Abstract
We use molecular dynamics simulation to investigate the early and developed stages of surface condensation. We find that the liquid-vapor and solid-liquid interfacial thermal resistances depend on the properties of solid and fluid, which are time-independent, while the condensate bulk thermal resistance depends on the condensate thickness, which is time-dependent. There exists intrinsic competition between the interfacial and condensate bulk thermal resistances in timeline and the resultant total thermal resistance determines the condensation intensity for a given vapor-solid temperature difference. We reveal the competition mechanism that the interfacial thermal resistance dominates at the onset of condensation and holds afterwards while the condensate bulk thermal resistance gradually takes over with condensate thickness growing. The weaker the solid-liquid bonding, the later the takeover occurs. This competition mechanism suggests that only when the condensate bulk thermal resistance is reduced after it takes over the domination can the condensation be effectively intensified. We propose a unified theoretical model for the thermal resistance analysis by making dropwise condensation equivalent to filmwise condensation. We further find that near a critical point (contact angle being ca. 153°) the bulk thermal resistance has the least opportunity to take over the domination while away from it the probability increases.
Similar content being viewed by others
Introduction
When a vapor is in contact with a solid surface at some temperature below the saturation temperature of the vapor, the vapor condenses to liquid on the surface, releasing to the surface the energy difference between the vapor and liquid states. The condensation mode is conventionally categorized as either dropwise or filmwise depending on the surface wettability. For a long time being, the general understanding of surface condensation has been acquired that dropwise condensation (DWC) and filmwise condensation (FWC) are two contrary modes and the former holds an order of magnitude more efficient in heat transfer than the latter1,2. The explanation has been given two-fold as: (1) the condensate bulk thermal resistance has been regarded as the crucial factor that determines the overall heat transfer performance and (2) the drop-shedding due to gravity makes DWC superior to FWC in reduction of condensate bulk thermal resistance. This understanding has been verified through plenty of experiments at conventional spatial and temporal scales. However, with the modern computer simulation methods3,4,5,6,7, cutting-edge experimental resorts8,9,10,11,12,13 and fast-developing nanomachining technologies14,15,16, the microscopic, even molecular-level, phenomena, characteristics and dynamics become observable, manipulatable and customizable. It has been demonstrated that the surface wettability, essentially determined by the solid-fluid interaction, dominates various interfacial phenomena, e.g. velocity slip6,7, temperature jump6,7, liquid layering11,12,13, contact angle3,9,17 and droplet jumping8,15,18. Therefore, the fundamental understanding of surface condensation needs to be elucidated by molecular-level insights.
Our recent work3, focused on the onset of surface condensation by molecular dynamics simulation and classical nucleation theory, has revealed the intrinsic connection between DWC and FWC. The solid-fluid interaction has been found as the origin to determine the surface condensation mode from the onset. A whole picture of condensation mode on surface with a wide range of wettability has been given, where DWC is taken as the transition mode between no-condensation and FWC. In this work, we continue to explore the insight of surface condensation after the onset, i.e. the early and developed stages. We transform DWC equivalent to FWC in terms of condensate bulk thermal resistance, enabling unified analysis of the complex phenomena. We reveal that the competition between the interfacial and condensate bulk thermal resistances determines the characteristics of time evolutions of different condensation modes and further the condensation intensities. The formation and transition mechanisms revealed in our previous work3 presents the spatial characteristics of unification of surface condensation while the competition mechanism revealed in this work presents its temporal characteristics. These characteristics clarify a fundamental insight of the phenomenon of surface condensation.
Results
We use molecular dynamics (MD) simulation to characterize the early and developed stages of surface condensation. A long cuboid simulation box is chosen to take the general feature that affect both DWC and FWC at these stages and to eliminate the vapor-liquid-solid contact-line of DWC (see Fig. 1). The fluid-fluid interaction is governed by the Lennard-Jones (L-J) potential function. The fluid-solid interaction is also described by the L-J potential function, where the parameter β measures the relative strength of fluid-solid bonding. A small value of β means low surface free energy and hydrophobicity while a large value of β means higher surface free energy and hydrophilicity6,7,19,20,21,22,23. The surface wettability is commonly interpreted by contact angle θ, a readily measureable quantity24,25,26. To establish the correlation between β and θ, we primarily conduct MD simulations at thermal equilibrium states of  (see Fig. 2), kB being the Boltzmann constant. The results show that θ decreases monotonically from 160° to 20° corresponding to θ from 0.1 to 0.7.
