Abstract
The polysaccharide capsule is the major virulence factor of Streptococcus pneumoniae (pneumococcus), a major human pathogen. The sequences in the promoter and coding regions of the capsule gene locus undergo extensive variations through the natural transformation-mediated horizontal gene transfer. The sequence variations in the coding region have led to at least 97 capsular serotypes. However, it remains unclear whether the sequence polymorphisms in the promoter region have any biological significance. In this study, we determined the sequences of the cps promoter region from 225 invasive pneumococcal isolates, and identified modular composition and remarkable inter-strain sequence variations in this region. The strain-to strain variations in the cps promoter are characterized by diversity in sequence and size, mosaic combinations of nucleotide polymorphisms and sequence modules, selective preservation of the sequence combinations, and promiscuous assortments of the sequences between the promoter and coding regions. Isogenic pneumococci carrying allelic variants of the cps promoter displayed significant differences in the transcription of the capsule genes, capsule production, adhesion to host epithelial cells, anti-phagocytosis and virulence in mouse bacteremia model. This study has thus indicated that the sequence polymorphisms in the cps promoter represent a novel mechanism for fine-tuning the level of encapsulation and virulence among S. pneumoniae strains.
Similar content being viewed by others
Introduction
Streptococcus pneumoniae (pneumococcus) is a Gram-positive bacterium that naturally resides in the human nasopharynx, but it causes a wide spectrum of bacterial infections including community-acquired pneumonia, otitis media, bacteremia, and meningitis1. Despite extensive use of antimicrobials and vaccines, S. pneumoniae remains as a major cause of bacterial infections worldwide, leading to approximately 1 million of death annually2,3. The polysaccharide capsule is the major virulence factor of S. pneumoniae, which enables the pneumococci to evade phagocytic killing by host immune system due to its negative charge and other biochemical properties4,5,6. However, the capsular polysaccharide (CPS) is immunogenic, and host immunity targeting the CPS is protective against the infection by the pneumococcus carrying the same CPS7. As a result of host immune selection, S. pneumoniae has evolved to produce at least 97 chemically and antigenically distinct types of the capsule (or serotypes) by frequent replacement of the CPS biosynthesis genes though acquisition of foreign DNA by natural genetic transformation8. Each type of CPS is synthesized and exported by a unique capsule gene (for type-37) or a set of the capsule genes (for all other types)9.
The capsule genes of all CPS types are clustered in the same locus of the genome between the dexB and aliA genes with the exception of the type-37 synthase gene (tts)5,9. The tts gene is located elsewhere on the chromosome due to degeneration of the original capsule genes in the dexB-aliA locus10. There are extensive inter-strain sequence variations in both the promoter and coding regions of the capsule locus as exemplified by the diversity in the locus size of different strains/types, which ranges from approximately 2.2 kilobases (kb) (type-37) to 30 kb (type-38) with an average of 21 kb9. The cps locus of the type-2 strain D39, the most extensively studied virulent S. pneumoniae strain, consists of 17 CPS-biosynthesis associated genes (20.316 kb in size)11. The sequence diversity in the coding region of the cps locus is responsible for the enormous diversity of the CPSs in chemical structure and antigenic property (a basis for pneumococcal serotyping).
Extensive sequence variations have also been described in the promoter region of the cps locus, which is defined as the intergenic sequence between dexB and cpsA (the first gene in the cps locus)12,13. Despite of its importance in the capsule production, the promoter region of S. pneumoniae is poorly characterized. As described in type-2 strain D3912, the typical promoter region consists of four basic modules upstream of the cpsA gene, the first gene in the capsule locus: insertional element (IE), repeat unit of pneumococcus (RUP), spacing sequence (SS), and a core promoter sequence that was previously defined in type-4 strain TIGR4 by Shainheit et al.14. The core promoter contains the predicted −10/−35 promoter motifs and the transcriptional start site14. Our previous study showed that the 17-gene cps locus in strain D39 is transcribed as an operon from the cps promoter region of the cps2A; the full transcription of the cps genes requires not only the core promoter, but also the other upstream modules (e.g., IE, RUP, and SS)12.
