Abstract
A recent study showed that posterior circulation plaques have a greater capacity for positive remodeling in a non-Asian population. We aimed to investigate if the features of plaques in the middle cerebral artery (MCA) were different from those in the basilar artery (BA) in a northern Chinese population. We retrospectively analysed the records of 71 consecutive patients with acute ischemic stroke. All patients had at least one MCA or BA plaque with early or mild (<50% stenosis) atherosclerosis identified using vessel wall magnetic resonance imaging. The remodeling ratio, eccentricity index, and plaque range were compared between MCA and BA plaques using multilevel analysis. A total of 101 plaques were included. There were 70 plaques located in the MCA and 31 plaques located in the BA. The features of non-advanced atherosclerotic plaques did not differ between the MCA and BA when accounting for the degree of stenosis or plaque burden in a northern Chinese population. Symptomatic plaques were associated with a higher eccentricity index and smaller plaque range than asymptomatic plaques under the same plaque burden. Further studies are warranted to investigate the progression of atherosclerosis in different intracranial arteries.
Similar content being viewed by others
Introduction
Intracranial atherosclerotic disease is one of the important causes of ischemic stroke or transient ischemic attack, especially in Asian, Hispanic and African populations1,2. Recently, with the rapid development of vessel wall magnetic resonance imaging (VWMRI) techniques, there has been increasing interest in studies of intracranial atherosclerosis. The use of VWMRI has greatly improved the understanding of the pathophysiology of atherosclerosis3. The plaque features on VWMRI may be important predictors of stroke and were shown to correlate with pathological findings4,5,6,7,8.
Atherosclerosis is a systemic disease that may involve several vascular beds, including coronary, peripheral, and renal arteries in addition to the cerebral arteries. However, a previous study showed that the carotid artery has a pattern of remodeling that was distinct from that of the femoral artery, which may be caused by differences in anatomy and blood flow9. A recently study also showed that there was a significant difference in arterial remodeling between the arteries of the intracranial posterior circulation and intracranial anterior circulation in a Western population10. We aimed to investigate if the features of plaques of the middle cerebral artery (MCA) were different from those of the basilar artery (BA) in the early and intermediate stages of atherosclerosis in a northern Chinese population.
Results
A total of 71 patients with 101 plaques were included in this study. There were 70 plaques located in the MCA and 31 plaques located in the BA. Among the 70 MCA plaques, 20 were classified as symptomatic, 6 were indeterminate, and 44 were asymptomatic. Among the 31 BA plaques, 11 were symptomatic, 12 were indeterminate, and 8 were asymptomatic (chi-squared test, P for trend = 0.022). Table 1 shows the characteristics of the patients. Table 2 shows the parameters of the MCA and BA plaques.
The inter-observer agreements (intraclass correlation coefficient) for wall area (WA), lumen area (LA), minimum wall thickness, maximum wall thickness, and plaque range were 0.93, 0.92, 0.89, 0.90, and 0.86, respectively.
Remodeling Ratio
The results of the null model showed that about 38% of the variance in remodeling ratio stems from differences between patients, clearly indicating that a multilevel model was required. The presence of significant between-group heterogeneity (P = 0.035) also indicated that a multilevel model was required (see Supplementary Table S1).
The level 2 explanatory variables of age, sex, hypertension, diabetes mellitus, dyslipidaemia, smoking, and history of coronary heart disease were added to the null model respectively. When these variables were added to the null model, the intercept kept almost unchanged, which suggested that the between-group variation could not be explained by the level 2 explanatory variables.
Level 1 explanatory variables, including the classification of plaque, location of plaque (MCA or BA), and degree of stenosis, were added to the null model. The results showed that the degree of stenosis was significantly associated with the remodeling ratio (P < 0.001) (Table 3 and Supplementary Table S2). The location of plaque (P = 0.768) and classification of plaque were not significantly associated with the remodeling ratio (Table 3 and Supplementary Table S2). The model fit of this model was better than that of the null model (P < 0.001) (see Supplementary Table S3). Testing results of the level 1 random slope coefficients suggested that none of the variables should be set as random slope. Thus, the above model is the final model. About 11% of the variance at level 1 and about 37% of the variance at level 2 can be explained by the final model (Table 3 and Supplementary Table S2).
