Abstract
A series of color tunable phosphors NaBa3La3Si6O20:Ce3+, Tb3+ were synthesized via the high-temperature solid-state method. NaBa3La3Si6O20 crystallizes in noncentrosymmetric space group Ama2 with the cell parameters of a = 14.9226(4) Å, b = 24.5215(5) Å and c = 5.6241(2) Å by the Rietveld refinement method. The Ce3+ ions doped NaBa3La3Si6O20 phosphors have a strong absorption band from 260 to 360 nm and show near ultraviolet emission light centered at 378 nm. The Ce3+ and Tb3+ ions coactivated phosphors exhibit color tunable emission light from deep blue to green by adjusting the concentration of the Tb3+ ions. An energy transfer of Ce3+ → Tb3+ investigated by the photoluminescence properties and lifetime decay, is demonstrated to be dipole–quadrupole interaction. These results indicate the NaBa3La3Si6O20:Ce3+, Tb3+ phosphors can be considered as potential candidates for blue-green components for white light emitting diodes.
Similar content being viewed by others
Introduction
By virtue of the special merits of high brightness, energy-efficient, life-durable, and environmentally friendly, the white light emitting diodes (w-LEDs) made from blue or near-ultraviolet (n-UV) emitting LEDs chips coated with phosphors have the potential to overtake incandescent and fluorescent lighting types1,2,3,4. In 1996, the w-LEDs fabricated from the blue-emitting InGaN LED chips combined with the yellow-emitting phosphors (YAG:Ce3+) were commercialized5. Unfortunately, this technology has the following problems: low color rendering index due to two-color mixing, and low color reproducibility due to the strong dependence of white color purity on the quality of phosphors6,7,8,9,10,11. To solve these problems, the w-LEDs had been fabricated employing blue, green and red emitting phosphors excited by a blue or n-UV chip12,13,14. However, the strong reabsorption of blue light by red and green phosphors reduces the luminescence efficiency in this system15,16. To overcome these disadvantages, vigorous attentions were received to exploit the emission-tunable phosphors with strong absorption in n-UV region17,18. Simultaneously, an energy transfer can obviously improve the luminescent efficiency and color reproducibility as well as widen the emission spectra of phosphors.
After years of efforts, a series of promising phosphors had been developed, such as fluorides19, silicates20,21, phosphates12, orthovanadates22,23, borates24,25, tungstates/molybdates26,27, nitrides28,29, aluminates30,31,32, etc. Among them, the silicate compounds as luminescent hosts were intensively studied because of their remarkable stability of physical and chemical properties, flexible crystal structures and relatively easy preparation process. Among rare earth ions, the Tb3+ ion is the best candidate for green component due to its predominant 5D4–7F5 transitions peaking at around 545 nm33. However, the electric dipole transitions within the 4f configurations of the Tb3+ ion is spin forbidden, resulting in the weak intensity of its absorption in the n-UV region and the narrow width. Thus, a suitable sensitizer is always necessary for the phosphors activated by the Tb3+ ion. It is well known that the Ce3+ ion is an excellent sensitizer transferring a part of its energy to an activator such as the Tb3+ ion depending on its lowest 5d state and broad absorption and emission bands from the allowed 4f → 5d transitions34. In this work, we reported a novel silicate host phosphor, NaBa3La3Si6O20:Ce3+, Tb3+ for the excitation by a n-UV LED chip, and the crystal structure, luminescent properties and energy transfer mechanism between the Ce3+ ion and the Tb3+ ion had been thoroughly investigated.
Results and Discussion
Crystal structure and phase formation
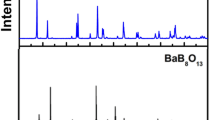
Figure 1(a) demonstrates the observed and calculated XRD patterns as well as their difference for the Rietveld refinement of NaBa3La3Si6O20. In the refinement, an initial structure model and atomic positions of NaBa3Eu3Si6O20 were adopted for the structure refinement35,36. NaBa3La3Si6O20 crystallizes in the noncentrosymmetric space group Ama2 and unit cell parameters are obtained as a = 14.9226(4) Å, b = 24.5215(5) Å and c = 5.6241(2) Å, which are slightly larger than those of NaBa3Eu3Si6O20 due to large ionic radius of La3+ ion37. As shown in Fig. 1(c), the basic structural units are distorted (SiO4)4− tetrahedra which are further linked by the Ba, La, and Na atoms to build a complex three-dimensional framework. The Na atoms which are surrounded by six oxygen adopt distorted pentagonal-pyramidal geometry, the Ba1 and Ba2 atoms coordinated to seven and eight oxygen are in distorted trigonal prism and cube configuration. In the structure of NaBa3La3Si6O20, there are two kinds of La sites, implying that there are two possible types of Ce3+ ions in the NaBa3La3Si6O20:Ce3+ samples. The La1 atoms are coordinated to seven oxygen atoms to form pentagonal bipyramid while the La2 surrounded by eight oxygen atoms are in square anti-prism environment (Fig. 1(d)).