(see Fig. 2), kB being the Boltzmann constant. The results show that θ decreases monotonically from 160° to 20° corresponding to θ from 0.1 to 0.7.
Schematic of simulation system for surface condensation.
The simulation size measures  . The saturated vapor (red) is at
. The saturated vapor (red) is at  . The solid wall (blue) is at the bottom and is at
. The solid wall (blue) is at the bottom and is at  . The periodic boundary condition is applied at the sides and the diffuse reflection boundary is applied at the top end. The vaper supply region (thickness
. The periodic boundary condition is applied at the sides and the diffuse reflection boundary is applied at the top end. The vaper supply region (thickness  ) is at the top. The surface wettability of solid wall changes from hydrophobicity to hydrophilicity by varying β from 0.1 to 0.7.
) is at the top. The surface wettability of solid wall changes from hydrophobicity to hydrophilicity by varying β from 0.1 to 0.7.
We perform MD simulations for various values of fluid-solid bonding strength under non-isothermal conditions that the solid temperature varies in the range of  and the saturated vapor temperature is fixed at
and the saturated vapor temperature is fixed at  . The transient density profiles (see Fig. 3a) show that on the surface with β = 0.1, where the fluid-solid interaction is very weak, the condensate bulk can hardly form. On the contrary, the condensate bulk is seen to form readily on the surface with β = 0.7, where the fluid-solid interaction is very strong, and to keep growing thicker with time, indicating a high condensation intensity. Generally, the condensate bulk grows faster for higher β but the growth decreases with time. The transient temperature profiles (see Fig. 3b) show that with the condensate bulk forming on surface, a local temperature gradient establishes spontaneously within the condensate bulk connecting the solid-liquid and liquid-vapor interfaces. The range of the locally linear temperature profile widens with increasing thickness of the condensate bulk. During the widening, the local temperature at the solid-liquid interface gradually decreases approaching the surface temperature Ts while the local temperature at the liquid-vapor interface basically holds close to the vapor bulk temperature Tv. Note that the locally linear temperature profile fails to establish for β = 0.1 but behaves more regularly for higher β. Generally, the temperature gradient increases with increasing β but decreases with time. Both the density and temperature profiles suggest that the condensation intensity becomes higher with increasing β but lower with time.
. The transient density profiles (see Fig. 3a) show that on the surface with β = 0.1, where the fluid-solid interaction is very weak, the condensate bulk can hardly form. On the contrary, the condensate bulk is seen to form readily on the surface with β = 0.7, where the fluid-solid interaction is very strong, and to keep growing thicker with time, indicating a high condensation intensity. Generally, the condensate bulk grows faster for higher β but the growth decreases with time. The transient temperature profiles (see Fig. 3b) show that with the condensate bulk forming on surface, a local temperature gradient establishes spontaneously within the condensate bulk connecting the solid-liquid and liquid-vapor interfaces. The range of the locally linear temperature profile widens with increasing thickness of the condensate bulk. During the widening, the local temperature at the solid-liquid interface gradually decreases approaching the surface temperature Ts while the local temperature at the liquid-vapor interface basically holds close to the vapor bulk temperature Tv. Note that the locally linear temperature profile fails to establish for β = 0.1 but behaves more regularly for higher β. Generally, the temperature gradient increases with increasing β but decreases with time. Both the density and temperature profiles suggest that the condensation intensity becomes higher with increasing β but lower with time.
As shown in Fig. 4, the condensation heat flux (qc) originates from the vapor bulk distant from the condensing surface. With the vapor molecules approaching and condensing into the condensate bulk, the energy difference between the vapor and liquid states is released at the liquid-vapor interface. qc then flows through the condensate bulk and across the solid-liquid interface via pure heat conduction (see linear temperature profile in Fig. 3b). Each step comes with a thermal resistance against qc. Therefore, the overall thermal resistance (Rtotal) consists of the liquid-vapor interfacial thermal resistance (Rlv), the condensate bulk thermal resistance (Rl) and the solid-liquid interfacial thermal resistance (Rsl). Correspondingly, there are temperature drops from vapor to solid ΔT, namely, ΔTlv, ΔT1 and ΔTsl. Essentially, the characteristics of density and temperature profiles are closely related to the thermal resistances. The uniformity in both density and temperature profiles indicates the region of vapor bulk. The sudden drop in density profile corresponds to Rlv, the local linearity in density and temperature profiles corresponds to R1 and the local temperature jump corresponds to Rsl. For a given vapor-to-solid temperature difference (ΔT), qc is determined as:

