoid synthesis
Peptoids were synthesized using standard submonomer solid-phase synthesis, as described previously12.
In vitro susceptibility studies
Minimum inhibitory concentrations (MIC) for the peptoids have been measured as described previously12.
Killing kinetics
The kinetics of antimicrobial activity against E. coli (ATCC 25922) were assessed at a peptoid concentration corresponding to 1×, 2× and 4 × MIC. Briefly, an overnight culture of bacteria was diluted 1:50 in fresh Mueller Hinton broth and regrown to an OD600 of 0.4 before diluting to a turbidity of 0.1. The bacterial suspension was added to a 96-well polystyrene flat bottomed plate containing the peptoid of interest in addition to known antibiotics. The plate was incubated without shaking at 37 °C for 180 minutes. Samples (100 μl) were taken at time 20, 40, 80, 120 and 180 minutes and diluted in ice cold 0.9% NaCl from which 100 μl was plated on LB agar plates. The plates were incubated for 18–24 hours at 30 °C and colony forming units (CFU) were counted. Sterility control was performed by plating 100 μl of MH broth and 0.9% NaCl. Plates where no detectable bacterial growth was observed were left for additional 18 hrs incubation.
Live/Dead Staining
Overnight culture of E. coli ATCC 25922 was diluted 100-fold in fresh MH broth and allowed to grow until it reached OD600 of 0.1. Duplicate samples of 1 ml each were transferred to eppendorf tubes and pelleted at 10.000 g for 8 minutes. One sample was resuspended in 1 ml 0.9% NaCl and the other in 50 μl 0.9% NaCl followed by addition of 950 μl 70% isopropyl alcohol to kill the bacterial. The tubes were left for 1 hour on ice with manual shaking every 15 minutes. The tube containing dead bacteria was pelleted at 12.000 rpm for 8 min and resuspended with 1 ml 0.9% NaCl to remove the isopropyl alcohol. Five different ratios corresponding to 0, 10, 50, 90 and 100% of live bacteria were prepared to obtain the standard curve for the analysis of bacterial viability when challenged by antimicrobials (Fig. 1, see Supplementary Material). Briefly, 100 μl of the staining solution (3 μl of 3.34 mM SYTO 9 and 3 μl of 20 mM propidium iodide in water) was added to 100 μl of the different bacteria ratios containing wells and incubated in dark for 15 minutes before reading on multi-detection macroplate reader Synergy HT. The green fluorescence (SYTO 9) corresponds to the amount of live bacteria and was excited at 485 nm and the emission detected at 528 nm. The red fluorescence (propidium iodide) was excited at 530 nm and detected at 645 nm which correspond to the amount of dead bacteria in the sample. Bacteria suspension challenged with antimicrobial for viability analysis were prepared by incubating 90 μl of bacteria (OD600 of 0.1) and 10 μl antimicrobials for 5 hours during which period samples were extracted in PCR tubes at 0, 10, 20, 40, 80 and 120 minutes and immediately put on ice. All PCR tubes were pelleted at 10.000 g for 8 minutes before being resuspended in cold 0.9% NaCl and placed on ice again. The content of each PCR tube was then added to individual wells of flat bottomed polystyrene 96 microtitter plate and mixed with 100 μl staining solution. After 15 minutes incubation in dark the fluorescence was measured using multi-detection microplate reader Synegry HT. The percentage of living cells was calculated using Equation 1 below.

Liposome preparation
Two different compositions of large unilamellar vesicles (LUVs) were made. To mimic the composition of bacterial membranes 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphoglycerol (POPG) and 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC) in a molar ratio of 7:3 was prepared. The second composition, a simple mimic of mammalian membranes, was made from POPC:POPG:Cholesterol in a molar ratio of 5:2:3. The indicated lipids were purchased from Avanti Lipids (Alabaster, Alabama). The lipid mixtures were initially mixed in chloroform, after which the solvent was removed under low pressure at 40 °C in a rotary evaporator over 2 hours. Ethanol (99.9%) was frequently added to remove residual organic solvent. The lipid mixture was then dissolved in 4 ml HEPES buffer (10 mM HEPES, 150 mM KCl, 0.03 mM CaCl2, 0.01 mM EDTA. pH 7.4) with 20 mM calcein (C0875, Sigma), for calcein containing liposomes. Multiple mixing and sonication steps for 5 minutes were performed to avoid aggregation. Subsequently, the lipid mixture was vigorously whirl-mixed every 10 minutes over a course of 1 hour and finally left at room temperature for an additional hour to allow the lipids to anneal. LUVs were prepared by extruding the lipid mixtures through double stacked 100 nm filters, a total of 10 times using Nitrogen powered extruder and 30 bars pressure. Calcein containing liposomes were loaded on Sephadex G-50 columns to separate encapsulated from free calcein and the elution was done using calcein free HEPES buffer. For verification of the size of the liposomes, dynamic light scattering (DLS) measurements were obtained on a Zetasizer Nano ZS (Malvern, Worcestershire, UK). Monodisperse liposomes of a size of 110 nm was measured by Malvern DTS v. 5.10 software.
