Abstract
Mitochondrial genetic variation shapes the expression of life-history traits associated with reproduction, development and survival, and has also been associated with the prevalence and progression of infectious bacteria and viruses in humans. The breadth of these effects on multifaceted components of health, and their link to disease susceptibility, led us to test whether variation across mitochondrial haplotypes affected reproductive success following an immune challenge in the form of a non-infectious pathogen. We test this, by challenging male and female fruit flies (Drosophila melanogaster), harbouring each of three distinct mitochondrial haplotypes in an otherwise standardized genetic background, to either a mix of heat-killed bacteria, or a procedural control, prior to measuring their subsequent reproductive performance. The effect of the pathogen challenge on reproductive success did not differ across mitochondrial haplotypes; thus there was no evidence that patterns of reproductive plasticity were modified by the mitochondrial genotype following a non-infectious pathogen exposure. We discuss the implications of our data, and suggest future research avenues based on these results.
Similar content being viewed by others
Introduction
It was long assumed that the genetic variation found within the mitochondrial genome would be neutral to selection1,2,3. This assumption was based on the rationale that selection should efficiently remove function-changing genetic variation from accumulating within the mitochondrial genome, given that the genome is haploid (and thus, all nucleotides will be constantly exposed to selection), and given that the products of this genome contribute to energy production and are therefore salient to eukaryotic life4,5. However, over the past two decades, it has become clear that mitochondrial genetic variation often contributes to the expression of traits entwined in organismal life-history, ranging from developmental rates6,7, to reproductive performance and longevity1,8,9,10,11. Additionally, polymorphisms within the mtDNA sequence have not only been associated with mitochondrial diseases12,13, but also with the risk of developing a range of other common late-onset diseases14,15.
In recent years, empirical evidence has been accumulating to suggest that phenotypic consequences of mitochondrial genetic variation might often be sex-specific12,16. Given the tight link between immunity, somatic development, and survival17, this contention is consistent with previously-reported mitochondrial genetic contributions to the expression of other key life-history traits4. However, specific evidence for a role of mitochondrial genetic involvement in the immune response remains sparse, and primarily comes from direct or indirect evidence in humans or mice, or from studies of cell lines, which have found associations between particular mitochondrial haplotypes and the expression patterns of nuclear genes involved in inflammation and apoptosis pathways pivotal to immune defense against invading pathogens15,18. For example, studies have linked certain mitochondrial haplotypes in humans to increased prevalence or disease progression of AIDS19, and other presumed auto-immune diseases such as Parkinson’s disease20 and Alzheimer’s14,21. Moreover, recent studies in humans have found associations between certain mtDNA haplogroups and the constitution of gut microbiomes, including infection with potentially pathogenic bacteria such as Streptococcus22. Taken together, these studies point to a role for the mitochondrial genome in regulating fitness responses in the face of stress introduced by the exposure to pathogens and parasites that are likely to be present in the natural environment.
Furthermore, a parallel set of evidence has been accumulating lately, which substantiates an evolutionary hypothesis that is often referred to as “Mother’s Curse”23. In theory, strict maternal inheritance of the mitochondrial genome means that the fate of mutations that accumulate within the mtDNA sequence will depend primarily on their phenotypic effects when expressed in females. This should enable the accumulation of male-harming, but female-benign, mutations in the mtDNA sequence24. This hypothesis has received empirical support from observations of mtDNA haplotypes that are associated with low male, but not female, fertility in hares25 and humans26. Further evidence for the hypothesis comes from recent experiments in Drosophila melanogaster, which have shown male-biased effects associated with mitochondrial genetic variation on fertility27,28, longevity11,29, and patterns of gene expression29,30; including identification of mtDNA mutations that confer male-specific decreases in infertility31,32,33,34.
Thus, there are emerging links between mitochondrial genetic variation, innate immune responses and life-history expression15,17,35, and these observations can be furthermore coupled with the recent evidence that mtDNA-mediated effects on the phenotype might be male-specific in expression, and that these effects might be particularly likely to shape the expression of male reproductive traits16. Hence, we therefore set out to explore whether the genetic variation found within the mitochondrial genome affects patterns of reproductive plasticity in response to a non-infectious pathogen challenge in the fruit fly Drosophila melanogaster, and furthermore, whether mitochondrial genetic effects on reproductive plasticity will be more pronounced in males.
To achieve this, we challenged male and female flies harbouring each of three distinct mtDNA haplotypes, with either a non-infectious pathogen challenge or a procedural control, and then measured their reproductive performance following this challenge. Using this approach, we were able to screen for gene-by-environment interactions involving mtDNA polymorphisms and the pathogen environment, on patterns of reproductive success, thus uncovering mtDNA-mediated effects on plasticity in reproductive success following the pathogen challenged. The mitochondrial haplotypes used in our study were chosen to maximize both genetic29 and phenotypic divergence11,27 between genetic strains, and have previously been demonstrated to influence male fertility, longevity, as well as patterns of nuclear and mitochondrial gene expression11,27,29,30.