Since qc holds through the thermal resistances, then we have

In this work, ΔT is calculated as:

and qc is calculated as:

where hv and h1 are the specific enthalpies in vapor and condensate bulks, respectively (see details in ref. 27);  is the condensed molecule flux, i.e. the time derivative of nc (the number of condensed molecules per unit area). In the simulations, we carefully monitor the time evolution of nc and find that nc perfectly follows a second-order exponential increasing function, as:
is the condensed molecule flux, i.e. the time derivative of nc (the number of condensed molecules per unit area). In the simulations, we carefully monitor the time evolution of nc and find that nc perfectly follows a second-order exponential increasing function, as:

where C0, C1, C2 and t1, t2 are all coefficients. For demonstration, the time evolutions of nc for the case of  and corresponding fitting curves are given in Fig. 5, from which we can clearly see that nc grows decreasingly with time, fitting the second-order exponential increasing function, and increases with increasing β.
and corresponding fitting curves are given in Fig. 5, from which we can clearly see that nc grows decreasingly with time, fitting the second-order exponential increasing function, and increases with increasing β.  is obtained by differentiation, as:
is obtained by differentiation, as:

where  (=
(= ) and
) and  (=
(= ) are coefficients. Apparently,
) are coefficients. Apparently,  follows a second-order exponential decreasing function. The time evolutions of nc and
follows a second-order exponential decreasing function. The time evolutions of nc and  are shown in the first and second panels of Fig. 6, from which we find that, for a same β, the condensation intensity increases with increasing ΔT as expected.
are shown in the first and second panels of Fig. 6, from which we find that, for a same β, the condensation intensity increases with increasing ΔT as expected.
The thickness of condensate bulk (δ) grows with condensation ongoing. For quantification, the liquid-side boundary of liquid-vapor interface is taken as the surface of the condensate bulk, which is the same as those in refs 28,29. We have tried both the density-based method30 and SRK equation-based method28 to determine the surface position and find that the results agree well with each other. The time evolutions of δ are shown in the third panel of Fig. 6.
Since qc can be calculated by Eq. (4) and ΔTlv, ΔTl and ΔTsl can be determined directly from the temperature profiles (see Fig. 3b), consequently, Rlv, Rl and Rsl are readily obtained based on Eq. (2). With condensation ongoing, Rl apparently increases with increasing δ, as shown in the lowermost panel of Fig. 6. By correlating R1 and nc to δ, we confirm  through that all data of R1 and nc fall well in linear functions of δ (see Fig. 7). It is implied that R1 and δ are also of the second-order exponential increasing functions of time as is nc. On the other hand, Rlv is supposed to be solely dependent on the thermodynamic state of fluid and
through that all data of R1 and nc fall well in linear functions of δ (see Fig. 7). It is implied that R1 and δ are also of the second-order exponential increasing functions of time as is nc. On the other hand, Rlv is supposed to be solely dependent on the thermodynamic state of fluid and  is known to be solely dependent on β. Therefore, for a given ΔT, the interfacial thermal resistances (
is known to be solely dependent on β. Therefore, for a given ΔT, the interfacial thermal resistances ( ) essentially remain constant. In the simulations, R1v is estimated to be
) essentially remain constant. In the simulations, R1v is estimated to be  for
for  and
and  for
for  while Rsl is estimated to be
while Rsl is estimated to be  for
for  and
and  for
for  . Clearly, Rlv is much smaller than
. Clearly, Rlv is much smaller than  . For demonstration, the time evolutions of Ri and Rl for
. For demonstration, the time evolutions of Ri and Rl for  and
and  are shown in Fig. 8. We can clearly see the competition between Ri (the time-independent component) and Rl (the time-dependent component) over time. At the initial period of time, Ri undoubtedly dominates in Rtotal and holds while with condensation ongoing Rl gradually increases, surpasses Ri and finally takes over the domination in Rtotal. Note that the condensation intensity is so low for β = 0.1 that Ri is not applicable and R1 keeps zero therefore these are not shown. By comparing Figs 6 and 8, we find that the moments Rl surpassing Ri are basically the inflection points in the time evolutions of
are shown in Fig. 8. We can clearly see the competition between Ri (the time-independent component) and Rl (the time-dependent component) over time. At the initial period of time, Ri undoubtedly dominates in Rtotal and holds while with condensation ongoing Rl gradually increases, surpasses Ri and finally takes over the domination in Rtotal. Note that the condensation intensity is so low for β = 0.1 that Ri is not applicable and R1 keeps zero therefore these are not shown. By comparing Figs 6 and 8, we find that the moments Rl surpassing Ri are basically the inflection points in the time evolutions of  . Apparently, the initially fast decreasing part of
. Apparently, the initially fast decreasing part of  is dominated by Ri while the afterwards slow decreasing part is dominated by Rl. Since Ri is due to inherent properties of solid and fluid, which cannot be changed during condensation, while Rl is linearly correlated to δ, which is artificially removable during condensation, it is concluded that the condensation could be effectively intensified by removing Rl out of Rtotal only when Rl has defeated Ri. This provide clear guidance on how to enhance condensation heat transfer using highly-customized nano-machining and nano-coating surfaces31,32,33.
is dominated by Ri while the afterwards slow decreasing part is dominated by Rl. Since Ri is due to inherent properties of solid and fluid, which cannot be changed during condensation, while Rl is linearly correlated to δ, which is artificially removable during condensation, it is concluded that the condensation could be effectively intensified by removing Rl out of Rtotal only when Rl has defeated Ri. This provide clear guidance on how to enhance condensation heat transfer using highly-customized nano-machining and nano-coating surfaces31,32,33.
Based on the above results that Rl grows as a second-order exponential increasing function of time while Ri is independent of time, we can finally achieve the following correlation from Eq. (1), as:

where  and
and  are coefficients. This indicates that qc is supposed to be an inversed second-order exponential decreasing function of time.
are coefficients. This indicates that qc is supposed to be an inversed second-order exponential decreasing function of time.
In addition, it is noteworthy that Rsl has been known to be correlated to β as a monotonically and nonlinearly decreasing function21,34,35. Our estimation of Rsl agrees well with the published data and we further successfully fit all the data-points into an exponential decreasing function (see Fig. 9).
Discussion
The simulation system in this work can be used to directly analogize FWC with one-dimensional characteristics but not DWC, with inherent three-dimensional characteristics. To reasonably carry out a theoretical analysis of thermal resistance between FWC and DWC, we propose a unified model, where the condensate bulk thermal resistance of DWC is made equivalent to that of FWC (see upper inset in Fig. 10). For the conductive heat transfer through the condensate bulk with a temperature difference ΔT1, the general equation has the form  . For FWC,
. For FWC,  therefore
therefore  (λ being the thermal conductivity of condensate bulk and taken to be constant for simplification). For DWC, we normally have
(λ being the thermal conductivity of condensate bulk and taken to be constant for simplification). For DWC, we normally have  , where
, where  ,
,  ,
,  and
and  are the condensation heat transfer rate, equivalent condensation heat flux, equivalent condensate bulk thermal resistance and equivalent heat transfer area, respectively. Based on the ideally spherical crown for a single droplet on solid surface (see lower inset in Fig. 10), the surfaces I and II are the isothermal surfaces and the distance (dε) between the two surfaces is the local thickness of the condensate bulk thermal resistance. The average value of dε is obtained as:
are the condensation heat transfer rate, equivalent condensation heat flux, equivalent condensate bulk thermal resistance and equivalent heat transfer area, respectively. Based on the ideally spherical crown for a single droplet on solid surface (see lower inset in Fig. 10), the surfaces I and II are the isothermal surfaces and the distance (dε) between the two surfaces is the local thickness of the condensate bulk thermal resistance. The average value of dε is obtained as:

where d is the diameter of the contact circle. Therefore,  is calculated by the integral mean value of A, as:
is calculated by the integral mean value of A, as:

where h is the height of the spherical crown. Note that  . On the other hand, for DWC, ΔT1 can be directly obtained by integrating the differential form of Fourier’s equation of heat conduction, as
. On the other hand, for DWC, ΔT1 can be directly obtained by integrating the differential form of Fourier’s equation of heat conduction, as

We now have the expression for  as
as

If we let  , i.e.
, i.e.  , and we define an equivalent factor f (=δ/h), then we have
, and we define an equivalent factor f (=δ/h), then we have

That means DWC with the height of h can be equivalently treated as FWC with the thickness of δ when Eq. (12) is satisfied. All the details of calculus are given in Supplementary Information. The curve of Eq. (12) is drawn in Fig. 10. We find that the curve starts from f = 0.5 at θ = 0° and then gradually increases with increasing θ (decreasing β) until it approaches around θ = 160°, after which it suddenly starts to grow increasingly towards infinity at θ = 180°. Note that f = 1 corresponds to θ ≈ 150°. This variation indicates that (1) the condensate bulk thermal resistance of a droplet with θ = 0° is equivalent to that of the condensate film with the thickness only half the height of the droplet (f = 0.5); (2) the condensate bulk thermal resistance of a droplet with θ = 180° is equivalent to that of the condensate film with infinite thickness (f → ∝); (3) the condensate bulk thermal resistance of a droplet with θ ≈ 150° is equivalent to that of the condensate film with the thickness being equal to the height of the droplet (f = 1); (4) the equivalent factor increases nonlinearly with increasing θ. Note that θ and β are not strictly linear to each other.
The curves of  ,
,  and
and  (see Fig. 11a) show that
(see Fig. 11a) show that  is solely dependent on δ while
is solely dependent on δ while  is dependent on both h and β.
is dependent on both h and β.  reduces decreasingly with increasing β and with an appreciable inflection point, which increases with increasing h. When h is small, e.g.
reduces decreasingly with increasing β and with an appreciable inflection point, which increases with increasing h. When h is small, e.g.  , there is a range of
, there is a range of  below Ri, e.g. β = 0.017∼0.3, meaning the latter overcomes the former. This range shrinks with increasing h. When h is large enough, e.g.
below Ri, e.g. β = 0.017∼0.3, meaning the latter overcomes the former. This range shrinks with increasing h. When h is large enough, e.g.  ,
,  is no longer below Ri at any value of β, meaning the former takes the absolute domination regardless of condensate shape or condensation mode. The curves of Rtotal (see Fig. 11b) basically shape the same way as in Fig. 11a because Rl and Ri constitute the overwhelming majority of Rtotal. Note that for FWC, Rtotal only includes Rsl, Rl and Rlv, while for DWC, a curvature-induced thermal resistance (Rcurv) exists and is estimated as
is no longer below Ri at any value of β, meaning the former takes the absolute domination regardless of condensate shape or condensation mode. The curves of Rtotal (see Fig. 11b) basically shape the same way as in Fig. 11a because Rl and Ri constitute the overwhelming majority of Rtotal. Note that for FWC, Rtotal only includes Rsl, Rl and Rlv, while for DWC, a curvature-induced thermal resistance (Rcurv) exists and is estimated as  , where ΔTcurv is calculated as36:
, where ΔTcurv is calculated as36:
Analytical results of surface condensation.
(a) Competition between interfacial thermal resistance (Ri) and condensate bulk thermal resistance (Rl) against fluid-solid bonding parameter (β) and θ; (b) Total thermal resistance (Rtotal) against β and θ; (c) Ratio of Rl to Rtotal against β and θ; (d) Transient variations of Rl and Ri against β and θ ( ).
).