(a) The XRD profiles for the Rietveld refinement of NaBa3La3Si6O20. (b) The XRD patterns of the NaBa3La3Si6O20, NaBa3La3Si6O20:0.007Ce3+ and NaBa3La3Si6O20:0.007Ce3+, yTb3+ phosphors. (c) The structure of unit cell of NaBa3La3Si6O20 along the c axis. (d) The coordination environments of La1 and La2 in NaBa3La3Si6O20.
Figure 1(b) shows the selected XRD patterns of the as-synthesized representative samples of NaBa3La3Si6O20 and NaBa3La3Si6O20:0.007Ce3+, yTb3+ (0 ≤ y ≤ 0.30) and the quantitative analysis of all the samples illustrate that the doping of Ce3+ or/and Tb3+ are successful (Supplementary Table S1). Also, it can be seen that all the diffraction peaks of the selected phosphors match well with the NaBa3La3Si6O20 phase. Even at high doping concentration of the Tb3+ ion (30%), the XRD patterns of phosphors are almost same with that of undoped phase, which illustrates the excellent stability and accommodation capacity for doped ions of crystal structure of the NaBa3La3Si6O20 host. The XRD profiles for the Rietveld refinement of the single element doped and co-doped samples and the coordination, occupancy and isotropic displacement parameter for all samples are listed (Supplementary Figs S1–S7, Tables S2–S9).
Photoluminescence properties and energy transfer
As shown in Fig. 2(a), the PLE spectra of the NaBa3La3Si6O20:0.007Ce3+ sample consist of three absorption bands centered at around 254, 282 and 331 nm, which arise from the electronic transitions between the ground state (2F5/2 and 2F7/2) and the levels of 5d excited split by crystal field of the Ce3+ ion38. Under the excitation wavelength of 331 nm, the Ce3+ ion doped NaBa3La3Si6O20 sample shows an asymmetric emission band extending from 340 to 500 nm with the maximum at 378 nm, indicating a possible spectral overlap originating from different luminescence centers. It is obvious that one type of Ce3+ ions gives rise to two emission band due to the transitions from the lowest 5d excited states to two ground states (2F7/2 and 2F5/2) respectively39. However, the emission band of the NaBa3La3Si6O20:0.007Ce3+ sample can be decomposed into four Gaussian components A–D peaking at 364, 380, 394 and 410 nm with the energy gaps between A and C is 2092 cm−1, that of B and D is 1926 cm−1, which are close to the theoretical value of 2000 cm−1 40,41. These results imply that there should be two kinds of Ce3+ ions, which is consistent with the previous investigation on the crystal structure that there are two kinds of different chemical environment of La3+ ions in the NaBa3La3Si6O20 host.
(a) The PLE (dark solid line) and PL spectra (red solid line) of the NaBa3La3Si6O20:0.007Ce3+ sample and the Gaussian peaks fitting (the green dashed lines of the Ce3+ (1) and the blue dashed lines of the Ce3+ (2)). (b) The PL spectra of the NaBa3La3Si6O20:xCe3+ samples with varying concentration of the Ce3+ ions.
As given in Fig. 2(b), the PL intensity of the NaBa3La3Si6O20:xCe3+ samples increases gradually with the increase of the doping concentration of the Ce3+ ions and reaches the maximum when the x value is 0.007, and then begins to decrease due to concentration quenching42. It is also indicated that the Ce3+ ion is a sensitizer for the Tb3+ ion and an energy transfer of Ce3+ → Tb3+ is crucial to enhance green emission of the Tb3+ ion and achieve color tunable emission light. Therefore, the optimal concentration of the Ce3+ ion in the NaBa3La3Si6O20:xCe3+ samples is confirmed to be 0.007.