Liposome quantification
Quantification of lipid content was performed using standard protocols described previously58. Initially, standard curve was obtained using 0, 20, 40, 60, 80, and 100 nmols of standard potassium phosphate solution (1 mM). Briefly, the tubes containing 0, 20, 40, 60, 80 and 100 μl of potassium phosphate buffer were dried in a heating block at 120 °C and 400 μl of perchloric acid (HClO4) was added. For determination of the lipid content of the prepared liposomes, 50 nmols were dried and 400 μl of perchloric acid was added. Digestion of the lipids was achieved by heating the suspension at 180 °C for up to 2 hours. After all samples have been cooled to room temperature, 1 ml of MilliQ water is added, followed by addition of 400 μl of ammonium molybdate (1.252 g/100 ml). All the tubes are vortexed and 400 μl of freshly prepared ascorbic acid (3% w/v) is added. The tubes are put to boiling water for 10 minutes and after they cool down, 1 ml is transferred to a 1 cm cuvettes and absorption is read using standard spectrophotometer at 797 nm. Using the standard curve equation, the concentration of liposome suspension was calculated before use.
Calcein release assay
Calcein release was done in a 96-well plate with shielded wells (MicroWell 96 optical bottom plate, NUNC, Roskilde, DK) as previously described16. Briefly, peptoids were diluted in 10 mM HEPES buffer and 100 μl were added to wells followed by addition of 80 μl of 45 μM liposomes suspension for a final liposome concentration of 20 μM. Immediately after addition the fluorescence was read using multi-detection microplate reader Synergy HT at an excitation wavelength of 485 nm and an emission wavelength of 520 nm over a course of 1 hour at 37 °C. Maximum calcein release was acquired using 10% Triton X-100 and release following peptoid exposure was calculated using equation 2, where F and Fo represent the initial and the final levels of fluorescence before and after peptoid addition, respectively, and Fmax is the fluorescence level after complete disruption of liposome by addition of detergent, 10% Triton X-100.

Carboxyfluorescein leakage assay
The permeabilizing activity studies of the peptides upon model membranes were performed as previously described59. This test is based on the property of self-quenching of carboxyfluorescein when in high concentrations, and high quantum yield when diluted. Stock solutions of the lipids were prepared in chloroform:ethanol mixture. Lipid films were obtained by evaporating the solvent from pre aliquoted mixtures of the stock solutions under a stream of N2 and submitted to vacuum for 2 hours. The lipid film is then resuspended in a 50 mM solution of carboxyfluorescein with Tris-HCl 20 mM and NaCl 300 mM and pH 7.4. The lipid suspension is extruded through two stacked polycarbonate filters with 100 nm pore size (Nuclepore, Maidstone, UK) to obtain the large unilamellar vesicles (LUV). At this stage, the suspension has 50 mM of carboxyfluorescein in the internal compartment of the LUV at the external environment. The LUV are separated from non-encapsulated carboxyfluorescein by a process of gel-filtration chromatography on a pre-packed Sephadex G-25 mini-column (GE Healthcare, Buckinghamshire, UK) equilibrated with the buffer. Lipids were further quantified by the Rouser method58. The total lipid concentration in the samples was 20 μM. The carboxyfluorescein release was determined same as calcein release, described earlier (Eq. 2).