Given that the previous studies have specifically linked mitochondrial genetic variation to components of the innate immune system and to the progression of auto-immune diseases, our specific goal of this study was to screen for interactions between the mitochondrial haplotype and the activation of host innate immune response on patterns of reproductive success in each of the sexes, without confounding effects associated with fighting an infection by alive and replicating pathogens36,37. Thus, the pathogen challenge used in our study consisted of a dose of “heat-killed”, and hence non-infectious bacteria, (Gram-negative bacterium Escherichia coli mixed with the Gram-positive bacterium Micrococcus luteus). It is well established that the maintenance and activation of immune function can be costly, and traded off against other life-history traits17,38,39,40, and that components of reproductive success have previously been shown to be sensitive to the species of heat-killed bacteria used in our study37,41,42, as well as to other immune elicitors (e.g. LPS)43,44. For example, an earlier study using a mix of E. coli and M. luteus recorded a trade-off between reproductive success (number of eggs) and the activation of a component of immunity (diptericin expression) in D. melanogaster41. Similar effects have been recorded in other invertebrate species that were challenged with heat-killed bacteria or other “dead” immune elicitors, for example red flour beetle (Tribolium castanaeum)45, mealworm beetles (Tenebrio molitor)46, and mosquitos (Anopheles gambiae)47. Thus, our approach has a strong precedent within the field of ecological immunity.
Results
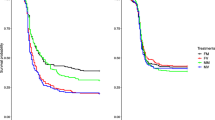
Following exposure to the pathogen challenge or procedural control, the reproductive success of females and males was measured across a four day period early in life. There was no effect of the mitochondrial haplotype on the reproductive success of either sex (male reproductive success was assessed in tester females with whom they had mated), nor were there any significant interactions between haplotype and pathogen treatment on reproductive success (Table 1, Fig. 1). Thus, we find no evidence that genetic polymorphisms within the mtDNA sequence affect patterns of reproductive plasticity in females or males. Likewise, no other interactions involving mtDNA haplotype were significant (Table 1).
Numbers above SE denote effect sizes of treatment vs. control per haplotype. (a) Female reproductive success, n = [pathogen challenge/control]: Japan = [23/19], Israel = [22/23], Dahomey = [21/21]. (b) Male reproductive success, n = [pathogen challenge/control]: Japan = [24/22], Israel = [22/26,] Dahomey = [17/19].
The pathogen challenge per se, however, imposed an initial cost on female reproductive success, with pathogen-challenged females exhibiting lower reproductive output around the 48 h time point following the pathogen challenge, relative to females that received the procedural control (Table 1a, Fig. 2a). However, pathogen-challenged females subsequently compensated for this negative effect by producing more offspring than their control counterparts, in the 72 to 96 h period following treatment (Table 1a, Fig. 2a). There was also an effect of female age at mating on reproductive output (Table 1a, Fig. 3), with one day older females producing slightly more offspring.
Note that the focal females (a) and males (b) are increasing in age across the four day mating assay, whereas the tester females and males are kept at a standardized (3–4 d) age throughout. (a) Female reproductive success, Nindividuals = [pathogen challenge/control]: [66/63]. (b) Male reproductive success, Nindividuals = [pathogen challenge/control]: [63/67].
In the male reproductive assay, there was a progressive reduction in reproductive success across the first 72 h of the reproductive assay (Fig. 2b). This decline is likely to have been caused by sperm limitation (given that the egg laying ‘tester’ females used were all of controlled age [3–4 d old] across the four days of the experiment).
Post-injection mortality (across the four days sampled) was invariably low and did not vary across the pathogen treatment in females (N treatment/control: NJap = 1/1, NIsr = 1/3, NDah = 0/2; Fisher Exact test, p = 1.0) or in males (N treatment/control: NJap = 2/2, NIsr = 5/3, NDah = 4/5; Fishers Exact test, p = 0.85).
Effect sizes (Hedges’ g), comparing means in pathogen-challenged versus control groups, across haplotypes, were invariably small for females and males (0 to 0.35; Table 2, Fig. 1).
Discussion
In recent years, the results of several studies have suggested that particular mitochondrial haplotypes are associated with an increased risk of developing certain infectious and auto-immune diseases in humans19,20,21,22,48, and that the mitochondria might play a role in the regulation of antibacterial and antiviral responses22,49. For example, humans harbouring the UK mtDNA haplogroup have been associated with higher abundances of Streptococcus than humans with other haplogroups22. Likewise, variation in gut microbiome profiles has been shown to correlate with mitochondrial haplogroup22. These associations are intriguing, given that it was traditionally thought that the function of the genes encoded by the mtDNA sequence was limited to the regulation of cellular respiration4,50.
