Abstract
Neoadjuvant Chemotherapy has been used for the stage III of non-small cell lung cancer (NSCLC) and has shown good clinical effects. However, the survival benefits of radiation therapy added in induction regimens remains controversial. We therefore conducted a meta-analysis of the published clinical trials to quantitatively evaluate the benefit of preoperative chemoradiotherapy. After searching the database of Pubmed, CNKI, EMBASE, ESMO, The Cochrane Library databases, The American Society of Clinical Oncology and Clinical Trials.gov. Trials were selected for meta-analysis if they provided an independent assessment of neoadjuvant chemoradiation and neoadjuvant chemotherapy, odds ratio(OR) for tumor downstaging, mediastinal lymph nodes pathological complete response and local control, hazard ratios (HRs) for 5-year survival and progression-free survival were pooled by the stata software version 12.0. Twelve studies involving 2,724 patients were identified, tumor downstaging (p = 0.01), mediastinal lymph nodes pathological complete responses (p = 0.028) and local control (P = 0.002) were achieved, when compared with neoadjuvant chemotherapy. The meta-analysis demonstrated neither 5-year survival nor progression-free-survival benefit in survival from adding radiation. In conclusion, the addition of radiotherapy into chemotherapy was not superior to neoadjuvant chemotherapy. The higher quality of trials need be investigated combining with the histopathological type and genotyping of lung cancer by clinicians.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related death in the world and non-small cell lung cancers (NSCLC) comprise more than 75% of all lung cancers. Approximately one-third of patients with non-small cell lung cancer (NSCLC) are diagnosed with locally advanced (stage III) disease1. The vast majority of patients with resectable N2 (ipsilateral lymph node involvement), some patients with N3 (contralateral mediastinal and upraclavicular lymph node involvement) NSCLC are offered surgery2, but survival remains disappointingly low even after complete resection. As part of a multimodality therapeutic approach, preoperative induction chemotherapy has been shown to eradication of distant micrometastases early and improve the survival as compared to resection alone3.
Phase II data from studies suggest that neoadjuvant chemoradiotherapy are active and well tolerated for patients with good performance status4. When compared to induction chemotherapy, whether neoadjuvant chemoradiationtherapy would confer a survival benefit had not been clearly demonstrated by inconsistent results of Phase III studies.
Shah and colleagues5 reported a meta-analysis comparing neoadjuvant chemoradiation therapy with chemotherapy alone for potentially operable stage IIIA NSCLC and found no benefit to neoadjuvant chemoradiation therapy over chemotherapy alone with respect to overall survival. Limited data including 1 randomized trials6 and 1 phase II trial7 are statistically integrated and analyzed in the meta-analysis, significant biases inherent in 2 retrospective studies also decreased the power of meta-analysis. After the literature search completion date (December 2010) of the report by Shah et al., new randomized trials displayed a different trend toward survival with chemoradiation were published recently; new clinical trials enrolled a substantial proportion of Stage IIIB patients with a high disease burden by improvement in radiotherapy (RT) technology. We perform a systematic review and meta-analysis again to ascertain whether the addition of preoperative radiotherapy to chemotherapy would improve survival outcome for NSCLC patients with stage III.
Materials and Methods
Eligibility criteria for meta-analysis
Randomized, non randomized and retrospective studies containing potentially operable patients with stage III NSCLC receiving induction chemotherapy and induction chemoradiotherapy were eligible for review. The following criteria for eligibility into this meta-analysis were set before collecting the articles. Odds ratio(OR) and confidence intervals (CIs) of the patients could be calculated at specific time intervals after surgery for tumor downstaging, mediastinal lymph nodes pathological complete response and local control, also the hazard ratios (HRs) and confidence intervals (CIs) for 5-year survival and progression-free survival (PFS) in the article. The articles were published in English between January 1990 and October 2015.