Scanning electron Microscopy (SEM)
Overnight culture (approx. 18 h at 37 °C) of E. coli ATCC 25922 was diluted 1:50 in fresh MH broth and regrown to an OD600 of 0.4 before diluting it to a turbidity of 0.1. Bacteria were treated with 1× and 4 × MIC concentrations of peptoids GN-2 Npm9 and GN-2 Nlys1–4 Ntrp5–8 prepared in MH Broth for 4 h at 37 °C in a microtitter plate. Samples were taken at two time points, one at 1 h and the second at 4 h incubation. To ensure enough cells for analysis a volume of 600 μl (3 × 200 μl) was polled into 1.5 ml Eppendorf tubes. The sample was then chemically fixed in 3% glutaraldehyde in MHB, pH adjusted to 7.3 at 4 °C for 16 hrs. After washing 3 times in distilled water, for 10 min each time, the sample was stained with 1% OsO4 at 4 °C for 16 hrs. Next, the sample was washed again, 3 times, and dried in ethanol series at 25 °C for 10 minutes for each ethanol step (30, 50, 70, 80, 90 and 100% ethanol). The 100% ethanol was replaced with acetone in three 10 minute steps: 30, 50 acetone and 100% acetone. After the acetone step, the sample was further dried in a Leica EM CPD300 onto a square piece of silicon wafer. The silicon substrate with the dried sample was attached onto an aluminium stub with a double sided C tape, and the sample was coated with 2 nm Pt in a Cressington 208HR High Resolution Sputter Coater. The sample was then imaged in an FEI Helios dual beam scanning electron microscope by monitoring the secondary electron signal at 2 keV and 43 pA with the through the lens detector. The size analysis was performed using the NIH public domain Image J.
Flow cytometry
Overnight culture of Escherichia coli ATCC 2592 was diluted 1:50 in fresh MH broth and allowed to grow until an OD600 of 0.1. This suspension is further 10-fold diluted and regrown till OD600 of 0.1 to ensure a uniform bacterial population. A volume of 90 μl of bacterial suspension was loaded to a flat bottomed 96-well Greiner plate. After extraction at zero minutes, 10 μl of the peptoid and antibiotics corresponding to 1× and 4 × MIC concentrations was loaded to the bacteria containing wells for a total volume of 100 μl. Samples were collected at 20, 40, 80,120 and 180 minutes and put on ice. The plate was sealed and incubated at 37 °C between sample extractions. Samples from wells containing bacteria without peptoids or antibiotics were taken at each time point. The samples were, whenever appropriate, centrifuged at 10.000 × g for 5 min at 4 °C. The supernatant was carefully removed and the bacteria were resuspended in 100 μl 10 mM Tris HCl pH 7.4. 1000 μl of ice cold 77% Ethanol was added before storing the samples at 4 °C until analysis. Rifampicin/Cephalexin samples were prepared by collecting 200 μl from the wells containing bacteria and the appropriate antimicrobial in an E-tube containing 45 μl of a mixture of Rifampicin (300 μg/ml) and Cephalexin (36 μg/ml). The tube was placed in a 37 °C shaking water bath for 2½ hours after which the samples were treated in the same way as the other samples before storing them at 4 °C. To monitor the bacterial growth the plate was read before each sample was extracted in a multi-detection plate reader Synergy HT. The following day, the samples were centrifuged at 10.000 × g for 5 min and the supernatant was carefully removed. The samples were stained for flow cytometry analysis with 140 μl of staining solution (90 μg/ml Mitramycin and 20 μg/ml Ethidium Bromide in 10 mM Tris pH 7.4, 10 mM MgCl2). For flow cytometry analysis A10 Bryte Flow Cytometer was used and around 20.000 events were included.
DNA binding assay
el retardation experiments were performed by mixing 100 ng of bacterial plasmid DNA (pBluescriptII SK+, # 212205 Stratagene) with different concentrations of both Peptoids 1 and 2 in 20 μl binding buffer (5% glycerol, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1 mM dithiothreitol, 20 mM KCl, and 50 μg/mL bovine serum albumin). The reaction mixtures were incubated for 1 h at room temperature before running 20 μl aliquot on a 1% agarose TAE (Tris-acetate-EDTA) gel using 1× TAE buffer in the electrophoresis chamber at 125 V.
Additional Information
How to cite this article: Mojsoska, B. et al. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 7, 42332; doi: 10.1038/srep42332 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
WHO. Antimicrobial resistance: overview of global report surveillance. (World Health Organization) (2014).
Outterson, K., Powers, J. H., Daniel, G. W. & McClellan, M. B. Repairing the broken market for antibiotic innovation. Health Aff (Millwood) 34, 277–285, doi: 10.1377/hlthaff.2014.1003 (2015).
Jenssen, H., Hamill, P. & Hancock, R. E. W. Peptide antimicrobial agents. Clinical microbiology reviews 19, 491, doi: 10.1128/Cmr.00056-05 (2006).