Generally, the critical distance RC between the Ce3+ ions can be calculated with the following equation given by Blasse43:

where V is the volume of unit cell, x is the critical concentration of doped ions, where the emission intensity of phosphors reaches the maximum, N is the number of host cations per unit cell. For the NaBa3La3Si6O20:0.007Ce3+ sample, N = 12, V = 2057.989 Å3, RC is calculated to be about 25.00 Å. Dexter noted a non-radiative energy transfer usually was attributed to exchange or multipole – multipole interaction in oxide phosphors and the exchange interaction was valid only when the Rc was shorter than 5 Å44. In consequence, the concentration quenching mechanism of the Ce3+ ions in the NaBa3La3Si6O20:xCe3+ samples is dominated by the multipole – multipole interaction.
Figure 3(a) depicts the PLE and PL spectra of the NaBa3La3Si6O20:0.20Tb3+ sample. The PLE spectrum monitored at 542 nm exhibits a broad absorption band centered at 268 nm from 200 to 300 nm and several peaks within the scope of 300 to 400 nm. The former excited peak is ascribed to 4f 8–4f 75d transition of the Tb3+ ion, while the latter peaks are from the intra-4f 8 transitions45,46. Under the excitation wavelength of 268 nm or 378 nm, the NaBa3La3Si6O20:0.20 Tb3+ sample emits green light with main peaks at 412, 435, 457, 488, 542, 581 and 622 nm, which can be ascribed to the 5D4–7FJ (J = 6, 5, 4 and 3) transitions of the Tb3+ ion. However, because the f–f absorption is a forbidden transition, only some narrow f–f transition lines locate in the excitation range of n-UV LED in spite of difficultly bumping the Tb3+ ion47. There is an overlap between the emission band (magenta line in Fig. 3(a)) of the Ce3+ ions and the f–f transition (olive line in Fig. 3(a)) absorption band of the Tb3+ ions, therefore, it is potential that the Ce3+ ions can be sensitizers to transfer energy to the Tb3+ ions to enhance their absorption. As shown in Fig. 3(b), the PL spectrum of the NaBa3La3Si6O20:Ce3+, Tb3+ phosphors exhibits broad emission bands corresponding to the allowed f–d transition of the Ce3+ ions and the 5D4–7FJ characteristic transitions of the Tb3+ ions. The emission intensity of the NaBa3La3Si6O20:Tb3+ samples under excitation wavelength of 268 nm is larger than that under 378 nm, because the intensity of the absorption peak centered at 268 nm is more intense than that at 378 nm. However, the emission light intensity monitored at 268 nm is less than that at 374 nm in the NaBa3La3Si6O20:Ce3+, Tb3+ phosphors. These results verify that it is the overlap between f-f transition (peaking at 374 nm) but not f-d transition (peaking at 268 nm) of the Tb3+ ions and the emission band of the Ce3+ ions induce the energy transfer. Figure 3(b) also shows the excitation spectrum of the NaBa3La3Si6O20:0.007Ce3+, 0.20Tb3+ phosphor monitored at 378 nm (the Ce3+ ions emission) is similar to that of at 542 nm (the Tb3+ ions emission) except the difference of luminous intensity, which provides another evidence for energy transfer of Ce3+ → Tb3+.
To further investigate the sensitized luminescence of the Tb3+ ions by the Ce3+ ions, the emission spectra of the NaBa3La3Si6O20:0.007Ce3+, yTb3+ phosphors were measured (Fig. 4). Although the amount of the Ce3+ ions is fixed, their emission intensity gradually decreases along with the increase of the concentration of the Tb3+ ions. The result indicates that a lot of Tb3+ ions as acceptors accelerate energy diffusion of donors, which speeds up the average transfer rate of Ce3+ → Tb3+.
Figure 5 and Table 1 show the variation of Commission International deL’Eclairage (CIE) chromaticity coordinates of the NaBa3La3Si6O20:0.007Ce3+, yTb3+ phosphors (y = 0, 0.05, 0.10, 0.15, 0.20, 0.25, 0.30) under excitation wavelength at 331 nm. The insets of Fig. 5 are the photographs of the NaBa3La3Si6O20:0.007Ce3+, yTb3+ phosphors with different amount Tb3+ ions in a 365 nm n-UV lamp box. These results indicate that the emission light color can be modulated from deep blue to green only by varying the contention of the Tb3+ ions. Therefore the NaBa3La3Si6O20:Ce3+, yTb3+ samples can be potential color-tunable phosphors for application in n-UV based WLED devices.