where Tsat is the saturation temperature, σst is the surface tension at Tsat,  is the latent heat and ρ1 is the condensate bulk density. In this work, rmin is estimated to be
is the latent heat and ρ1 is the condensate bulk density. In this work, rmin is estimated to be  , which means that when
, which means that when  the relative influence of Rcurv is less than 8%. Therefore, the effect of Rcurv can be hardly felt in Fig. 11b. For proportional analysis, the curves of
the relative influence of Rcurv is less than 8%. Therefore, the effect of Rcurv can be hardly felt in Fig. 11b. For proportional analysis, the curves of  (
( for DWC and
for DWC and  for FWC) are shown in Fig. 11c. We can clearly see that there exists a minimum for all curves corresponding to a common critical value of
for FWC) are shown in Fig. 11c. We can clearly see that there exists a minimum for all curves corresponding to a common critical value of  (
( ). This critical point actually corresponds to f = 1 in Fig. 10 with small deviation caused by Rcurv. This means that for a given equivalent thickness to FWC, f = 1 guarantees the minimal Rl/Rtotal. The reason is two-sided: (1) When β > βcr (θ < θcr), f < 1 leads to decreasing Rl but Rtotal decreases due to more decreasing Ri (see Fig. 10) therefore the resultant Rl/Rtotal increases; (2) When
). This critical point actually corresponds to f = 1 in Fig. 10 with small deviation caused by Rcurv. This means that for a given equivalent thickness to FWC, f = 1 guarantees the minimal Rl/Rtotal. The reason is two-sided: (1) When β > βcr (θ < θcr), f < 1 leads to decreasing Rl but Rtotal decreases due to more decreasing Ri (see Fig. 10) therefore the resultant Rl/Rtotal increases; (2) When  (
( ), f > 1 leads to more increasing Rl though Rtoatl also increases due to increasing Ri (see Fig. 10) therefore the resultant Rl/Rtotal also increases. This point could shed some light on the design and optimization of superhydrophobic surfaces for DWC, where the so-called self-propelled droplet-jumping (removal of Rl) takes place15,37,38,39. Based on the above-acquired second-order exponential correlation of Rl against time, we show the competition between Rl and Ri over time in Fig. 11d. We find that for FWC, Rl can easily defeats Ri with time lapse while for DWC, the probability decreases with decreasing β (increasing θ).
), f > 1 leads to more increasing Rl though Rtoatl also increases due to increasing Ri (see Fig. 10) therefore the resultant Rl/Rtotal also increases. This point could shed some light on the design and optimization of superhydrophobic surfaces for DWC, where the so-called self-propelled droplet-jumping (removal of Rl) takes place15,37,38,39. Based on the above-acquired second-order exponential correlation of Rl against time, we show the competition between Rl and Ri over time in Fig. 11d. We find that for FWC, Rl can easily defeats Ri with time lapse while for DWC, the probability decreases with decreasing β (increasing θ).
To clearly illustrate our competition mechanism, a schematic presentation is given in Fig. 12. We show the typical evolutions of Rl and Ri, corresponding to DWC with θ > 90°, DWC with θ < 90° and FWC (θ = 0°), in the timeline. Generally, Rl grows decreasingly with time in a second-order exponential way while Ri generally keeps constant. We define the time when Rl surpasses Ri as the takeover time (tto) and use it as the threshold between the early and developed stages. For DWC with θ > 90°, the solid-fluid interaction is very weak (small β) leading to large Ri. On the other hand, weak solid-fluid interaction hinders the mass and heat transfer hence weakens the condensation intensity leading to slowly increasing Rl. The resultant tto is much delayed. For DWC with θ < 90°, the solid-fluid interaction is strong (large β) leading to small Ri. On the other hand, strong solid-fluid interaction enhances the mass and heat transfer hence strengthens the condensation intensity leading to quickly increasing Rl. The resultant tto is advanced. For FWC, the solid-fluid interaction is very strong (larger β) leading to tiny Ri. On the other hand, very strong solid-fluid interaction significantly strengthens the condensation intensity leading to more quickly increasing Rl. The resultant tto is extremely early. We find that the period of early stage is elongated with decreasing β. Note that β being extremely small (θ → 180°) suggests infinite period of early stage. Since heat transfer with fixed temperature difference can be enhanced by reducing the thermal resistance, we emphasize that with fixed ΔT only in the developed stage the condensate is removed can the condensation be effectively enhanced. Otherwise, the heat transfer is still dominated by Ri and the removal is supposed to be in vain.