Mojsoska, B. & Jenssen, H. Peptides and Peptidomimetics for Antimicrobial Drug Design. Pharmaceuticals (Basel) 8, 366–415, doi: 10.3390/ph8030366 (2015).
Hilpert, K. et al. Screening and characterization of surface-tethered cationic peptides for antimicrobial activity. Chemistry & biology 16, 58–69, doi: 10.1016/j.chembiol.2008.11.006 (2009).
Miller, S. M. et al. Proteolytic studies of homologous peptide and N-substituted glycine peptoid oligomers. Bioorganic & medicinal chemistry letters 4, 2657–2662, doi: http://dx.doi.org/10.1016/S0960-894X(01)80691-0 (1994).
Simon, R. J. et al. Peptoids: a modular approach to drug discovery. Proceedings of the National Academy of Sciences of the United States of America 89, 9367–9371 (1992).
Chongsiriwatana, N. P. et al. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proceedings of the National Academy of Sciences of the United States of America 105, 2794–2799, doi: 10.1073/pnas.0708254105 (2008).
Czyzewski, A. M. et al. In Vivo, In Vitro, and In Silico Characterization of Peptoids as Antimicrobial Agents. PloS one 11, e0135961, doi: 10.1371/journal.pone.0135961 (2016).
Godballe, T., Nilsson, L. L., Petersen, P. D. & Jenssen, H. Antimicrobial beta-peptides and alpha-peptoids. Chemical biology & drug design 77, 107–116, doi: 10.1111/j.1747-0285.2010.01067.x (2011).
Tan, N. C., Yu, P., Kwon, Y. U. & Kodadek, T. High-throughput evaluation of relative cell permeability between peptoids and peptides. Bioorganic & medicinal chemistry 16, 5853–5861, doi: 10.1016/j.bmc.2008.04.074 (2008).
Mojsoska, B., Zuckermann, R. N. & Jenssen, H. Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrobial agents and chemotherapy 59, 4112–4120, doi: 10.1128/AAC.00237-15 (2015).
Smith, P. T., Huang, M. L. & Kirshenbaum, K. Osmoprotective polymer additives attenuate the membrane pore-forming activity of antimicrobial peptoids. Biopolymers 103, 227–236, doi: 10.1002/bip.22588 (2015).
Murphy, J. E. et al. A combinatorial approach to the discovery of efficient cationic peptoid reagents for gene delivery. Proceedings of the National Academy of Sciences of the United States of America 95, 1517–1522 (1998).
Goodson, B. et al. Characterization of novel antimicrobial peptoids. Antimicrobial agents and chemotherapy 43, 1429–1434 (1999).
Godballe, T., Mojsoska, B., Nielsen, H. M. & Jenssen, H. Antimicrobial activity of GN peptides and their mode of action. Biopolymers, doi: 10.1002/bip.22796 (2015).
Bang, J. K., Nan, Y. H., Lee, E. K. & Shin, S. Y. A Novel Trp-rich Model Antimicrobial Peptoid with Increased Protease Stability. B Korean Chem Soc 31, 2509–2513, doi: 10.5012/bkcs.2010.31.9.2509 (2010).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nature reviews. Immunology 3, 710–720, doi: 10.1038/nri1180 (2003).
Jindal, H. M. et al. Antimicrobial Activity of Novel Synthetic Peptides Derived from Indolicidin and Ranalexin against Streptococcus pneumoniae. PloS one 10, e0128532, doi: 10.1371/journal.pone.0128532 (2015).
Hawkey, P. M. Mechanisms of quinolone action and microbial response. The Journal of antimicrobial chemotherapy 51 Suppl 1, 29–35, doi: 10.1093/jac/dkg207 (2003).
Takahashi, H., Palermo, E. F., Yasuhara, K., Caputo, G. A. & Kuroda, K. Molecular design, structures, and activity of antimicrobial peptide-mimetic polymers. Macromolecular bioscience 13, 1285–1299, doi: 10.1002/mabi.201300126 (2013).
Jahnsen, R. D., Haney, E. F., Franzyk, H. & Hancock, R. E. Characterization of a proteolytically stable multifunctional host defense peptidomimetic. Chemistry & biology 20, 1286–1295, doi: 10.1016/j.chembiol.2013.09.007 (2013).