Collection of published studies
PubMed, Embase, the Cochrane Library (Issue 4, 2007) Databases and the American Society of Clinical Oncology (2002–2015) online conference proceedings were searched in October 2015, Clinical Trials.gov (http://clinical trials.gov) were searched to identify ongoing studies. Relevant articles and abstracts were selected and reviewed by two reviewers, and reference lists were searched for additional trials.
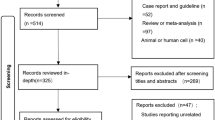
The keywords “non-small cell lung cancer or carcinoma, non-small cell lung or NSCLC” and “induction therapy or chemotherapy or chemoradiotherapy” and “resection or surgery”, hit 2045 citations, the relevant clinical studies were manually selected based on titles and summary analyses. Articles reporting studies unrelated to our question were excluded, and finally only twelve studies (two abstract) were found to fulfill all of our eligibility criteria (Fig. 1).
Statistical analysis
The Stata software version 12.0 (Stata Corporation, College Station, TX, USA) was used to carry out the meta-analysis. HR and OR with 95% CI was used to combine the data. When these statistical variables were not provided, they were calculated from available numerical data or Kaplan–Meier survival curve8. This assumption was tested by performing Chi-squared Q-tests for heterogeneity. A P-value greater than 0.05 for the Q-test indicated lack of heterogeneity among studies, so the fixed-effects model was used for meta-analysis. Otherwise, Dersimonian –Laird random-effect method was used9,10. We also quantified the effect of heterogeneity using I2 statistic which measured the degree of heterogeneity.I2 value ranges from 0% to 100%.
Both Begg’s funnel plot and Egger’s test were performed to assess the publication bias. A sensitivity analysis, in which one study was removed at a time, was performed to evaluate result stability.
Results
We identified 2045 abstracts of which 16 were further assessed for eligibility by full text, After excluding 4 repeated reports, a total of 12 studies involving 2724 patients served as data sources for the present meta-analysis6,7,10,11,12,13,14,15,16,17,18,19, including 8 randomized control trial, 4 retrospective studies (Table 1). The PRISMA flow Diagram for the selection and inclusion of studies is presented in Fig. 1. The main characteristics of the eligible publications are reported in Table 2.
2 studies demonstrated a survival benefit to adding induction radiation to induction chemotherapy versus induction chemotherapy alone, 7 studies did not support it, and 3 studies had no data. In the aspect of disease-free survival, 3 studies indicated that induction chemoradiotherapy were superior to induction chemotherapy, 5 studies supported not, and 4 studies have no data. Subgroup analysis was based on study design, 2 published abstracts and 2 retrospective reviews were unable to be incorporated into the meta-analysis due to lack of available data.
We can extract the OR and 95%CI through the existing data in 6 randomized controlled trials of 12 studies, the meta-analysis demonstrated induction chemoradiation have benefit in tumor downstaging (OR = 0.75, p = 0.001) and mediastinal lymph nodes pathological complete response (OR = 0.72, p = 0.001) compared with induction chemotherapy (Fig. 2). OR and 95% CI are extracted through the existing data in 5 randomized controlled trials of 12 studies, induction chemoradiation also have benefit in local control (OR = 0.64, p = 0.002) (Fig. 3).
As assessed for hazard ratio of 5-year survival and progression-free survival, the useful data for calculation were obtained directly from the original articles or had to be extrapolated from Kaplan–Meier survival curve. 2 published abstracts and 2 retrospective reviews were unable to be incorporated into the meta-analysis due to lack of available data. Trials by Girard et al. and Pless et al. were exclude, as Girard et al. only provided 3-year survival date and sequential chemo-radiation was administered in trial conducted by Pless et al.