Based on sequence variation in the cps promoter region, Moscoso et al. identified 26 sequence organizations (SOs) from the sequences of the cps promoter from 115 S. pneumoniae sequence entries; the majority of the SOs consists of one or more IEs, a RUP, and a conserved sequence (covering the core promoter and spacing sequence)13. However, it remains completely undefined whether the inter-strain allelic variations in the cps promoter region have any impact on pneumococcal encapsulation and virulence. In this study, we performed a comprehensive sequencing analysis of the cps promoter region in 225 invasive isolates of S. pneumoniae. Among the cps promoter variants of these isolates, our data revealed: 1) extensive variations in sequence and size, 2) mosaic combinations of nucleotide polymorphisms and sequence modules, 3) selective preservation of the sequence combinations, and 4) promiscuous assortments of the promoter sequences with those of the coding region. Functional analysis of the selected promoter sequences uncovered significant impact of the allelic variations in this region on the transcription of the cps genes, capsule production, adhesion to host epithelial cells, anti-phagocytosis and virulence in isogenic pneumococcal backgrounds. These data indicate that the allelic variations in the cps promoter serve as a novel mechanism for modulating the amount of pneumococcal capsule, pathogen-host interaction and virulence.
Results
The cps promoter of invasive pneumococcal isolates contains distinctive combinations of nucleotide polymorphisms and sequence modules
There are extensive strain-to-strain sequence variations in the cps promoter region of S. pneumoniae, or the intergenic region between the dexB and cpsA genes12,13. However, it remains completely unclear whether the promoter allelic variations are relevant to the biological behaviors of this pathogen, particularly to its encapsulation and virulence. This study attempted to evaluate potential pathobiological impact of the promoter polymorphisms in invasive pneumococci. Because many existing sequence entries of the cps promoter in the public databases did not provide the necessary background information of the source strains (e.g., serotype and tissue/geographic source), which was essential for our investigation of invasive pneumococci, we performed sequencing analysis of the cps promoter region from 225 invasive isolates of S. pneumoniae. These isolates represented at least 30 capsular serotypes (1, 4, 6A, 6B, 7F, 8, 9A, 9V, 10, 10A, 11, 12F, 14, 15A, 15B, 15C, 16F, 18C, 19A, 19F, 20, 22F, 23B, 23F, 29, 33, 33F, 34, and 35B), including the vast majority of the serotypes covered by the current pneumococcal vaccines15. Because the capsule genes of the type-3 strains are completely different from and not comparable with those of all the other types, our sequence analysis focused only on the non-type-3 isolates.
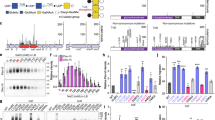
These isolates displayed various levels of sequence variations in the cps promoter region, reflected by differences in the arrangement of sequence modules, molecular size, and nucleotide polymorphisms (Fig. 1 and Fig. S1). As represented in Fig. 1A, there were several unique arrangements of sequence modules (e.g., IE, RUP, SS, and core promoter) in this region. All of the sequences contained a relatively conserved segment in the 3′ ends (approximately 85 bp) although polymorphisms were still present virtually in all of the strains. This region consists of a partial SS module (~26 bp), the core promoter (~35 bp), and the ribosome binding site-containing 5′ untranslated segment (~24 bp) (Fig. 2). The upstream IE, RUP, and 5′ SS modules are highly variable in sequence and arrangement, which were responsible for the diversity in the size among the cps promoter variants. The RUP module was present in the vast majority of the invasive isolates, but its relative position was not a constant (Fig. 1). RUP was positioned between the IE and SS modules in the vast majority of the isolates (Fig. 1A, rows 1–3), but it was also found upstream of the IE module in certain isolates (Fig. 1A, 9N, 14–1, and 20). RUP was entirely absent from 20 out of the 225 invasive isolates (9%) tested in this work.
(A) Sequence diversity among the strains representing the 22 WZY-dependent IPD types. (B) Sequence diversity among the five type-6B promoter strains used in Figs 6 and 7. (C) Independence of the promoter sequence from the coding region (capsular type). Sequence modules are indicated with colored strips at the bottom. The size of each sequence (bp) and serotype of the source strain are indicated at the left and right sides, respectively. The numbers following the serotype icons indicated the different strains of the same serotypes.
The region covering the RUP, spacing sequence, and core promoter modules were aligned with the sequences from the strains illustrated in Fig. 1A by the Cluster W method58. The IE module is not fully presented due to their extensive sequence differences and space limit. Partial IE module is included for each of the strains lacking the RUP module immediately upstream of the SS module. The promoter motifs (−10 and −35), transcriptional start site identified in type-2 strain D3912, cpsA start codon (SC) were indicated at the both of the second panel.