Our study constitutes an effort to complement such association analyses conducted in humans, in that we experimentally screened for interactions between the mtDNA genotype and the pathogen environment on the expression of reproductive success in fruit flies, utilising a design in which we were able to fully disentangle mitochondrial genetic from nuclear genetic effects. To achieve this, we harnessed experimentally engineered and replicated strains, in which mtDNA haplotypes were placed alongside a standardized background. We, however, found no evidence for gene-by-environment interactions involving mtDNA haplotypes and the pathogen treatment in either sex, and effects sizes across haplotypes were generally small. This suggests that genetic variation harboured within the mitochondrial genome does not interact with components of the innate immune response to perceived bacterial infection, to affect the outcomes of reproduction, at least not across the haplotypes screened here, or using the immune elicitor employed here, in this species.
In this experiment, we limited our assay to three mitochondrial haplotypes that had previously been shown to diverge molecularly (in terms of number of SNPs separating each pair of haplotypes), and phenotypically for longevity and reproductive success11,27,29. However, we detected no effects of mtDNA haplotype on the reproductive performance of males or females. While the lack of effect in females can be reconciled by the prediction that purifying selection should remove non-neutral mtDNA polymorphisms that affect female function16,24, the lack of an effect in males is surprising. This is because maternal transmission of mtDNA should in theory lead to the build-up of male harming (but female benign) mtDNA mutations16,24, and prior evidence has indicated male-biased mtDNA mutations typically affect components of male reproductive performance25,27,30,32. On this, we note that Yee et al.28 showed larger mitochondrial genetic effects on male fertility when fertility was measured in the presence of sperm competition with rival males, than when fertility was measured in the absence of inter-male competition. Thus, the lack of a recorded mtDNA-mediated effect on male reproductive success in our study, may stem from the nature of our design in which reproductive success was assayed in a non-competitive setting.
Our results show that the direct costs to female reproductive success, imposed by the pathogen challenge, were temporary in nature, primarily affecting females during the first 48 h post-treatment prior to a compensatory effect starting after the 72 h mark. The magnitude of this decrease in female reproductive success, and subsequent compensation, was however not contingent on the mitochondrial haplotype. This compensatory effect likely reflects the nature of the pathogen challenge, in which the pathogens (E. coli and M. luteus) had been heat-killed prior to its use. This was our intention – to induce costs to the host brought about by upregulation of the innate immune system per se, rather than costs brought about by pathogenic effects to the host of harbouring an infectious and replicating pathogen. While our results here suggest that the direct negative effects of this pathogen treatment on female reproductive success were short-lived, previous studies using very similar approaches have recently documented effects associated with exposure to a heat-killed pathogen challenge that extended across generations and shaped the expression of offspring reproductive success37,42.
We note the possibility that negative effects associated with the pathogen challenge might have affected the mating rates of flies across the treatment (pathogen challenged versus procedural control), potentially inflating or confounding the observed differences between treatments. While we were unable to track mating rates across the experiment associated with the pathogen treatment and control, we were able to assess whether the pathogen-challenged individuals were more likely not to have mated at all during the experiment relative to the control-treated individuals. Indeed, our data did not show any biases in the number of zero reproductive events in flies subjected to the pathogen treatment relative to flies subjected to the control (total number of individuals generating zero offspring: Npathogen challenge = 5; Ncontrol = 3, Odds ratio = 1.80, p = 0.49; also see Supplementary material Table S2 for a breakdown across days). These results suggest that the pathogen-treatment did not impede the capacity of treated flies to copulate and produce fertile offspring. We do, however, acknowledge that the pathogen treatment might have altered the remating behaviour of females (i.e. remating rates over and above the one mating per female), potentially affecting reproductive outcomes. While it was traditionally thought that female D. melanogaster enter a strong refractory period following mating that lasts up to 24 hr51,52,53, some studies have shown that many females will mate a second time when exposed to males following a shorter period54, with one study reporting up to 50% of females remating within six hours55. That said, we believe mating rates in our study were likely to have been low across both treatments, given females were exposed to low levels of mating interactions, cohabiting with just one other male in each 24 hour period of the 4 day assay. Finally, we note that the natural mating system of D. melanogaster is one where females mate multiply (allowing matings with the same and different males) within relatively short periods of time54,55, as was possible in our experimental design (i.e. matings with same and different mates, across a 96 h time period). Assessing the effects of the pathogen treatment in the presence of the natural mating rate is, we believe, the ecologically-relevant context in which to have measured the reproductive consequences of this treatment.