Energy transfer mechanism
In general, the energy transfer from a sensitizer to an activator in oxide may take place via exchange interaction or electric multipolar interaction48. The separation distance RCe–Tb can be also estimated from equation (1). Here, x is the total concentration of the Ce3+ and Tb3+ ions, where the luminescence intensity of sensitizer is half of that in samples lack of activator. For the NaBa3La3Si6O20:Ce3+, Tb3+ phosphors, the value of  and
and  is about 0.021 and 0.75 respectively, thus RCe–Tb is calculated to be about 7.5 Å. Since exchange interaction was restricted to distances of about 4 Å, the energy transfer mechanism of Ce3+ → Tb3+ should may be electric multipolar interaction43,44.
is about 0.021 and 0.75 respectively, thus RCe–Tb is calculated to be about 7.5 Å. Since exchange interaction was restricted to distances of about 4 Å, the energy transfer mechanism of Ce3+ → Tb3+ should may be electric multipolar interaction43,44.
According to Dexter’s energy transfer expressions of multipolar interaction and Reisfeld’s approximation, the following relation can be given as42,48,49,50,51:

where η0 and η are the luminescence quantum efficiency of the Ce3+ ions in absence and presence of the Tb3+ ions, n = 6, 8 and 10 are corresponding to dipole – dipole, dipole – quadrupole and quadrupole – quadrupole interactions, respectively. The value ƞ0/ ƞ is approximately estimated by the ratio of related luminescence intensity I0/I, I0 is the intrinsic luminescence intensity of the Ce3+ ions, and I is the luminescence intensity of the Ce3+ ions in presence of the Tb3+ ions. Figure 6(a–d) illustrates the relationships between I0/I and  as well as I0/I and
as well as I0/I and  . The R2 value is reasonable in Fig. 6(b,c), implying the energy transfer of Ce3+ → Tb3+ may occur via dipole–dipole or dipole–quadrupole interaction. However, Sommerdijk stated the probability of energy transfer of Ce3+ → Tb3+ via electric dipole-dipole interaction was less likely, therefore, dipole – quadrupole interaction should mainly contribute to energy transfer of Ce3+ → Tb3+ 52.
. The R2 value is reasonable in Fig. 6(b,c), implying the energy transfer of Ce3+ → Tb3+ may occur via dipole–dipole or dipole–quadrupole interaction. However, Sommerdijk stated the probability of energy transfer of Ce3+ → Tb3+ via electric dipole-dipole interaction was less likely, therefore, dipole – quadrupole interaction should mainly contribute to energy transfer of Ce3+ → Tb3+ 52.
In order to further validate the energy transfer process, the room temperature decay curves for the 4f-5d (centered at 378 nm) transition of the Ce3+ ions in NaBa3La3Si6O20:0.007Ce3+, yTb3+ (y = 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30) excited at 330 nm are shown in Fig. 7 For existing two types of Ce3+ ions in topic phosphors, the decay curves should be well fitted with a typical two exponential function53:

where I(t) and I0 are the luminescence intensity at time t, A1 and A2 are the fitting constants, τ1 and τ2 represent the decay time for the exponential components. Then the average lifetime (τ*) can be calculated to be 24.5, 22.6, 21.7, 20.5, 20.0, 19.2 and 17.3 by the following formula40,54:

The decay time of the Ce3+ ions decreases as increase of the concentration of the Tb3+ ions, which strongly demonstrates the energy transfer of Ce3+ → Tb3+.
Subsequently the energy levels model for the energy transfer processes of Ce3+ → Tb3+ was investigated. As given in Fig. 8(a), the Ce3+ ion absorbs light firstly, then it jumps from the ground states (2F5/2) to the excited states (5d energy levels), subsequently the excited state Ce3+ ion returns to the lowest level of 5d levels by giving off excess energy to its surroundings, eventually goes back to the 2F7/2 or 2F5/2 ground states by a radiative process. The energy transfer efficiency of Ce3+ → Tb3+ should increase as the increase of the concentration of the Tb3+ ions due to more neighboring Tb3+ ions around the Ce3+ ions. Finally the energy level transitions of 5D4 to 7FJ (J = 3, 4, 5 and 6) produce the characteristic emission of the Tb3+ ions.