The cps promoter sequences of many isolates also exhibited remarkable differences in molecular size, particularly in the IE and RUP modules. IE represents the most variable segment in the cps promoter region, ranging from >200 bp to <3,000 bp, and is primarily responsible for the size differences among the isolates. The longest promoter sequence (3,862 bp) was identified in type-6B isolate TH2882 (Fig. 1A, 6B-1), which is identically present in the genome of strain 670-6B (accession CP002176). Although this sequence was highly divergent from the counterparts of many other strains due to its long IE and lack of the RUP module, it is structurally similar to that of type-11A strain TH4849 (Fig. 1A, 11A; 2,418 bp). The latter lacked a 5′ insertion element (1,441 bp) of strain TH2882, which was responsible for the size difference between the two sequences. The sequence from type-35B isolate TH2733 had the shortest sequence (560 bp in size) (Fig. 1A, type-35B). This sequence consisted of an extremely truncated IE module (192 bp), a 107-bp RUP, a 61-bp spacing sequence, and the core promoter.
Intriguingly, there is no apparent association between the capsule types and the modular/sequence features of the cps promoter. First, the cps promoter sequences from the strains within the same serotype could be as divergent as those from the strains with different capsular types. As exemplified in Fig. 1B and Fig. S2, the cps promoter sequences from the five type-6B strains remarkably differed in size and modular arrangement. For example, the sequence of strain TH2882 (Fig. 1B, 6B-1) was 2–3 times larger than those of the other 6B strains. Instead, this sequence is more similar to the cps promoter sequence of type-11A strain TH4849 (Fig. 1A, 11A). Along this line, unlike the other type-6B isolates, strain TH2787 (Fig. 1B, 6B-2) positioned its RUP module in front of the IE module, resembling the modular structure of the promoter sequences in the type-9N, -14, and -20 strains (Fig. 1A). Second, pneumococcal strains with different types of the capsule carried similar promoter sequences in the capsule locus. As exemplified in Fig. 1C and Fig. S3, the capsule locus in strains of types 6B (6B-3, 6B-5), 9V, 14, 16F, 19F, and 23F possessed highly homologous promoter sequences as reflected by their similarities in molecular size and modular arrangement. Promiscuous assortments of the promoter sequences with those of the coding region in these invasive isolates implicate that the promoter and coding regions of the cps locus are able to undergo independent assortments by horizontal gene transfer through natural genetic transformation.
Lastly, there are many minor polymorphisms throughout the four basic modules of the entire promoter among these isolates, which include nucleotide replacements, insertions, and deletions (Fig. 2, Figs S2 and S3). Interestingly, some of the sequence changes occurred in the predicted -10 promoter motif, although the vast majority of the isolates had a sequence of 5′-TATAAT-3′. However, these nucleotide polymorphisms in the cps promoter, as well as the arrangements of the sequence modules, are individually shared by many isolates although the combinations of these changes resulted in the mosaic pattern. These lines of evidence suggest that the allelic variations in the cps promoter have been maintained and shared by the pneumococcal strains as a function of selective pressure in humans. Taken together, our sequencing result has uncovered extensive variations in sequence and modular structure of the cps promoter. The sequence and modular features of the cps promoter are not associated with the boundary of the capsular types (the coding region in the capsule locus), suggesting that the promoter and coding regions of the cps locus are able to undergo independent assortments by horizontal gene transfer through natural genetic transformation.
Strain-to-strain sequence variations in the cps promoter of the major invasive pneumococcal disease (IPD) types alter the transcription of the capsule genes
Our recent study has shown that the full transcription of the cps operon and capsule production depend on the four sequence modules in the cps promoter region12. Based on the extensive sequence variations in these promoter modules, particularly in the IE and RUP (Fig. 1), we hypothesized that the inter-strain sequence polymorphisms in the cps promoter affect the transcription of the capsule gene operon. To test this possibility, we initially determined the transcriptional activity of the cps promoter sequences from the strains representing the 22 major IPD serotypes in the pneumococcal vaccines using a luciferase-based transcriptional reporter system. The entire sequence between the dexB stop codon and cpsA start codon was amplified by PCR from each target strain using the same primer pair (except for the sequence in the type-3 strain), fused to the upstream of the luciferase gene in the pIB166 shuttle plasmid, and transformed into D39, a type-2 virulent Avery strain16. The type-3 cps promoter was amplified with the primer sequences in the upstream region of the ugd (cps3D) gene9. The relevant information for the reporter construction is provided in Table S2.