In sum, our key predictions were not supported. The genetic variation found across three distinct mitochondrial haplotypes had no effect on patterns of reproductive plasticity in response to a non-infectious pathogen challenge, in either sex. However, given the previously-reported links between mtDNA haplotypes in humans with abundances of certain pathogens22,49, and given the emerging conceptual link between the mitochondria and the innate immune system22,49, we suggest that further tests of the capacity for the mtDNA haplotype to influence the ability of an individual to withstand or tolerate exposure to environmental pathogens, are warranted. Such tests should use an experimental framework that has the power to unambiguously partition mitochondrial genetics from nuclear genetic effects, as employed here. We believe that the next step should be to also include a challenge involving a live pathogen, to examine the interplay between the mitochondrial genotype and the pathogen environment, over and above effects attributable to those of activation and upregulation of the host immune system per se, on a range of parameters including proximate (immune parameter) and ultimate (longevity, reproductive fitness) traits. Furthermore, it will be worthwhile investigating the role of epistatic interactions between mitochondrial and nuclear genetic polymorphisms, in moderating such effects, given that we know that almost all components of mitochondrial function rely on coordinated interactions between genes that span mitochondrial and nuclear genomes50,56.
Methods
Mitochondrial fly strains
The focal flies were sourced from three different strains, each of which harboured a different mitochondrial haplotype, sourced originally from Japan, Israel, and Dahomey. These strains are regularly screened at diagnostic markers to ensure their mitochondrial genetic identity is maintained, without contamination. The mitochondrial haplotypes were chosen to maximize levels of genetic divergence (minimum pairwise divergence between haplotypes is 18 SNPs, Supplementary material Table S129), and phenotypic divergence (based on previously reported effects on male fertility and longevity between each11,27).
Each mitochondrial haplotype had been extracted and placed in an isogenic nuclear background, w1118 (Bloomington stock no. 5905, isogenic for chromosome 1, 2, and 3, and constructed by John Roote, Cambridge, UK) by David Clancy in 200457. Upon arrival in our lab in 2007, each of these mitochondrial strains was split into two independent duplicates, and then virgin females of each mitochondrial strain duplicate were backcrossed to males of the w1118 strain for a further 75 generations. Isogenicity of the nuclear background was guaranteed by propagating the w1118 strain via mating one pair of full-siblings each generation11. All strains were treated, and later screened, for Wolbachia to ensure that they were free of infection57,58. Flies were reared under a 12:12 h light/dark cycle, at 25 °C, on a potato-dextrose-agar-medium59 and with ad libitum live yeast, at controlled egg densities (40 eggs per vial). All populations were propagated using four days old females.
Experimental procedure
Between 20 and 25 virgins of each sex were collected per duplicate of each mitochondrial strain, and stored across two vials containing 10 flies each. These were the focal flies in the experiment. We also collected virgin flies of standardized age (3–4 days old), of each sex, from the w1118 isogenic strain, to be used as ‘tester’ flies that mated with focal flies during the experiment. When focal flies were three-to-four days old, females (N3day = 37, N4day = 92 across all mtDNA haplotypes) and males (N3day = 45, N4day = 85 across all mtDNA haplotypes) were subjected to either a pathogen challenge or procedural control, administered by microinjection into the abdomen (using the nano-injector “Nanoject”, Drummond Scientific Company, Broomall, PA, USA). The control consisted of 41.4 nl of PBS (Sigma Aldrich tablet P4417, pH 7.4), and the pathogen challenge consisted of 41.4 nl of a mix of equal volume heat-killed Gram-negative (Escherichia coli, strain K12, OD600 = 1.0, corresponding to ~27.5 × 106 CFU per fly) and heat-killed Gram-positive (Micrococcus luteus strain, A204, OD600 = 0.1, corresponding to ~1.1 × 106 CFU per fly) bacteria re-suspended in PBS (supplied by Micromon Genomics, biotechnology and diagnostics, Monash University). In brief, a single colony of E. coli and M. luteus had been inoculated in Nutrient broth, and incubated at 37 °C, followed by overnight shaking (105 r.p.m.). The resulting cultures were used to inoculate Nutrient broths again, followed by shaking (at 37 °C, 150 r.p.m.) until reaching the desired concentrations, upon which cell pellets were washed once in PBS and harvested by centrifugation. Cell pellets were re-suspended in PBS, and viability checks conducted. Finally, bacteria were heat-killed at 60 °C for 1 h for the E. coli, and 4 h for M. luteus, which was verified successful by bacterial growth test on Nutrient agar. Final OD and volumes were verified again before the onset of the experiment, using a spectrophotometer (UV/VIS SP8001, Metertech inc., Taiwan), and using starting volumes of 2 ml for the E. coli and 1.2 ml for the M. luteus. Pathogen treatments were administered under CO2 sedation, which was never administered for more than a maximum of three minutes per fly.