We point out that this work is more focused on the early stage of surface condensation when Ri is yet comparable to Rl. As shown in Fig. 11a, given sufficiently long time, Rl will defeat all the other thermal resistances and eventually become dominant in Rtotal. This is the developed stage of surface condensation, where the conventional understanding becomes applicable (see Introduction). We also point out that Fig. 12 provides us a whole picture of the transitional roles of thermal resistances for surface condensation. This work, in line with our recent work on formation and transition mechanisms of condensation mode3, further bridges the early stage to developed stage of surface condensation in terms of time evolutions of thermal resistances.
In summary, we use MD simulation to investigate the characteristics of condensate growth in the early and developed stages of surface condensation. We divide the thermal resistances into interfacial and condensate bulk components. The interfacial thermal resistances solely depend on the properties of solid and fluid, which are time-independent. However, the condensate bulk thermal resistance and also the condensate thickness and the number of condensed molecules, which are time-dependent, are all second-order exponential increasing functions of time. The condensation heat flux is supposed to be an inversed second-order exponential decreasing function of time. With condensation ongoing, there exists an intrinsic competition between the interfacial and condensate bulk thermal resistances and the resultant total thermal resistance determines the condensation intensity under a given vapor-solid temperature difference. The competition mechanism works as that the interfacial thermal resistance dominates at the onset of condensation and holds afterwards while the condensate bulk thermal resistance gradually takes over with condensate thickness growing. The takeover time is longer for a smaller β. This competition mechanism suggests that only when the condensate bulk thermal resistance is removed after it takes over the domination can the condensation be effectively intensified. Otherwise, the effectiveness could be little. To fairly carry out the thermal resistance analysis, we propose a unified theoretical model by making DWC equivalent to FWC. The theoretical results indicate that near the critical point ( ,
,  ), the condensate bulk thermal resistance has the least opportunity to take over the domination while away from it the probability increases. By implementing the correlation of condensate bulk thermal resistance with time, we find that for FWC the condensate bulk thermal resistance can easily defeats the interfacial thermal resistance over time while for DWC the probability decreases with increasing contact angle, i.e. decreasing β. We reveal the transitional roles of thermal resistances over time in surface condensations, which provides full understanding of the insight of the surface condensation for different modes over a whole range of conditions and bridges the early stage to developed stage of surface condensation.
), the condensate bulk thermal resistance has the least opportunity to take over the domination while away from it the probability increases. By implementing the correlation of condensate bulk thermal resistance with time, we find that for FWC the condensate bulk thermal resistance can easily defeats the interfacial thermal resistance over time while for DWC the probability decreases with increasing contact angle, i.e. decreasing β. We reveal the transitional roles of thermal resistances over time in surface condensations, which provides full understanding of the insight of the surface condensation for different modes over a whole range of conditions and bridges the early stage to developed stage of surface condensation.
Methods
The fluid-fluid interaction is governed by the Lennard-Jones (L-J) potential function, as:

where r is the intermolecular separation, σ and ε are the length and energy scales, respectively40. The function is truncated by the cut-off radius  , beyond which the interactions are not considered. The fluid-solid interaction is also described by the L-J potential function with length scale
, beyond which the interactions are not considered. The fluid-solid interaction is also described by the L-J potential function with length scale  and energy scale
and energy scale  , where the parameter β measures the relative strength of fluid-solid bonding.
, where the parameter β measures the relative strength of fluid-solid bonding.
The solid wall, i.e. the condensing surface, is set at the bottom of the simulation box and represented by three layers of solid molecules forming a (111) plane of a face-centered cubic lattice with the lattice constant  . Neighboring solid molecules are connected by Hookean springs with the constant
. Neighboring solid molecules are connected by Hookean springs with the constant  41. Two extra layers of solid molecules are set below the three layers. The lower is stationary as a frame while the upper is governed by the Langevin thermostat, as:
41. Two extra layers of solid molecules are set below the three layers. The lower is stationary as a frame while the upper is governed by the Langevin thermostat, as:

where pi is the momentum of the ith solid molecule;  is the damping constant42, fi is the sum of the forces acting on the ith solid molecule, Fi is a random force, of which each component is sampled from the Gaussian distribution with zero mean value and variance
is the damping constant42, fi is the sum of the forces acting on the ith solid molecule, Fi is a random force, of which each component is sampled from the Gaussian distribution with zero mean value and variance  (
( is the time step, where
is the time step, where  is the time scale, m being the mass of a fluid molecule)41,42. The periodic boundary condition is applied at the sides and the diffuse reflection boundary is applied at the top end.
is the time scale, m being the mass of a fluid molecule)41,42. The periodic boundary condition is applied at the sides and the diffuse reflection boundary is applied at the top end.
In each run, a relaxation period of 200 τ is used to keep the vapor saturated at  , followed by the condensation period of
, followed by the condensation period of  with the solid temperature at a value in the range of
with the solid temperature at a value in the range of  and the saturated vapor temperature at
and the saturated vapor temperature at  , respectively. Extra vapor molecules are supplied through the upmost supply region (thickness lz/10) by the USHER algorithm43 immediately when the density within the supply region is lower than its initial saturation value. During the condensation period, the temperature in the supply region is controlled at
, respectively. Extra vapor molecules are supplied through the upmost supply region (thickness lz/10) by the USHER algorithm43 immediately when the density within the supply region is lower than its initial saturation value. During the condensation period, the temperature in the supply region is controlled at  by the Langevin thermostat20,44. The surface wettability of solid wall changes from hydrophobicity to hydrophilicity by varying β from 0.1 to 0.7.
by the Langevin thermostat20,44. The surface wettability of solid wall changes from hydrophobicity to hydrophilicity by varying β from 0.1 to 0.7.
Additional Information
How to cite this article: Sun, J. and Wang, H. S. On the early and developed stages of surface condensation: competition mechanism between interfacial and condensate bulk thermal resistances. Sci. Rep. 6, 35003; doi: 10.1038/srep35003 (2016).
References
Rose, J. W. Dropwise condensation theory and experiment: a review. Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy 216, 115–128 (2002).
Schmidt, E., Schurig, W. & Sellschopp, W. Versuche über die Kondensation von Wasserdampf in Film- und Tropfenform. Forsch. Ing. 1, 53–63 (1930).
Sheng, Q., Sun, J., Wang, Q., Wang, W. & Wang, H. S. On the onset of surface condensation: Formation and transition mechanisms of condensation modes. Sci Rep 6, 30764 (2016).
Cheng, Y. P., Xu, J. L. & Sui, Y. Numerical investigation of coalescence-induced droplet jumping on superhydrophobic surfaces for efficient dropwise condensation heat transfer. Int J Heat Mass Tran 95, 506–516 (2016).
Nam, Y., Kim, H. & Shin, S. Energy and hydrodynamic analyses of coalescence-induced jumping droplets. Appl Phys Lett 103 (2013).
Sun, J., Wang, W. & Wang, H. S. Dependence between velocity slip and temperature jump in shear flows. J Chem Phys 138, 234703 (2013).
Sun, J., Wang, W. & Wang, H. S. Dependence of nanoconfined liquid behavior on boundary and bulk factors. Phys Rev E 87, 023020 (2013).
Narhe, R. D., Khandkar, M. D., Shelke, P. B., Limaye, A. V. & Beysens, D. A. Condensation-induced jumping water drops. Phys Rev E 80 (2009).
Chen, L., Yu, J. P. & Wang, H. Convex nanobending at a moving contact line: the missing mesoscopic link in dynamic wetting. ACS Nano 8, 11493–11498 (2014).
Yu, J. P., Wang, H. & Liu, X. Direct measurement of macro contact angles through atomic force microscopy. Int J Heat Mass Tran 57, 299–303 (2013).
Magnussen, O. M. et al. X-ray reflectivity measurements of surface layering in liquid mercury. Phys Rev Lett 74, 4444–4447 (1995).
Regan, M. J. et al. Surface layering in liquid gallium - an X-ray reflectivity study. Phys Rev Lett 75, 2498–2501 (1995).
Huisman, W. J. et al. Layering of a liquid metal in contact with a hard wall. Nature 390, 379–381 (1997).