The energy transfer efficiency ƞT from the Ce3+ ions to the Tb3+ ions can be calculated according to the following equation55:

where I0 and Iy are the emission light intensity of the sensitizer with and without an activator, respectively. In the NaBa3La3Si6O20:0.007Ce3+, yTb3+ samples, the Ce3+ ion is a sensitizer and the Tb3+ ion is an activator. The ƞT values can be calculated as 7.06%, 16.71%, 28.95%, 37.04%, 48.95%, 78.31%, as a function of y (y = 0.15, 0.30, 0.45, 0.60, 0.75, 0.90), respectively (Fig. 8(b)). The energy transfer of Ce3+ → Tb3+ is consistent with the conclusion that the energy transfer efficiency increases as the increase of the concentration of the Tb3+ ions due to more neighboring Tb3+ ions around the Ce3+ ions and is equivalent to that of the reported K2MgSiO4:Ce3+, Tb3+ silicate phosphor56.
Conclusion
A series of novel NaBa3La3Si6O20:Ce3+, Tb3+ phosphors were prepared by solid state method. The energy transfer processe of Ce3+ → Tb3+ has been demonstrated to be dipole – quadrupole interaction. The tunable colors from deep blue to green can be realized by varying the doping concentration of the Tb3+ ions under the irradiation of 331 nm. These results demonstrate the as-prepared NaBa3La3Si6O20:Ce3+, Tb3+ samples can act as potential n-UV based w-LED phosphors.
Experimental Section
Compounds synthesis
The NaBa3La3Si6O20:Ce3+, Tb3+ phosphors were synthesized by high temperature solid state method. Na2CO3 (A.R.), BaCO3 (A.R.), SiO2 (A.R.), La2O3 (99.99%), CeO2 (99.99%) and Tb4O7 (99.99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. All of the initial chemicals were used without further purification. Stoichiometric amounts of the above-mentioned chemicals were ground thoroughly by an agate mortar, packed tightly in an alumina crucible. The temperature of the furnace was heated up to 500 °C at a rate of 60 °C/h, then held for 24 h to preheat the mixture in air atmosphere. After the mixture was ground once again, the temperature was increased to 930 °C at a rate of 60 °C/h and held for 100 h with four intermittent grindings. Finally, the prepared phosphors were cooled to room temperature and reground into resulting phosphors. For convenient expression, NaBa3La3−3x–3yCe3xTb3ySi6O20 is abbreviated as NaBa3La3Si6O20:xCe3+, yTb3+. For example, NaBa3La2.55Ce0.15Tb0.30Si6O20 is denoted as NaBa3La3Si6O20:0.05Ce3+, 0.10Tb3+.
Material characterization
The powder XRD measurements were taken on a Bruker D8 X-ray diffractometer with a Cu Kα source (λ = 1.5418 Å) in the angular range from 5° to 80° with a scanning step of 0.15. The structure refinement was carried out with the General Structure Analysis (GSAS) and EXPGUI software57,58. XRD Rietveld profile refinements of the structural models were performed using the General Structure Analysis (GSAS) software. The photoluminescence (PL) and photoluminescence excitation (PLE) spectra were obtained by an FLS-980 fluorescence spectrophotometer equipped with a 450 W Xe light source. The photoluminescence lifetime curves were measured on an FLS-920 fluorescence spectrophotometer equipped with a laser as light source. All measurements were performed at room temperature. The element analyses of samples were performed by the (X-ray fluorescence) XRF method on a thermo ARL ADVANTXP+ apparatus.
Additional Information
How to cite this article: Jia, Z. and Xia, M. Blue-green tunable color of Ce3+/Tb3+ coactivated NaBa3La3Si6O20 phosphor via energy transfer. Sci. Rep. 6, 33283; doi: 10.1038/srep33283 (2016).
References
Schubert, E. F. & Kim, J. K. Solid-state light sources getting smart. Science 308, 1274–1278 (2005).
Shur, M. S. & Zukauskas, R. Solid-state lighting: toward superior illumination. P. Ieee. 93, 1691–1703 (2005).
Narukawa, Y. et al. Recent progress of high efficiency white LEDs. Phys. Status. Solidi. A 204 (2007).
Tsao, J. Y., Coltrin, M. E., Crawford, M. H. & Simmons, J. A. Solid-state lighting: an integrated human factors, technology, and economic perspective. P. Ieee. 98, 1162–1179 (2010).