The aim of using a treatment consisting of a mix of Gram-positive and Gram-negative bacteria was to stimulate both main pathways of the Drosophila immune system; Gram-negative bacteria primarily activates the Imd immune pathway and Gram-positive bacteria primarily the Toll pathway36,60. Both these pathways are responsible for activating the production of antimicrobial peptides (AMPs) that are used in defense against bacteria and fungi36,60,61. Previous studies on Drosophila have demonstrated effects of both M. luteus and E. coli on gene or gene products associated with immune function62, and some studies have shown that these bacteria activate specific immune defense functions (e.g. phagocytosis)60,63. There are also demonstrated effects on phenotypic parameters37,41. Hence, we anticipated that the administration of a mix of Gram-positive and Gram–negative bacteria would facilitate an elevated response to the pathogen challenge, due to a greater perceived threat to survival36,60, which could possibly generate a longer lasting cost43.
Moreover, by using a heat-killed pathogen challenge rather than a live pathogen, we ensured any response to the treatment could be primarily traced to the host response per se, and not the indirect effects of pathogenesis induced by replicating pathogens37,44. Previous pilot experiments have indicated that the doses used for the pathogen challenge decreased reproductive success of Drosophila females (Supplementary Material, Fig. S1), and previous experiments identified transgenerational fitness effects tied to a challenge with M. luteus37 and by a mix of M. luteus and E. coli in fruit flies42.
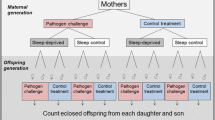
Following the microinjections, flies were given 24 h recovery time during which time they cohabited with other flies of the same sex in shared vials (mean: 4.4 ± 1.5 S.E. per vial), after which time they were each placed in separate vials and subjected to a four day mating trial (i.e. Day 1 covering the 24–48 h time interval post-injection [referred to as 24 h mark in graphs based on the start of the interval], Day 2 covering the 48–72 h time interval post-injection [referred to as 48 h mark in graphs], and so forth), as outlined in Fig. 4, and described below.
Focal females of each mitochondrial strain duplicate were transferred to a new vial each day for four consecutive 24 h periods, to allow ovipositing. A virgin ‘tester’ male (from the w1118 strain) of standardized age (3–4 days old) was provided to each focal female at each of the 4 consecutive 24 h periods, thus ensuring focal females could mate freely with four different males over the course of the assay. Furthermore, the reproductive assays of the focal females represent measures of early life reproductive success, and commenced when the focal females were three to four days of age. These assays of early life reproductive success provide good estimates of reproductive success over early to mid-life, since reproductive success of both females and males declines with age. Our previous work has indeed confirmed a strong correlation between reproductive success scored across the first four days post-mating and that scored across the first 10 days (R2 = 0.82, Fisher z = 1.49, n = 300, p = <0.0001)37. Likewise, a previous study using two of the same mitochondrial strains (but which, similarly to our assay on male reproductive success, sampled progressively aging males, but who were mated with a [new] standardized aged female each day) to those that were used, here also recorded the same pattern of declining reproductive success across ten days of egg laying28. Eleven days later, the number of adult offspring eclosing from each vial was counted, and thus female reproductive success was estimated as the number of offspring each female produced over a 96 h mating and ovipositing window.
Male reproductive success was recorded using a similar design, with the exception that males were given two standardized aged (3–4 d old) virgin tester females (of the w1118 strain) to mate with during each consecutive 24 h period. At the end of each 24 h mating bout, each female was transferred to an individual vial, and 11 d later, the number of offspring eclosing from each of these individual vials, as well as the vials in which the matings took place, were counted. Because each focal male was provided with two tester females per 24 h period, male reproductive scores are derived from the reproductive success of eight tester females, and were thus generally higher in magnitude than the reproductive scores of the focal female reproductive assays (see Fig. 4).
Statistics
All statistical analyses were run in R 3.1.1 (R Development Core Team 2012)64. Data were zero-inflated, and models assuming a Poisson distribution over-dispersed. Zero-inflation of data was verified both graphically, by conducting a Vuong-test of fixed effects, and by conducting simulations of 95% confidence intervals around the zero values generated by a Poisson models that were corrected for over-dispersion by adding an observation-level random effect. The latter generated 2.5% and 97.5% confidence intervals (CI) of 4–17 in females, and CI of 73–125 in males. Both cases displayed a total number of zeros outside these confidence interval ranges (67 out of 516 data points in females, and 159 out of 520 data points in males). The zero-inflated models adopted here allow for the zero data to be a mix of structural and sampling zeros, thus accounting for zero values that originated from females that mated but failed to produce offspring, and females that may not have mated in the first instance. Hence, in both sexes, we fitted a negative binomial distribution to a zero-inflated model. However, in females, the NB2 fit (variance = μ(1 + μ∕k) was more appropriate, whereas in males, NB1 fit (variance = ϕμ) produced the better-fitting model based on AIC values65.
For both males and females, the response variable was the number of offspring produced, and fixed factors were the pathogen treatment (challenge, control), mtDNA haplotype (Japan, Israel, Dahomey), female age at mating (3 and 4 d), and day of ovipositing (i.e. focal females, or tester females in the male assay). While the interaction of key interest was between the pathogen treatment and mtDNA haplotype (since this would allow us to screen for mtDNA-mediated effects on reproductive plasticity), we explored all possible interactions up to first order. Random effects were the strain duplicate nested within mitochondrial strain, the vial identity nested within mitochondrial strain duplicate, and individual identity.