We conducted a meta analysis of the above 4 randomized controlled trials that administered concurrent chemoradiation using a random effect model. The forest Figure shows no benefit to induction chemoradiotherapy versus induction chemotherapy alone in 5-year OS (HR = 0.89, P = 0.44) nor in PFS (HR = 0.74, P = 0.26) (Fig. 4); Using a fixed effects model for 2 retrospective reviews (I2 = 0%), The meta analysis demonstrated no statistically significant benefit to the addition of radiation to induction chemotherapy versus induction chemotherapy alone in OS (HR = 0.77, P = 0.24) nor PFS (HR = 0.73, P = 0.20) (Fig. 5).
There was no indication of publication bias from either Egger’s or Begg’s tests in 5-year survival (Begg p = 0.308; Egger p = 0.267) nor progression-free survival (Begg p = 0.296; Egger p = 0.331). A sensitivity analysis, in which one study was removed at a time, was performed to evaluate result stability. The corresponding pooled OR and HRs were not significantly altered, suggesting stability of our result.
Discussion
Multimodality therapy is preferable in most subsets of patients with stage III lung cancer. This heterogeneous group of patients can be treated with surgery, chemotherapy, radiation, or both20. For individuals with good performance scores, neoadjuvant therapy, followed by surgery, was commonly offered treatment strategy.
Neoadjuvant therapy including induction chemoradiotherapy is feasibly and effective in increasing the resectability of the tumor by decreasing its size21,22, some studies demonstrated that the addition of radiotherapy to induction chemotherapy improve overall survival compared with induction chemotherapy alone in stage III NSCLC patients, however, other studies did not showed a survival benefit, nor does the result of recent meta-analysis support the findings5, which limits the strength of recommendations. We attempted to evaluate and synthesize the available data to provide clinicians with summarized evidence-based information to guide them in taking care of patients with stage III disease.
Induction chemoradiotherapy was well tolerated by stage IIIA or IIIB individuals with good performance scores. Most trials included in the meta-analysis have not observed a difference in surgical complications between the two preoperative regimens. The final result in our meta-analysis shows a benefit of neoadjuvant chemoradiation to the patients with stage III non-small-cell lung cancer in local control, tumor downstaging and mediastinal lymph nodes pathological complete response (pCR). However, the addition of radiotherapy into chemotherapy was not superior to neoadjuvant chemotherapy alone in terms of progression-free survival and 5-year survival.
Induction chemoradiotherapy has multiple potential advantages over chemotherapy alone. The published results showed that the addition of radiation to the preoperative regimen increased the local control rate, which is an important aim of induction treatment23.
Although complete resection shown to be a major prognostic factor for survival in multiple studies. The high down-staging rate, pathological complete response and the absence of treatment-related death in CRS arm did not translated into a longer PFS and OS of patients with stage III NSCLC who underwent pulmonary resection, numerous limitations of these trials may hinder a fair assessment of the role of radiation therapy in the induction phase of treatment. Several trials were small and clearly underpowered to detect meaningful differences in outcomes. Phase II trials of Higgins14 and Pezzetta13 had the limitations as any retrospective analysis. Some of the randomized trials utilized different chemotherapy regimens between the two treatment arms, chemotherapy regiment directly affect pre-existing micrometastases and induces high rates of response for potentially resectable disease. The complicated treatment of patients with postoperative recurrence and metastasis may also affect the result.
The addition of radiotherapy may be not worthwhile in order to improve over survival. Evidence available for meta-analysis were not in favour of it. For patients with good performance status and advanced local disease requiring maximal shrinkage to facilitate complete resection, we recommend considering radiotherapy in combination with chemotherapy in the induction phase of treatment. However, the best therapeutic plan should be achieved through the multidisciplinary cooperation of a team specialized in lung cancer.
Future trials are needed to investigate the roles of individualized chemotherapy and surgery in particular cohorts or settings24, and it would be ideal utilize an identical chemotherapy regimen in each treatment. The increasing availability of antibody therapy and tyrosine kinase inhibitors for the treatment of lung cancer might offer new options25, Future trials will evaluate the role of targeted therapies in this setting to increase systemic control while decreasing hematological toxicity rates of combined treatment.