Bando, K., Sakano, K., Noguchi, Y. & Shimizu, Y. Development of high-bright and pure-white LED Lamps. J. Illum. Eng. Soc. 22, 1–5 (1998).
Hye Oh, J., Ji Yang, S. & Rag Do, Y. Healthy, natural, efficient and tunable lighting: four-package white LEDs for optimizing the circadian effect, color quality and vision performance. Light-Sci. Appl. 3, e141 (2014).
Setlur, A. A., Heward, W. J., Hannah, M. E. & Happek, U. Incorporation of Si4+–N3− into Ce3+-Doped Garnets for Warm White LED Phosphors. Chem. Mater. 20, 6277–6283 (2008).
Setlur, A. A. et al. Crystal chemistry and luminescence of Ce3+-doped Lu2CaMg2(Si,Ge)3O12 and its use in LED based lighting. Chem. Mater. 18, 3314–3322 (2006).
Lü, W. et al. A novel efficient Mn4+ activated Ca14Al10Zn6O35 phosphor: application in red-emitting and white LEDs. Inorg. Chem. 53, 11985–11990 (2014).
Wang, L. et al. Enriching red emission of Y3Al5O12:Ce3+ by codoping Pr3+ and Cr3+ for improving color rendering of white LEDs. Opt. Express. 18, 25177–25182 (2010).
Lee, S.-P., Huang, C.-H., Chan, T.-S. & Chen, T.-M. New Ce3+-activated thiosilicate phosphor for LED lighting—synthesis, luminescence studies, and applications. ACS Appl. Mat. Inter. 6, 7260–7267 (2014).
Hao, Z. et al. White light emitting diode by using α-Ca2P2O7:Eu2+, Mn2+ phosphor. Appl. Phys. Lett. 90, 261113 (2007).
Yang, W.-J. & Chen, T.-M. Ce3+∕Eu2+ codoped Ba2ZnS3: A blue radiation-converting phosphor for white light-emitting diodes. Appl. Phys. Lett. 90, 171908 (2007).
Kim, J. S. et al. White-light generation through ultraviolet-emitting diode and white-emitting phosphor. Appl. Phys. Lett. 85, 3696–3698 (2004).
Piao, X., Horikawa, T., Hanzawa, H. & Machida, K.-i. Characterization and luminescence properties of Sr2Si5N8:Eu2+ phosphor for white light-emitting-diode illumination. Appl. Phys. Lett. 88, 161908 (2006).
Lee, S. H., Park, J. H., Son, S. M., Kim, J. S. & Park, H. L. White-light-emitting phosphor: CaMgSi2O6:Eu2+, Mn2+ and its related properties with blending. Appl. Phys. Lett. 89, 221916 (2006).
Xia, Z. G. et al. Chemical unit cosubstitution and tuning of photoluminescence in the Ca2(Al1−xMgx)(Al1−xSi1+x)O7:Eu2+ phosphor. J. Am. Chem. Soc. 137, 12494–12497 (2015).
Bai, G., Tsang, M.-K. & Hao, J. Tuning the luminescence of phosphors: beyond conventional chemical method. Adv. Opt. Mater. 3, 431–462 (2015).
Xia, Z. & Liu, R.-S. Tunable blue-green color emission and energy transfer of Ca2Al3O6F:Ce3+,Tb3+ phosphors for near-UV white LEDs. J. Phys. Chem. C 116 (2012).
Zhang, X., Zhou, L., Pang, Q., Shi, J. & Gong, M. Tunable luminescence and Ce3+ → Tb3+ → Eu3+ energy transfer of broadband-excited and narrow line red emitting Y2SiO5:Ce3+, Tb3+, Eu3+ phosphor. J. Phys. Chem. C 118, 7591–7598 (2014).
Xia, Z. G. et al. Tuning of photoluminescence by cation nanosegregation in the (CaMg)x(NaSc)1−xSi2O6 solid solution. J. Am. Chem. Soc. 138, 1158–1161 (2016).
Kang, F. et al. Red photoluminescence from Bi3+ and the influence of the oxygen-vacancy perturbation in ScVO4: a combined experimental and theoretical study. J. Phys. Chem. C 118, 7515–7522 (2014).
Kang, F., Peng, M., Zhang, Q. & Qiu, J. Abnormal anti-quenching and controllable multi-transitions of Bi3+ luminescence by temperature in a yellow-emitting LuVO4:Bi3+ phosphor for UV-Converted white LEDs. Chem–Eur J. 20, 11522–11530 (2014).