We also explored interactions between random effects and fixed effects. However, in no cases did random × fixed effect interactions significantly contribute to the models, and as a result, they were excluded from the analysis. Models were evaluated by removing one parameter at a time from the full model, and assessing the associated change in deviance using log-likelihood ratio tests. Model simplification then proceeded by sequentially dropping the non-significant terms (two-tailed test, α = 0.05) that had the least effect on the model, and using the resulting AIC values of all possible models to select the best-fitting model (best model highlighted in bold in Table 1).
Additional Information
How to cite this article: Nystrand, M. et al. No effect of mitochondrial genotype on reproductive plasticity following exposure to a non-infectious pathogen challenge in female or male Drosophila. Sci. Rep. 7, 42009; doi: 10.1038/srep42009 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
James, A. & Ballard, J. Mitochondrial genotype affects fitness in Drosophila simulans. Genetics 164, 187–194 (2003).
Dowling, D. K., Abiega, K. C. & Arnqvist, G. Temperature-specific outcomes of cytoplasmic-nuclear interactions on egg-to-adult development time in seed beetles. Evolution 61, 194–201, doi: 10.1111/j.1558-5646.2007.00016.x (2007).
Meiklejohn, C. D. et al. An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in Drosophila . PLoS Genet. 9, e1003238, doi: 10.1371/journal.pgen.1003238 (2013).
Dowling, D. K., Friberg, U. & Lindell, J. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 23, 546–554, doi: 10.1016/j.tree.2008.05.011 (2008).
Rand, D. M., Haney, R. A. & Fry, A. J. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 19, 645–653, doi: 10.1016/j.tree.2004.10.003 (2004).
Dowling, D. K., Friberg, U., Hailer, F. & Arnqvist, G. Intergenomic epistasis for fitness: within-population interactions between cytoplasmic and nuclear genes in Drosophila melanogaster. Genetics 175, 235–244, doi: 10.1534/genetics.105.052050 (2007).
Immonen, E., Collet, M., Goenaga, J. & Arnqvist, G. Direct and indirect genetic effects of sex-specific mitonuclear epistasis on reproductive ageing. Heredity (Edinb.) 116, 338–347, doi: 10.1038/hdy.2015.112 (2016).
Dowling, D. K., Meerupati, T. & Arnqvist, G. Cytonuclear interactions and the economics of mating in seed beetles. Am. Nat. 176, 131–140, doi: 10.1086/653671 (2010).
Maklakov, A., Friberg, U., Dowling, D. & Arnqvist, G. Within-population variation in cytoplasmic genes affects female life span and aging in Drosophila melanogaster. Evolution 60, 2081–2086 (2006).
Zhu, C.-T., Ingelmo, P. & Rand, D. M. G × G × E for Lifespan in Drosophila: Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity. PLoS Genet. 10, e1004354, doi: 10.1371/journal.pgen.1004354 (2014).
Camus, M. F., Clancy, David J. & Dowling, Damian K. Mitochondria, Maternal Inheritance, and Male Aging. Curr. Biol. 22, 1717–1721 (2012).
Dowling, D. K. Evolutionary perspectives on the links between mitochondrial genotype and disease phenotype. Biochim. Biophys. Acta Gen. Subj. 1840, 1393–1403, doi: 10.1016/j.bbagen.2013.11.013 (2014).
Wallace, D. C. The mitochondrial genome in human adaptive radiation and disease: On the road to therapeutics and performance enhancement. Genetics 354, 169–180, doi: 10.1016/j.gene.2005.05.001 (2005).
Hudson, G., Gomez-Duran, A., Wilson, I. J. & Chinnery, P. F. Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases. PLoS Genet. 10, e1004369, doi: 10.1371/journal.pgen.1004369 (2014).
Arnoult, D., F., S., Tattoli, I. & Girardin, S. E. Mitochondria in innate immunity. EMBO. Rep. 12, 901–910 (2011).
Beekman, M., Dowling, D. K. & Aanen, D. K. The costs of being male: are there sex-specific effects of uniparental mitochondrial inheritance? Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 369, 20130440, doi: 10.1098/rstb.2013.0440 (2014).
Sheldon, B. & Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 (1996).
Kenney, M. C. et al. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: insights into mitochondrial-nuclear interactions. Hum. Mol. Genet. 23, 3537–3551, doi: 10.1093/hmg/ddu065 (2014).
Hendrickson, S. L. et al. Mitochondrial DNA Haplogroups influence AIDS Progression. AIDS (London, England) 22, 2429–2439, doi: 10.1097/QAD.0b013e32831940bb (2008).
van der Walt, J. M. et al. Mitochondrial Polymorphisms Significantly Reduce the Risk of Parkinson Disease. Am. J. Hum. Genet. 72, 804–811 (2003).