Unanswered questions remain about definitive chemoradiotherapy, including the optimal chemotherapy agents; dose, duration, density of chemotherapy26, radiation fractionation and radiation dose27,28,29,30. However, we were not sure tumor cells would be more sensitive to radiotherapy or chemotherapy when diagnosed, especially for squamous cell carcinoma patients31, best modality for assessing response in advanced disease utilizing 18F-FDG PET/CT has been testing32, studies on identifying markers to predict the response to CRT should be pursued33,34, moreover, strategies to select patients for the most appropriate therapy according to the molecular profile of individual tumors could contribute to further improvements in treatment outcome. In conclusion, clinicians needed to investigate the higher quality of trials combined with the histopathological type and genotyping of lung cancer, and probe the best method of treatment for resectable Stage III Non-Small-Cell Lung Cancer.
Additional Information
How to cite this article: Guo, S. x. et al. Neoadjuvant Chemoradiotherapy vesus Chemotherapy alone Followed by Surgery for Resectable Stage III Non-Small-Cell Lung Cancer: a Meta-Analysis. Sci. Rep. 6, 34388; doi: 10.1038/srep34388 (2016).
References
Yang, P. et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 128, 452–462 (2005).
Riquet, M. et al. Long-term survival with surgery as part of a multimodality approach for N3 lung cancer. Eur J Cardiothorac Surg 44, 1117 (2013).
Nakamura, H., Kawasaki, N., Taguchi, M. & Kabasawa, K. Role of preoperative chemotherapy for non-small-cell lung cancer: a meta-analysis. Lung Cancer 54, 325–329 (2006).
Seder, C. W. et al. Stage IIIA Non-Small Cell Lung Cancer: Morbidity and Mortality of Three Distinct Multimodality Regimens. Ann Thorac Surg 95, 1708–1716 (2013).
Shah, A. A. et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 93, 1807–1812 (2012).
Thomas, M. et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 9, 636–648 (2008).
Girard, N. et al. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 69, 86–93 (2010).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17, 2815–2834 (1998).
Bohning, D. et al. Some general points in estimating heterogeneity variance with the DerSimonian-Laird estimator. Biostatistics 3, 445–457 (2002).
Toyooka, S. et al. Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 15, 954–960 (2012).
Fleck, J., Carmargo, J. & Godoy, D. Chemoradiation therapy(CRT) versus chemotherapy (CT) alone as a neoadjuvant treatment for stage III non-small cell lung cancer (NSCLC). Preliminary report of a phase III prospective randomized trial. Proc Am Soc Clin Oncol 12, 333 (1993).
Sauvaget, J., Rebischung, J. & Vannetzel, J. & al., e. Phase III study of neo-adjuvant MVP versus MVP plus chemo radiation in stage III NSCLC. Proc Am Soc Clin Oncol 19, 495A (2000).
Pezzetta, E. et al. Comparison of neoadjuvant cisplatin-based chemotherapy versus radiochemotherapy followed by resection for stage III (N2) NSCLC. Eur J Cardiothorac Surg 27, 1092–1098 (2005).
Higgins, K. et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 75, 1462–1467 (2009).
Li, J. et al. Prognostic factors and long term results of neo adjuvant therapy followed by surgery in stage IIIA N2 non-small cell lung cancer patients. Ann Thorac Med 4, 201–207 (2009).
Katakami, N. et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 118, 6126–6135 (2012).
Yang, H., Yao, F., Zhao, Y. & Zhao, H. Clinical outcomes of surgery after induction treatment in patients with pathologically proven N2-positive stage III non-small cell lung cancer. J Thorac Dis 7, 1616–1623 (2015).
Yang, C. F. et al. Adding radiation to induction chemotherapy does not improve survival of patients with operable clinical N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 150, 1484–1493 (2015).