Eccentricity Index
The results of the null model indicated that a multilevel model was required for the outcome measures of the eccentricity index (see Supplementary Table S4). The same flow was used to identify the variables that were associated with the eccentricity index. The level 1 explanatory variables of the classification of plaque, location of plaque, and plaque burden were included in the final model. The results showed that the location of plaque (P = 0.387) and plaque burden (P = 0.156) were not significantly associated with the eccentricity index (Table 3 and Supplementary Table S5). Symptomatic plaques were associated with a significantly higher eccentricity index (P = 0.028) than asymptomatic plaques.
Plaque Range
The results of the null model indicated that a multilevel model was required for the plaque range (see Supplementary Table S6). The level 1 explanatory variables of the classification of plaque, location of plaque, and plaque burden were included in the final model. The results showed that the plaque burden was positively associated with plaque range (P = 0.037) (Table 3 and Supplementary Table S7). Symptomatic plaques were associated with a significantly smaller plaque range (P = 0.042) than asymptomatic plaques.
Additional Analysis
Eight MCA plaques and two BA plaques were excluded because the degree of stenosis was ≥ 50%. An additional analysis was performed including these 10 plaques. Supplementary Table S8 shows that the results were essentially unchanged.
Discussion
By using multilevel model, this study found that the remodeling ratio, eccentricity index, and plaque range were not significantly different between MCA and BA plaques with the same degree of stenosis or plaque burden in a northern Chinese population. Symptomatic plaques were associated with a higher eccentricity index and smaller plaque range than asymptomatic plaques.
The multilevel model, also referred to as mixed-effects models, random coefficient models, random effects models, or variance component models, has become a popular method in many fields of research that have nested data structures11,12. Both statistical and conceptual problems may exist if multilevel model is not considered for data with a multilevel structure. For our study, variables at the plaque level are level 1 variables, which including classification of plaque, location of plaque, degree of stenosis, and plaque range. Variables at the patient level are level 2 variables, which including age and sex. Because more than one plaque from the same patient may exist and be included, and different plaques within the same patient may have similar features, multilevel model should be considered. Furthermore, the results of the null models for the remodeling ratio, eccentricity index, and plaque range in this study showed that a multilevel model is necessary.
By using multilevel model, Qiao et al. recently reported that posterior circulation plaques have a higher remodeling ratio than anterior circulation plaques in a non-Asian population10, which was inconsistent with our study. However, some important level 1(lesion level) variables such as plaque classification and degree of stenosis were not included in their model. On the other hand, only MCA and BA plaques were included in our study. Furthermore, it is very plausible that some positively remodeled vertebrobasilar arteries could simply be early or developing atherosclerotic “fusiform” aneurysms, which are well known to predominantly affect the posterior circulation. The high remodeling ratio of posterior circulation plaques in the previous study might be attributed to the fact that some of the included posterior circulation plaques may be in the at early or developing stages of atherosclerotic “fusiform” aneurysms.
The formula deduced from the remodeling model (see Supplementary Fig. S1) and data from the plaques (see Supplementary Fig. S2) suggested that the remodeling ratio is positively associated with the plaque burden and inversely associated with the degree of stenosis. Therefore, to investigate if the remodeling ratio differs between MCA and BA plaques, degree of stenosis or plaque burden must be accounted for. Because the degree of stenosis is the most common parameter measured in atherosclerotic lesions, it was included (i.e. adjusted for) in our model. In other words, we determined whether the remodeling ratio was different between MCA and BA plaques with the same degree of stenosis; the results suggest that it was not (Table 3, P = 0.768). A prospective study to investigate the progression of MCA and BA atherosclerosis may further answer the question of whether the remodeling ratio is different between MCA and BA plaques9,13.
Previous studies showed that positive remodeling is associated with symptomatic plaque; however, this was not confirmed in our study14,15. Qiao et al. reported that culprit plaques were associated with positive remodeling when compared with both non-culprit and indeterminate plaques or non-culprit plaques alone. However, they reported that the association became non-significant after adjusting for the plaque burden10. In our study, the remodeling ratio was not associated with the classification of plaque when examined using a one-way analysis of variance (P for trend = 0.909) or multivariate analysis (Table 3). The different methods of plaque classification and/or statistical analysis may account for the controversial result.
Eccentricity is another important feature of atherosclerotic plaques. Previous studies showed that for carotid plaques, larger maximum wall thickness and eccentric plaques were associated with the occurrence of subsequent cerebrovascular events16,17. For intracranial vascular disease, eccentric wall thickening has been used to distinguish intracranial atherosclerosis from other etiologies18,19. A recent in vitro study showed that eccentric plaques were associated with a non-significant higher risk of cerebrovascular events compared with concentric plaques (30.77% vs. 17.39%; n = 72, P = 0.241)5. Our study showed that symptomatic plaques were associated with a significantly higher eccentricity index than asymptomatic plaques after adjusting for the plaque burden (P = 0.028).