Hein-Kristensen, L., Knapp, K. M., Franzyk, H. & Gram, L. Bacterial membrane activity of alpha-peptide/beta-peptoid chimeras: influence of amino acid composition and chain length on the activity against different bacterial strains. BMC microbiology 11, 144, doi: 10.1186/1471-2180-11-144 (2011).
Fisher, L. M. et al. Ciprofloxacin and the fluoroquinolones. New concepts on the mechanism of action and resistance. The American journal of medicine 87, 2S–8S (1989).
HsuChen, C. C. & Feingold, D. S. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry 12, 2105–2111 (1973).
Tossi, A., Sandri, L. & Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55, 4–30, doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M (2000).
Tiozzo, E., Rocco, G., Tossi, A. & Romeo, D. Wide-spectrum antibiotic activity of synthetic, amphipathic peptides. Biochemical and biophysical research communications 249, 202–206, doi: 10.1006/bbrc.1998.9114 (1998).
Smith, P. T., Huang, M. L. & Kirshenbaum, K. Osmoprotective Polymer Additives Attenuate the Membrane Pore-Forming Activity of Antimicrobial Peptoids. Biopolymers, doi: 10.1002/bip.22588 (2014).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395, doi: 10.1038/415389a (2002).
Ramamoorthy, A. et al. Cell selectivity correlates with membrane-specific interactions: a case study on the antimicrobial peptide G15 derived from granulysin. Biochimica et biophysica acta 1758, 154–163, doi: 10.1016/j.bbamem.2006.02.014 (2006).
Bera, S. et al. Biophysical insights into the membrane interaction of the core amyloid-forming Abeta40 fragment K16-K28 and its role in the pathogenesis of Alzheimer’s disease. Physical chemistry chemical physics: PCCP 18, 16890–16901, doi: 10.1039/c6cp02023b (2016).
Sengupta, D., Leontiadou, H., Mark, A. E. & Marrink, S. J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochimica et biophysica acta 1778, 2308–2317, doi: 10.1016/j.bbamem.2008.06.007 (2008).
Saravanan, R. et al. Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnology and bioengineering 111, 37–49, doi: 10.1002/bit.25003 (2014).
Subbalakshmi, C. & Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS microbiology letters 160, 91–96 (1998).
Rokitskaya, T. I., Kolodkin, N. I., Kotova, E. A. & Antonenko, Y. N. Indolicidin action on membrane permeability: carrier mechanism versus pore formation. Biochimica et biophysica acta 1808, 91–97, doi: 10.1016/j.bbamem.2010.09.005 (2011).
Fjell, C. D., Hiss, J. A., Hancock, R. E. & Schneider, G. Designing antimicrobial peptides: form follows function. Nature reviews. Drug discovery 11, 37–51, doi: 10.1038/nrd3591 (2012).
Huang, H. W. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochimica et biophysica acta 1758, 1292–1302, doi: 10.1016/j.bbamem.2006.02.001 (2006).
Russell, A. L. et al. Spectroscopic and thermodynamic evidence for antimicrobial peptide membrane selectivity. Chemistry and physics of lipids 163, 488–497, doi: 10.1016/j.chemphyslip.2010.03.009 (2010).
Neale, C., Hsu, J. C., Yip, C. M. & Pomes, R. Indolicidin binding induces thinning of a lipid bilayer. Biophysical journal 106, L29–31, doi: 10.1016/j.bpj.2014.02.031 (2014).
Lundquist, A., Wessman, P., Rennie, A. R. & Edwards, K. Melittin-lipid interaction: a comparative study using liposomes, micelles and bilayer disks. Biochimica et biophysica acta 1778, 2210–2216, doi: 10.1016/j.bbamem.2008.05.009 (2008).
Killian, J. A. & von Heijne, G. How proteins adapt to a membrane-water interface. Trends in biochemical sciences 25, 429–434 (2000).
Arakha, M., Saleem, M., Mallick, B. C. & Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep 5, 9578, doi: 10.1038/srep09578 (2015).
Chileveru, H. R. et al. Visualizing attack of Escherichia coli by the antimicrobial peptide human defensin 5. Biochemistry 54, 1767–1777, doi: 10.1021/bi501483q (2015).
Hartmann, M. et al. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrobial agents and chemotherapy 54, 3132–3142, doi: 10.1128/AAC.00124-10 (2010).
Spratt, B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proceedings of the National Academy of Sciences of the United States of America 72, 2999–3003 (1975).