Peng, M. & Wondraczek, L. Bi2+-doped strontium borates for white-light-emitting diodes. Opt. Lett. 34, 2885–2887 (2009).
Guo, C., Luan, L., Xu, Y., Gao, F. & Liang, L. White light–generation phosphor Ba2Ca(BO3)2:Ce3+, Mn2+ for light-emitting diodes. J. Electrochem. Soc. 155, J310–J314 (2008).
Su, Y., Li, L. & Li, G. Synthesis and optimum luminescence of CaWO4-based red phosphors with codoping of Eu3+ and Na+. Chem. Mater. 20, 6060–6067 (2008).
Yan, S. et al. Enhanced red emission in CaMoO4:Bi3+, Eu3+. J. Phys. Chem. C 111, 13256–13260 (2007).
Pust, P. et al. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 13, 891–896 (2014).
Xia, Z. G., Miao, S. H., Molokeev, M. S., Chen, M. Y. & Liu, Q. L. Structure and luminescence properties of Eu2+ doped LuxSr2−xSiNxO4−x phosphors evolved from chemical unit cosubstitution. J. Mater. Chem. C 4, 1336–1344 (2016).
Liu, X. et al. Single-phased white-emitting 12CaO·7Al2O3:Ce3+, Dy3+ phosphors with suitable electrical conductivity for field emission displays. J. Mater. Chem. 22, 16839–16843 (2012).
Lü, W. et al. Tunable color of Ce3+/Tb3+/Mn2+-coactivated CaScAlSiO6 via energy transfer: a single-component red/white-emitting phosphor. Inorg. Chem. 52, 3007–3012 (2013).
Lü, W. et al. Tunable full-color emitting BaMg2Al6Si9O30:Eu2+,Tb3+,Mn2+ phosphors based on energy transfer. Inorg. Chem. 50, 7846–7851 (2011).
Opstelte, J. j., Radielov, D. & Wanmaker, W. L. Choice and evaluation of phosphors for application to lamps with improved color rendition. J. Electrochem. Soc. 120, 1400–1408 (1973).
Nag, A. & Kutty, T. R. N. Photoluminescence due to efficient energy transfer from Ce3+ to Tb3+ and Mn2+ in (Sr3AlSiO20)-Si-10. Mater. Chem. Phys. 91, 524–531 (2005).
Heyward, C. C., McMillen, C. D. & Kolis, J. W. Hydrothermal growth of lanthanide borosilicates: a useful approach to new acentric crystals including a derivative of cappelenite. Inorg. Chem. 54 (2015).
Sanjeewa, L. D. et al. Hydrothermal synthesis, structure, and property characterization of rare earth silicate compounds: NaBa3Ln3Si6O20 (Ln = Y, Nd, Sm, Eu, Gd). Solid. State. Sci. 48, 256–262 (2015).
Shannon, R. D. & Prewitt, C. T. Effective Ionic radii in oxides and fluorides. Acta. Crystallogr. B 25, 925 (1969).
Dorenbos, P. The 5d level positions of the trivalent lanthanides in inorganic compounds. J. Lumin. 91, 155–176 (2000).
Park, H. K., Oh, J. H., Kang, H., Zhang, J. & Do, Y. R. Hybrid 2D photonic crystal-assisted Lu3Al5O12:Ce ceramic-plate phosphor and free-standing red film phosphor for white LEDs with high color-rendering index. ACS Appl. Mat. Inter. 7, 4549–4559 (2015).
Shang, M. et al. Blue emitting Ca8La2(PO4)6O2:Ce3+/Eu2+ phosphors with high color purity and brightness for white LED: soft-chemical synthesis, luminescence, and energy transfer properties. J. Phys. Chem. C 116, 10222–10231 (2012).
Lian, Z. et al. Crystal structure refinement and luminescence properties of Ce3+ singly doped and Ce3+/Mn2+ co-doped KBaY(BO3)2 for n-UV pumped white-light-emitting diodes. RSC Adv. 3, 16534–16541 (2013).
Dexter, D. L. & Schulman, J. H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 22, 1063–1070 (1954).
Blasse, G. Energy transfer in oxidic phosphors. Philips. Res. Rep. 24, 131 (1969).
Dexter, D. L. A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836–850 (1953).