We also introduced a new parameter related to plaques, the plaque range. We found that symptomatic plaques had a smaller plaque range than asymptomatic plaques after adjusting for the plaque burden. However, a recent published study showed that plaques with <50% wall involvement were significantly associated with a higher possibility of asymptomatic plaques than plaques with >50% wall involvement20. The conflicting results may be attributed to the different methodologies between these two studies. Notably, the plaque burden was adjusted for in our study. The authors explained that plaques with >50% wall involvement are more advanced when compared with plaques with <50% wall involvement20, which suggests that the adjustment for the plaque burden is necessary. Another likely explanation for the conflicting results is that the plaque range may be simply acting as a surrogate measure of plaque eccentricity, rather than as a truly independent variable.
There are several limitations of this study. First, a 2D technique may lead to overestimation of thickness measurements because of partial volume effects10. Second, because the purpose of this study was to investigate plaque features in the early and mild stages of atherosclerosis, the results of our study may not be generalized to lesions causing ≥50% stenosis. Third, plaque features along the long axis of the artery were not considered in this study. Last and most importantly, the tapering of the vessel was not considered when calculating the parameters of the remodeling ratio, plaque burden, or degree of stenosis. This is a potential source for significant measurement bias, which may have a substantial impact on the results, particularly when the reference slice is distant from the pathological slice.
In conclusion, by using multilevel model, this study found that the remodeling ratio, eccentricity index, and plaque range were not significantly different between non-advanced MCA and BA plaques after controlling for the degree of stenosis or plaque burden in a northern Chinese population. Symptomatic plaques were associated with a higher eccentricity index and smaller plaque range than asymptomatic plaques. A prospective study to investigate the progression of MCA and BA atherosclerosis may further answer the question of whether plaque features are different between MCA and BA plaques.
Methods
Study Population
We retrospectively collected consecutive patients who were admitted to the Department of Neurology of the First Hospital of Jilin University between January 2015 and January 2016 with the following characteristics: (1) patients with acute ischemic stroke confirmed by diffusion-weighted imaging within 7 days of symptom onset; (2) patients with atherosclerotic plaques in the MCA or BA that were identified using VWMRI; (3) patients with at least two vascular risk factors18; (4) patients without evidence of cardioembolism; and (5) the 2D T2-weighted VWMRI images were of sufficiently good quality to analyse. Only atherosclerotic lesions of the MCA or BA that caused less than 50% stenosis were included. Patients with evidence of moyamoya disease, vasculitis, reversible cerebral vasoconstriction syndrome, or dissection according to the criteria from previous reports were excluded18,19. The institutional review board of the First Hospital of Jilin University approved this retrospective study. Written informed consent was waived because this was a retrospective study and was approved by the institutional review board. The methods were carried out in accordance with the approved guidelines.
Imaging Protocol
Since almost all of the VWMRI examinations were performed on a 3 T Siemens Trio MR scanner (Siemens Healthcare, Erlangen, Germany) between January 2015 and January 2016, the only qualifying patient that was examined using the Achieva 3 T scanner (Philips Healthcare, Eindhoven, The Netherlands) was excluded. The VWMRI protocol included 3D time-of-flight MR angiography (repetition time, 21 ms; echo time, 4 ms; slice thickness, 0.50 mm; flip angle, 18°; field of view, 215 mm × 195 mm), 3D T1-weighted SPACE (Sampling Perfection with Application optimized Contrast using different angle Evolutions) (oblique coronal plane acquisition; variable refocusing flip angle; repetition time, 550 ms; echo time, 18 ms; field of view = 210 mm × 210 mm; voxel size = 0.8 mm × 0.8 mm × 0.8 mm; with or without contrast enhancement), and 2D T2-weighted VWMRI (repetition time, 3600 ms; echo time, 56 ms; slice thickness, 2 mm; flip angle, 120°; field of view, 130 mm × 130 mm; 512 × 512 matrix). Two-dimensional T2-weighted VWMRI was obtained perpendicular to the long axis of the BA (approximately 13 slices) or the M1 segments of the relevant MCA (11 slices) and contralateral MCA (11 slices). A 12-channel head coil was used for the above sequences.