Ienco, E. C. et al. May “Mitochondrial Eve” and Mitochondrial Haplogroups Play a Role in Neurodegeneration and Alzheimer’s Disease? Int. J. Alzheimers Dis. 2011, 11, doi: 10.4061/2011/709061 (2011).
Ma, J. et al. mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics 15, 1–14, doi: 10.1186/1471-2164-15-257 (2014).
Gemmell, N. J., Metcalf, V. J. & Allendorf, F. W. Mother’s curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19, 238–244, doi: 10.1016/j.tree.2004.02.002 (2004).
Frank, S. A. & Hurst, L. D. Mitochondria and male disease. Nature 383, 224–224 (1996).
Smith, S., Turbill, C. & Suchentrunk, F. Introducing mother’s curse: low male fertility associated with an imported mtDNA haplotype in a captive colony of brown hares. Mol. Ecol. 19, 36–43, doi: 10.1111/j.1365-294X.2009.04444.x (2010).
Ruiz-Pesini, E. et al. Human mtDNA Haplogroups Associated with High or Reduced Spermatozoa Motility. Am. J. Hum. Genet. 67, 682–696, doi: 10.1086/303040 (2000).
Yee, W. K. W., Sutton, K. L. & Dowling, D. K. In vivo male fertility is affected by naturally occurring mitochondrial haplotypes. Curr. Biol. 23, R55–R56, doi: 10.1016/j.cub.2012.12.002 (2013).
Yee, W. K., Rogell, B., Lemos, B. & Dowling, D. K. Intergenomic interactions between mitochondrial and Y-linked genes shape male mating patterns and fertility in Drosophila melanogaster. Evolution 69, 2876–2890, doi: 10.1111/evo.12788 (2015).
Camus, M. F., Wolf, Jochen B. W., Morrow, Edward H. & Dowling, Damian K. Single Nucleotides in the mtDNA Sequence Modify Mitochondrial Molecular Function and Are Associated with Sex-Specific Effects on Fertility and Aging. Curr. Biol. 25, 2717–2722, doi: 10.1016/j.cub.2015.09.012 (2015).
Innocenti, P., Morrow, E. H. & Dowling, D. K. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848, doi: 10.1126/science.1201157 (2011).
Clancy, D. J., Hime, G. R. & Shirras, A. D. Cytoplasmic male sterility in Drosophila melanogaster associated with a mitochondrial CYTB variant. Heredity (Edinb.) 107, 374–376, doi: 10.1038/hdy.2011.12 (2011).
Dowling, D. K., Tompkins, D. M. & Gemmell, N. J. The Trojan Female Technique for pest control: a candidate mitochondrial mutation confers low male fertility across diverse nuclear backgrounds in Drosophila melanogaster. Evol. Applic. 8, 871–880, doi: 10.1111/eva.12297 (2015).
Wolff, J. N., Tompkins, D. M., Gemmell, N. J. & Dowling, D. K. Mitonuclear interactions, mtDNA-mediated thermal plasticity, and implications for the Trojan Female Technique for pest control. Sci. Rep. 6, 30016, doi: 10.1038/srep30016 (2016).
Patel, M. R. et al. A mitochondrial DNA hypomorph of cytochrome oxidase specifically impairs male fertility in Drosophila melanogaster. eLife 5, e16923, doi: 10.7554/eLife.16923 (2016).
West, A. P., Shadel, G. S. & Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 11, 389–402 (2011).
Lemaitre, B. & Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743, doi: 10.1146/annurev.immunol.25.022106.141615 (2007).
Nystrand, M. & Dowling, D. K. Transgenerational interactions involving parental age and immune status affect female reproductive success in Drosophila melanogaster. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 281, doi: 10.1098/rspb.2014.1242 (2014).
Kraaijeveld, A. & Godfray, H. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280 (1997).
Lochmiller, R. & Deerenberg, C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 (2000).
Zuk, M. & Stoehr, A. Immune defense and host life history. Am. Nat. 160, S9–S22 (2002).
Zerofsky, M., Harel, E., Silverman, N. & Tatar, M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103–108 (2005).
Nystrand, M., Cassidy, E. J. & Dowling, D. K. Transgenerational plasticity following a dual pathogen and stress challenge in fruit flies. BMC Evol. Biol. 16, 1–11, doi: 10.1186/s12862-016-0737-6 (2016).
McKean, K., Yourth, C., Lazzaro, B. & Clark, A. The evolutionary costs of immunological maintenance and deployment. BMC Evol. Biol. 8, 76 (2008).
Nystrand, M. & Dowling, D. K. Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J. Evol. Biol. 27, 876–888, doi: 10.1111/jeb.12364 (2014).