Mason, D. J., Power, E. G., Talsania, H., Phillips, I. & Gant, V. A. Antibacterial action of ciprofloxacin. Antimicrobial agents and chemotherapy 39, 2752–2758 (1995).
Lewin, C. S. & Amyes, S. G. The role of the SOS response in bacteria exposed to zidovudine or trimethoprim. J Med Microbiol 34, 329–332, doi: 10.1099/00222615-34-6-329 (1991).
Lutkenhaus, J. Regulation of cell division in E. coli. Trends in genetics: TIG 6, 22–25 (1990).
Gottfredsson, M., Erlendsdottir, H., Sigfusson, A. & Gudmundsson, S. Characteristics and dynamics of bacterial populations during postantibiotic effect determined by flow cytometry. Antimicrobial agents and chemotherapy 42, 1005–1011 (1998).
Phillips, I., Culebras, E., Moreno, F. & Baquero, F. Induction of the SOS response by new 4-quinolones. The Journal of antimicrobial chemotherapy 20, 631–638 (1987).
Mukherjee, A., Cao, C. & Lutkenhaus, J. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 95, 2885–2890 (1998).
Piddock, L. J. & Walters, R. N. Bactericidal activities of five quinolones for Escherichia coli strains with mutations in genes encoding the SOS response or cell division. Antimicrobial agents and chemotherapy 36, 819–825 (1992).
Wickens, H. J., Pinney, R. J., Mason, D. J. & Gant, V. A. Flow cytometric investigation of filamentation, membrane patency, and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrobial agents and chemotherapy 44, 682–687 (2000).
Tamayo, M., Santiso, R., Gosalvez, J., Bou, G. & Fernandez, J. L. Rapid assessment of the effect of ciprofloxacin on chromosomal DNA from Escherichia coli using an in situ DNA fragmentation assay. BMC microbiology 9, 69, doi: 10.1186/1471-2180-9-69 (2009).
Novo, D. J., Perlmutter, N. G., Hunt, R. H. & Shapiro, H. M. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrobial agents and chemotherapy 44, 827–834 (2000).
Hale, J. D. & Hancock, R. E. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert review of anti-infective therapy 5, 951–959, doi: 10.1586/14787210.5.6.951 (2007).
Fjell, C. D. et al. Identification of novel antibacterial peptides by chemoinformatics and machine learning. Journal of medicinal chemistry 52, 2006–2015, doi: 10.1021/jm8015365 (2009).
Rouser, G., Fkeischer, S. & Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 494–496 (1970).
Alvarez, C. et al. Binding of sea anemone pore-forming toxins sticholysins I and II to interfaces–modulation of conformation and activity, and lipid-protein interaction. Chemistry and physics of lipids 122, 97–105 (2003).
Acknowledgements
This work was funded by The Danish Council for Independent Research (grant #10-085287). We also acknowledge the Molecular Foundry, the work of which was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, and Professor Lars Holm Ogendal (Niels Bohr Institute, Copenhagen University) for dynamic light scattering measurements of our liposomes.
Author information
Authors and Affiliations
Contributions
Biljana Mojsoska (B.M.) and Håvard Jenssen (H.J.) designed the experiments. B.M. conducted the experiments. Gustavo Carretero designed and conducted the experiments regarding CR release and assisted in conducing calcein release experiments. Sylvester Larsen conducted the gel retardation assay. Ramona Mateiu took the SEM images and did the analysis of size distribution. B.M. and H.J. analysed and interpreted data. B.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mojsoska, B., Carretero, G., Larsen, S. et al. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci Rep 7, 42332 (2017). https://doi.org/10.1038/srep42332
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42332
- Springer Nature Limited
This article is cited by
-
Silver Nanoparticle-Aminogylcosides Conjugation for Enhanced Control of Pathogenic E. Coli O157:H7
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
In Vitro Biological Evaluation and Mechanism of Action of Peptoid Analogue Based on Cationic, Amphipathic Peptide A-12
International Journal of Peptide Research and Therapeutics (2023)
-
Novel small molecules affecting cell membrane as potential therapeutics for avian pathogenic Escherichia coli
Scientific Reports (2018)
-
Novel Imidazole and Methoxybenzylamine Growth Inhibitors Affecting Salmonella Cell Envelope Integrity and its Persistence in Chickens
Scientific Reports (2018)
-
Rational design of syn-safencin, a novel linear antimicrobial peptide derived from the circular bacteriocin safencin AS-48
The Journal of Antibiotics (2018)