Classification of Plaque
Each plaque was classified as either symptomatic, indeterminate, or asymptomatic according to criteria adapted from previous studies10,14. A plaque was deemed to be symptomatic if there was an ischemic stroke or transient ischemic attack in the region supplied by the corresponding artery within the preceding four weeks. The plaque should also have been the the only or most stenotic lesion in the corresponding artery. A plaque was deemed to be asymptomatic if there was no history of ischemic stroke or transient ischemic attack in the regions supplied by the corresponding artery. A plaque was deemed to be indeterminate if it was not the most stenotic lesion within the corresponding artery and there was an ischemic events in the regions supplied by the corresponding artery. For patients with a single subcortical infarction in the territory of the MCA, the plaque was classified as indeterminate if the infarction was not in the striatocapsular area21. For patients with a single small deep pontine infarction, the plaque was classified as indeterminate22.
Measurement of Plaque
Images from 2D T2-weighted VWMRI were used to perform all measurements. For each atherosclerotic lesion, the cross-sectional slice with the highest degree of stenosis was selected to measure the parameters. For patients with more than one plaque in the same artery, all of the plaques were analyzed. A normal slice (i.e. without plaque on VWMRI) proximal to the selected slice was chosen as the reference site. If there was no such slice, a normal slice distal to the selected slice was chosen as the reference site14,15,21.
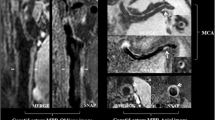
The plaque measurements were performed by two experienced neuroradiologists, each with approximately 3 years of VWMRI reading experience. Quantitative plaque measurement software (MRI-PlaqueView, VPDiagnostics Inc., Seattle, WA) was used to measure the parameters including LA, WA, outer wall area (OWA = LA + WA), minimum wall thickness, and maximum wall thickness (Figs 1 and 2). The lumen counter and outer wall counter of the selected lesion slice and the reference slice were traced in a semi-automated manner by using the software (Fig. 2).
Magnetic resonance images of the vessel wall of a basilar artery plaque.
The lumen area and wall area as measured using the MRI-PlaqueView software are 15.03 mm2 and 24.21 mm2 for the lesion slice (a) and 16.04 mm2 and 13.85 mm2 for the reference slice (b). The maximum wall thickness and minimum wall thickness measured using MRI-PlaqueView for the lesion slice are 2.24 mm and 0.75 mm. The distribution range of the plaque measured using RadiAnt DICOM Viewer software is 133.8°. Therefore, the remodeling ratio, eccentricity index, and plaque range for this plaque are 1.31, 0.67, and 133.8°, respectively.
In previous studies, the plaque burden was defined as the ratio of the wall area to the outer wall area10. We believed that this ratio or the WA alone would not be appropriate for comparisons of the MCA and BA because the diameter and wall thickness of the normal MCA and BA are different. Consequently, WAlesion/WAreference was used as the plaque burden in our study (Fig. 1), a ratio that has been referred to as the wall area index in previous studies23,24. The arterial remodeling ratio was defined as the ratio of the OWA of the selected lesion site (OWAlesion) to the OWA of the reference site (OWAreference) (Fig. 1). The eccentricity index was defined as follows: (maximum wall thickness−minimum wall thickness)/maximum wall thickness (Fig. 1)5.
We loaded the images of lesions to a DICOM viewer (RadiAnt DICOM Viewer, version 2.2.8; Meixant, Poznan, Poland). The distribution range of the plaque was calculated using the angle tool (Figs 1 and 2). The centre was determined manually based on the normal part of the wall. The edges of the plaque were determined visually.
Statistical Analysis
Quantitative data are expressed as mean ± SD. Qualitative data are expressed as the number and percentages. For quantitative data, two-sample Student’s t (for normally distributed data) or Mann–Whitney U tests were used. The chi-squared or Fisher’s exact tests were used for qualitative data. The eccentricity index, remodeling ratio, and plaque range were compared between the MCA and BA plaques using multilevel model to adjust for the effect of including more than one lesion from a single patient10. The explanatory variables included level 1 explanatory variables (including the classification of plaque, location of plaque, degree of stenosis, and plaque burden) and level 2 explanatory variables (including age, sex, hypertension, diabetes mellitus, dyslipidaemia, smoking, and history of coronary heart disease). Inter-observer agreement for plaque measurements were examined using the intraclass correlation coefficient. The level of statistical significance was set at P < 0.05. SAS 9.3 (SAS institute, Cary, NC, USA) was used to perform the analysis.
Additional Information
How to cite this article: Niu, P.-P. et al. Vessel wall differences between middle cerebral artery and basilar artery plaques on magnetic resonance imaging. Sci. Rep. 6, 38534; doi: 10.1038/srep38534 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wong, L. K. Global burden of intracranial atherosclerosis. Int J Stroke 1, 158–159 (2006).