Roth, O. & Kurtz, J. The stimulation of immune defence accelerates development in the red flour beetle (Tribolium castaneum). J. Evol. Biol. 21, 1703–1710, doi: 10.1111/j.1420-9101.2008.01584.x (2008).
Sadd, B. et al. Modulation of sexual signalling by immune challenged male mealworm beetles (Tenebrio molitor, L.): evidence for terminal investment and dishonesty. J. Evol. Biol. 19, 321–325, doi: 10.1111/j.1420-9101.2005.01062.x (2006).
Ahmed, A. M., Baggott, S. L., Maingon, R. & Hurd, H. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae . Oikos 97, 371–377, doi: 10.1034/j.1600-0706.2002.970307.x (2002).
Wallace, D. C. Mitochondria as Chi. Genetics 179, 727–735, doi: 10.1534/genetics.104.91769 (2008).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557, doi: 10.1038/nature14156 (2015).
Wolff, J. N., Ladoukakis, E. D., Enríquez, J. A. & Dowling, D. K. Mitonuclear interactions: Evolutionary consequences over multiple biological scales. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 369, doi: 10.1098/rstb.2013.0443 (2014).
Manning, A. A Sperm Factor Affecting the Receptivity of Drosophila Melanogaster Females. Nature 194, 252–253, doi: 10.1038/194252a0 (1962).
Manning, A. The control of sexual receptivity in female Drosophila. Anim. Behav. 15, 239–250, doi: 10.1016/0003-3472(67)90006-1 (1967).
Brown, W., Bjork, A., Schneider, K. & Pitnick, S. No evidence that polyandry benefits females in Drosophila melanogaster. Evolution 58, 1242–1250 (2004).
Fricke, C., Green, D., Mills, W. E. & Chapman, T. Age-dependent female responses to a male ejaculate signal alter demographic opportunities for selection. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 280, doi: 10.1098/rspb.2013.0428 (2013).
van Vianen, A. & Bijlsma, R. The adult component of selection in Drosophila melanogaster: some aspects of early-remating activity of females. Heredity (Edinb.) 71, 269–276 (1993).
Dobler, R., Rogell, B., Budar, F. & Dowling, D. K. A meta-analysis of the strength and nature of cytoplasmic genetic effects. J. Evol. Biol. 27, 2021–2034, doi: 10.1111/jeb.12468 (2014).
Clancy, D. J. Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 7, 795–804, doi: 10.1111/j.1474-9726.2008.00428.x (2008).
O’Neill, S. L., Giordano, R., Colbert, A. M., Karr, T. L. & Robertson, H. M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89, 2699–2702 (1992).
Williams, B. R., Heerwaarden, B., Dowling, D. K. & SgrÒ, C. M. A multivariate test of evolutionary constraints for thermal tolerance in Drosophila melanogaster . J Evol. Biol. 25, doi: 10.1111/j.1420-9101.2012.02536.x (2012).
Nehme, N. T. et al. Relative Roles of the Cellular and Humoral Responses in the Drosophila Host Defense against Three Gram-Positive Bacterial Infections. PLoS One 6, e14743, doi: 10.1371/journal.pone.0014743 (2011).
Lemaitre, B., Reichhart, J.-M. & Hoffmann, J. A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 94, 14614–14619 (1997).
Rutschmann, S. et al. The Rel Protein DIF Mediates the Antifungal but Not the Antibacterial Host Defense in Drosophila. Immunity 12, 569–580, doi: 10.1016/S1074-7613(00)80208-3 (2000).
Elrod-Erickson, M., Mishra, S. & Schneider, D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 10, 781–784, doi: 10.1016/S0960-9822(00)00569-8 (2000).
R Development Core Team. (R Foundation for Statistical Computing, Vienna, Austria, 2012).
Skaug, H. J., Fournier, D. A., Nielsen, A., Magnusson, A. & Bolker, B. M. Package glmmADMB: Generalized linear mixed models using AD Model Builder. R package version 0.7.3. http://r-forge.r-project.org/projects/glmmadmb/ (2012).
Acknowledgements
This research was supported by both the Australian Research Council (Australian Postdoctoral Fellowship and Discovery Project, DP110104965) and a Monash University Margaret Clayton Women in Research Postdoctoral Fellowship to M.N. Construction and maintenance of mitochondrial strains were supported by an Australian Research Council grant to DKD (DP1092897).
Author information
Authors and Affiliations
Contributions
M.N. designed and coordinated the study, conducted statistical analyses, and wrote the manuscript; E.C. carried out lab work, and contributed to design; D.K.D. helped design the study and write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nystrand, M., Cassidy, E. & Dowling, D. No effect of mitochondrial genotype on reproductive plasticity following exposure to a non-infectious pathogen challenge in female or male Drosophila. Sci Rep 7, 42009 (2017). https://doi.org/10.1038/srep42009
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42009
- Springer Nature Limited