Pless, M. et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 386, 1049–1056 (2015).
Ramnath, N. et al. Treatment of Stage III Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 143, e314S–e3140 (2013).
Uramoto, H. et al. The long-term outcomes of induction chemoradiotherapy followed by surgery for locally advanced non-small cell lung cancer. Case Rep Oncol 7, 700–710 (2014).
Kawaguchi, K. et al. Trimodality therapy for lung cancer with chest wall invasion: initial results of a phase II study. Ann Thorac Surg 98, 1184–1191 (2014).
Pottgen, C. et al. Prognostic model for long-term survival of locally advanced non-small-cell lung cancer patients after neoadjuvant radiochemotherapy and resection integrating clinical and histopathologic factors. BMC Cancer 15, 363 (2015).
Shien, K. et al. Lower lobe origin is a poor prognostic factor in locally advanced non-small-cell lung cancer patients treated with induction chemoradiotherapy. Mol Clin Oncol 3, 706–712 (2015).
Hotta, K. et al. Gefitinib Combined With Standard Chemoradiotherapy in EGFR-Mutant Locally Advanced Non-Small-Cell Lung Cancer: The LOGIK0902/OLCSG0905 Intergroup Study Protocol. Clin Lung Cancer 17, 75–79 (2016).
Singhal, N. et al. Oral vinorelbine and cisplatin with concomitant radiotherapy in stage III non-small-cell lung cancer: an open-label phase II multicentre trial (COVeRT study). Anticancer Drugs 26, 1083–1088 (2015).
Bharadwaj, S. C. et al. Higher Versus Standard Preoperative Radiation in the Trimodality Treatment of Stage IIIa Lung Cancer. Ann Thorac Surg 100, 207–213 discussion 213-4 (2015).
Cheung, P. et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst 106, 164 (2014).
Maguire, J. et al. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur J Cancer 50, 2939–2949 (2014).
Sher, D. J., Fidler, M. J., Seder, C. W., Liptay, M. J. & Koshy, M. Relationship Between Radiation Therapy Dose and Outcome in Patients Treated With Neoadjuvant Chemoradiation Therapy and Surgery for Stage IIIA Non-Small Cell Lung Cancer: A Population-Based, Comparative Effectiveness Analysis. Int J Radiat Oncol Biol Phys 92, 307–316 (2015).
Brodin, O. et al. Comparison of induction chemotherapy before radiotherapy with radiotherapy only in patients with locally advanced squamous cell carcinoma of the lung. The Swedish Lung Cancer Study Group. Eur J Cancer 32a, 1893–1900 (1996).
Chaft, J. E. et al. Adaptive neoadjuvant chemotherapy guided by F-FDG-PET in resectable non-small-cell lung cancers: the NEOSCAN trial. J Thorac Oncol 12, 104 (2015).
Walker, M. J. et al. Discovery and Validation of Predictive Biomarkers of Survival for Non-small Cell Lung Cancer Patients Undergoing Radical Radiotherapy: Two Proteins With Predictive Value. EBioMedicine 2, 839–848 (2015).
Han, S. et al. Analysis of Clinical and Dosimetric Factors Influencing Radiation-Induced Lung Injury in Patients with Lung Cancer. J Cancer 6, 1172–1178 (2015).
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (No. 81660395).
Author information
Authors and Affiliations
Contributions
S.x.G., Y.J. and F.f.T. conceived the study design, S.x.G., Y.J. and Y.l.C. performed the analyses. S.x.G, Y.J., Y.C. and Q.y.Z.: Statistical analyses and paper writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, S., Jian, Y., Chen, Y. et al. Neoadjuvant Chemoradiotherapy vesus Chemotherapy alone Followed by Surgery for Resectable Stage III Non-Small-Cell Lung Cancer: a Meta-Analysis. Sci Rep 6, 34388 (2016). https://doi.org/10.1038/srep34388
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34388
- Springer Nature Limited