Li, X., Mao, L. & Ma, X. Dynamic behavior of water droplet impact on microtextured surfaces: the effect of geometrical parameters on anisotropic wetting and the maximum spreading diameter. Langmuir 29, 1129–1138 (2013).
Miljkovic, N. et al. Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano Lett 13, 179–187 (2013).
Boreyko, J. B., Zhao, Y. & Chen, C.-H. Planar jumping-drop thermal diodes. Appl Phys Lett 99 (2011).
Niu, D. & Tang, G. H. The effect of surface wettability on water vapor condensation in nanoscale. Sci Rep 6, 19192 (2016).
Enright, R. et al. How coalescing droplets jump. ACS Nano 8, 10352–10362 (2014).
Thompson, P. A. & Robbins, M. O. Origin of stick-slip motion in boundary lubrication. Science 250, 792–794 (1990).
Thompson, P. A. & Troian, S. M. A general boundary condition for liquid flow at solid surfaces. Nature (London) 389, 360–362 (1997).
Xue, L., Keblinski, P., Phillpot, S. R., Choi, S. U. S. & Eastman, J. A. Two regimes of thermal resistance at a liquid-solid interface. J Chem Phys 118, 337–339 (2003).
Dammer, S. M. & Lohse, D. Gas enrichment at liquid-wall interfaces. Phys Rev Lett 96 (2006).
Liu, C. & Li, Z. Molecular dynamics simulation of composite nanochannels as nanopumps driven by symmetric temperature gradients. Phys Rev Lett 105, 174501 (2010).
Shirtcliffe, N. J., McHale, G., Atherton, S. & Newton, M. I. An introduction to superhydrophobicity. Adv Colloid Interface 161, 124–138 (2010).
Drelich, J., Chibowski, E., Meng, D. D. & Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 7, 9804–9828 (2011).
Atefi, E., Mann, J. A. & Tavana, H. A robust polynomial fitting approach for contact angle measurements. Langmuir 29, 5677–5688 (2013).
Sun, J., He, Y. L. & Tao, W. Q. Molecular dynamics-continuum hybrid simulation for condensation of gas flow in a microchannel. Microfluid Nanofluid 7, 407–422 (2009).
Meland, R., Frezzotti, A., Ytrehus, T. & Hafskjold, B. Nonequilibrium molecular-dynamics simulation of net evaporation and net condensation, and evaluation of the gas-kinetic boundary condition at the interphase. Phys Fluids 16, 223–243 (2004).
Meland, R. Molecular exchange and its influence on the condensation coefficient. J Chem Phys 117, 7254–7258 (2002).
Wang, Z. J., Chen, M. & Guo, Z. Y. A molecular study on the liquid-vapor interphase transport. Microscale Therm Eng 7, 275–289 (2003).
Enright, R., Miljkovic, N., Alvarado, J. L., Kim, K. & Rose, J. W. Dropwise condensation on micro- and nanostructured surfaces. Nanosc Microsc Therm 18, 223–250 (2014).
Rykaczewski, K. et al. Dropwise condensation of low surface tension fluids on omniphobic surfaces. Sci Rep 4 (2014).
Das, A. K., Kilty, H. P., Marto, P. J., Andeen, G. B. & Kumar, A. The use of an organic self-assembled monolayer coating to promote dropwise condensation of steam on horizontal tubes. J Heat Trans-T ASME 122, 278–286 (2000).
Maruyama, S. & Kimura, T. A study on thermal resistance over a solid-liquid interface by the molecular dynamics method. Therm Sci Eng 7, 63–68 (1999).
Wang, C. S., Chen, J. S., Shiomi, J. & Maruyama, S. A study on the thermal resistance over solid-liquid-vapor interfaces in a finite-space by a molecular dynamics method. Int J Therm Sci 46, 1203–1210 (2007).
Le Fevre, E. J. & Rose, J. W. In Proceedings of the 3rd International Heat Transfer Conference Vol. 2 362–375 (AIChE, Chicago, USA, 1966).
Chen, C.-H. et al. Dropwise condensation on superhydrophobic surfaces with two-tier roughness. Appl Phys Lett 90 (2007).
Boreyko, J. B. & Chen, C.-H. Self-propelled dropwise condensate on superhydrophobic surfaces. Phys Rev Lett 103 (2009).
Boreyko, J. B. & Chen, C.-H. Self-propelled jumping drops on superhydrophobic surfaces. Phys Fluids 22 (2010).
Allen, M. P. & Tildesley, D. J. Computer simulation of liquids. (Clarendon Press, 1987).
Yi, P., Poulikakos, D., Walther, J. & Yadigaroglu, G. Molecular dynamics simulation of vaporization of an ultra-thin liquid argon layer on a surface. Int J Heat Mass Tran 45, 2087–2100 (2002).
Maruyama, S. In Advances in numerical heat transfer vol. 2 (eds W. J. Minkowycz & E. M. Sparrow ) Ch. 6, 189–226 (Tayler & Francis, 2000).
Delgado-Buscalioni, R. & Coveney, P. V. USHER: an algorithm for particle insertion in dense fluids. J Chem Phys 119, 978–987 (2003).
Thompson, P. A. & Robbins, M. O. Shear flow near solids: Expitaxial order and flow boundary conditions. Phys Rev A 41, 6830–6837 (1990).
Acknowledgements
Financial supports from the National Natural Science Foundation of China (51406205), the Beijing Natural Science Foundation (3142021) and the Engineering and Physics Science Research Council (EPSRC) of the UK (EP/L001233/1) are acknowledged.
Author information
Authors and Affiliations
Contributions
J.S. developed the code, performed the simulations, prepared the manuscript, analyzed the results and reviewed the manuscript. H.S.W. analyzed the results and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, J., Wang, H. On the early and developed stages of surface condensation: competition mechanism between interfacial and condensate bulk thermal resistances. Sci Rep 6, 35003 (2016). https://doi.org/10.1038/srep35003
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35003
- Springer Nature Limited


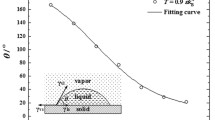



 . The simulations are carried out in thermodynamic equilibrium state at
. The simulations are carried out in thermodynamic equilibrium state at  and
and  with
with  . The insets are for
. The insets are for  .
.
 ).
).

 ).
).
 ), thickness of condensate (δ) and condensate bulk thermal resistance (Rl) in the condensation period.
), thickness of condensate (δ) and condensate bulk thermal resistance (Rl) in the condensation period.


 ) with fluid-solid bonding parameter (β).
) with fluid-solid bonding parameter (β).