Holmstedt, C. A., Turan, T. N. & Chimowitz, M. I. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 12, 1106–1114 (2013).
Bodle, J. D. et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 44, 287–292 (2013).
Turan, T. N. et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 237, 460–463 (2014).
Yang, W. J. et al. In Vitro Assessment of Histology Verified Intracranial Atherosclerotic Disease by 1.5T Magnetic Resonance Imaging: Concentric or Eccentric? Stroke 47, 527–530 (2016).
Chen, X. Y., Wong, K. S., Lam, W. W. & Ng, H. K. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 9, E19 (2014).
Alexander, M. D. et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry 87, 589–597 (2016).
Jiang, Y. et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 249, 10–16 (2016).
Bianda, N. et al. Progression of human carotid and femoral atherosclerosis: a prospective follow-up study by magnetic resonance vessel wall imaging. Eur Heart J 33, 230–237 (2012).
Qiao, Y. et al. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 47, 434–440 (2016).
Peugh, J. L. & Enders, C. K. Using the SPSS mixed procedure to fit cross-sectional and longitudinal multilevel models. Educational and Psychological Measurement 65, 811–835 (2005).
Nilsson, K. et al. Associations between workplace affiliation and phlebotomy practices regarding patient identification and test request handling practices in primary healthcare centres: a multilevel model approach. BMC Health Serv Res 15, 503 (2015).
Gutierrez, J. & Elkind, M. S. Letter by Gutierrez and Elkind Regarding Article, “Patterns and Implications of Intracranial Arterial Remodeling in Patients With Stroke”. Stroke 47, e86 (2016).
Xu, W. H. et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 212, 507–511 (2010).
Zhao, D. L. et al. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci 22, 700–704 (2015).
Takaya, N. et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke 37, 818–823 (2006).
Ohara, T. et al. Eccentric stenosis of the carotid artery associated with ipsilateral cerebrovascular events. AJNR Am J Neuroradiol 29, 1200–1203 (2008).
Mossa-Basha, M. et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 46, 1567–1573 (2015).
Ahn, S. H. et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke 46, 697–703 (2015).
Dieleman, N. et al. Magnetic Resonance Imaging of Plaque Morphology, Burden, and Distribution in Patients With Symptomatic Middle Cerebral Artery Stenosis. Stroke 47, 1797–1802 (2016).
Ryoo, S., Lee, M. J., Cha, J., Jeon, P. & Bang, O. Y. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke 46, 2815–2821 (2015).
Klein, I. F. et al. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke 41, 1405–1409 (2010).
Chung, J. W. et al. Echoing Plaque Activity of the Coronary and Intracranial Arteries in Patients With Stroke. Stroke 47, 1527–1533 (2016).
Chung, J. W., Hwang, J., Lee, M. J., Cha, J. & Bang, O. Y. Previous Statin Use and High-Resolution Magnetic Resonance Imaging Characteristics of Intracranial Atherosclerotic Plaque: The Intensive Statin Treatment in Acute Ischemic Stroke Patients With Intracranial Atherosclerosis Study. Stroke 47, 1789–1796 (2016).
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (Grant No. 2016YFC1301603) and Changbai mountain scholars, Jilin Provincial government to Yi Yang.
Author information
Authors and Affiliations
Contributions
Y.Y. (Yi Yang) conceived and designed the study. Y.L. (Yun Luo), Z.N.G., and H.J. collected the information of patients. P.P.N., Y.Y. (Yao Yu), H.W.Z., and Y.Y. (Yi Yang) wrote the manuscript. P.P.N. performed the statistical analyses. H.W.Z. and Y.L. (Yang Liu) analyzed the images. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Niu, PP., Yu, Y., Zhou, HW. et al. Vessel wall differences between middle cerebral artery and basilar artery plaques on magnetic resonance imaging. Sci Rep 6, 38534 (2016). https://doi.org/10.1038/srep38534
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38534
- Springer Nature Limited
This article is cited by
-
Influence of angioplasty and stenting on intracranial artery stenosis: preliminary results of high-resolution vessel wall imaging evaluation
European Radiology (2022)
-
Peri-thrombus vascular hyperintensity sign: detection of intracranial thrombus location and length in acute ischemic stroke
Japanese Journal of Radiology (2020)
-
Toward Improving Safety in Neurosurgery with an Active Handheld Instrument
Annals of Biomedical Engineering (2018)