Bourcet, J. C. & Fong, F. K. Quantum efficiency of diffusion limited energy-transfer in La1−X−YCexTbyPO4 . J. Chem. Phys. 60, 34–39 (1974).
Liang, C. et al. A novel tunable blue-green-emitting CaGdGaAl2O7:Ce3+, Tb3+ phosphor via energy transfer for UV-excited white LEDs. Dalton Trans. 44, 8100–8106 (2015).
Ryan, J. L. & Jorgense, C. k. Absorption spectra of octahedral lanthanide hexahalides. J. Phys. Chem. 70, 2845 (1966).
Reisfeld, R., Greenber, E., Velapold, R. & Barnett, B. Luminescence quantum efficiency of Gd and Tb in borate glasses and mechanism of energy-transfer between them. J. Chem. Phys. 56, 1698 (1972).
Lahoz, F., Martin, I. R., Mendez-Ramos, J. & Nunez, P. Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: an infrared-laser induced upconversion study. J. Chem. Phys. 120, 6180–6190 (2004).
Huang, C. H. & Chen, T. M. A novel single-composition trichromatic white-light Ca3Y(GaO)3(BO3)4:Ce3+, Mn2+, Tb3+ phosphor for UV-Light emitting diodes. J. Phys. Chem. C 115, 2349–2355 (2011).
Jia, Y. C., Qiao, H., Zheng, Y. H., Guo, N. & You, H. P. Synthesis and photoluminescence properties of Ce3+ and Eu2+-activated Ca7Mg(SiO4)4 phosphors for solid state lighting. Phys. Chem. Chem. Phys. 14, 3537–3542 (2012).
Verstegen, J. M. P. J., Sommerdijk, J. L. & Verriet, J. G. Cerium and terbium luminescence in LaMgAl11O19 . J. Lumin. 6, 425–431 (1973).
Lephoto, M. A. et al. Synthesis and characterization of BaAl2O4:Eu2+ co-doped with different rare earth ions. Physica. B 407, 1603–1606 (2012).
Zhu, G. et al. Ca5La5(SiO4)3(PO4)3O2:Ce3+, Mn2+: A color-tunable phosphor with efficient energy transfer for white-light-emitting diodes. J. Electrochem. Soc. 158, J236–J242 (2011).
Paulose, P. I., Jose, G., Thomas, V., Unnikrishnan, N. V. & Warrier, M. K. R. Sensitized fluorescence of Ce3+/Mn2+ system in phosphate glass. J. Phys. Chem. Solids 64, 841–846 (2003).
Xia, Y. F. et al. Luminescence properties and energy transfer in K2MgSi4:Ce3+, Tb3+ as a green phosphor. Mater. Express. 6, 37–44 (2016).
Larson, A. C. & Dreele, R. B. von GASA, General Structure Analysis System. (LANSCE, MS-H805). Los Alamos National Laboratory Los Alaoms, NM (1994).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001).
Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51502307) and Key Laboratory of Coordination Chemistry and Functional Materials in Universities of Shandong, Dezhou University.
Author information
Authors and Affiliations
Contributions
M.X. developed the idea and supervised the project, Z.J. and M.X. conducted the experiments and wrote the paper. All authors discussed the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jia, Z., Xia, M. Blue-green tunable color of Ce3+/Tb3+ coactivated NaBa3La3Si6O20 phosphor via energy transfer. Sci Rep 6, 33283 (2016). https://doi.org/10.1038/srep33283
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33283
- Springer Nature Limited
This article is cited by
-
Morphology and optical properties of wet chemistry synthesized submicron CePO4:Tb3+ hollow spheres
Chemical Papers (2024)
-
Radiative properties of green-emitting Ho3+ doped Y2SiO5 system: exploring the potential use as a phosphor
Journal of Materials Science: Materials in Electronics (2023)
-
A potential red-light-emitting Pr3+-doped novel silicate phosphors: Judd–Ofelt analysis and photoluminescence properties
Journal of Materials Science: Materials in Electronics (2023)
-
Luminescent and colorimetric properties of the sol–gel derived mono-phase Dy3+ doped silicate-based phosphor for w-LED applications
Journal of Sol-Gel Science and Technology (2022)
-
Ce3+-doped silicate-based down-conversion phosphors: investigation on synthesis, structure and photoluminescence properties
Journal of Materials Science: Materials in Electronics (2018)









 (b)
(b)  (c)
(c)  (d)
(d)  .
.