When compared with the luciferase activity of the type-2 cps promoter (as a reference), the cps reporter constructs showed variable levels of transcriptional activity (Fig. 3A and Table S4). The type-3 construct showed the highest transcription activity among all of the 22 cps reporters with a 6.58-fold increase above the reference level. Although the margins of the variations were relatively small, 10 additional reporters displayed relatively lower levels of luciferase activity, such as those of types 1 (21.0%), 6B (46.0%), 7F (48.3%), 8 (41.3%), 9V (38.6%), 12F (55.3%), 19A (43.5%), 22F (33.9%), 23F (40.4%), and 33F (34.2%). The remaining 10 constructs showed a level of luciferase activity similar to that of the D39 (e.g., 4, 9N, 10A, 11A, 14, 15B, 17F, 18C, 19F, and 20). The reporter experiment suggested that sequence diversity in the cps promoter among different strains/types affects the transcription of the cps operon.
(A) Assessment of promoter activity by a luciferase reporter. Each promoter sequence was fused to the 5′ end of a promoterless luciferase gene in the pIB166 shuttle plasmid, and transformed in strain D39. The promoterless plasmid (vector) was used as a negative control. Transcriptional activity of each cps promoter sequence was quantified by measuring luminescence level of the mid-log culture inoculated with the pneumococci carrying the appropriate reporter construct. Each reporter was identified with the serotype of its source strain. The sequence of the first strain was used for each of the serotypes with multiple strains listed in Fig. 1. The values represent the percent luciferase activities of the reporters relative to the luciferase activity readout of the D39 (first bar) promoter construct. (B) The qRT-PCR readouts of the cps2A transcripts from the promoter replacement D39 derivatives of 13 IPD serotypes. The promoterless derivative TH4525 described in our previous study was used as a negative control. The mRNA levels were presented as relative values to that of D39s. (C) Same as panel B, except that cps2E was tested. The sequence of the first strain was used in both the luciferase and promoter replacement experiments for each of the serotypes with multiple strains listed in Fig. 1. The values were the means ± standard errors of the results from triplicate samples as compared with those of the type 2. *p < 0.05, **<0.01 and ***<0.001.
We subsequently sought to verify the transcriptional impact of the cps promoter sequence polymorphisms with a series of isogenic promoter replacement derivatives of strain D39, with a focus on the promoter sequences displaying significant differences in the reporter test (Fig. 3A). The replacement strains were constructed by individually replacing the dexB-cps2A region of D39 with the corresponding region from the target strains through counter selection. The transcriptional activities of the “transgenic” promoters were assessed by determining the mRNA levels of cps2A and cps2E in the resulting D39 derivatives by quantitative real-time PCR (qRT-PCR). The cps2A and cps2E were previously used as the transcriptional readouts of the cps operon12,14. The cps2A primers revealed significant differences between the type-2 and all of the 12 cps promoter sequences except for those of types 18C, 22F, and 33F (Fig. 3B). The cps2E amplification identified much fewer significant hits with significant differences in 6 of the 12 cps promoter sequences (types 3, 8, 14, 19A, 19F, and 23F) (Fig. 3C). Consistent with the luciferase reporter data, the strain containing the type-3 promoter displayed the highest transcription levels of both cps2A (by 1.76-fold) and cps2E (by 1.54-fold). Although the overall transcriptional patterns of the cps2A and cps2E were similar for most of the cps promoter replacement strains, discrepancy was observed with the cps2A and cps2E results with the strains containing the csp promoter sequences of types 1, 4, 6B, and 11A. As compared with the parent strain, these strains showed significantly higher levels of cps2A (Fig. 3B) but not cps2E (Fig. 3C). Given both cps2A and cps2E are co-transcribed12, this discrepancy may reflect uneven degradation of different regions in the cps mRNA. As exemplified by the cps promoter sequence of types 1, 4, 6B, 11A, 14, 19A, 19F, 22F, 23F, and 33F, we also observed apparent inconsistency between the results of the luciferase reporter and qRT-PCR experiments, which could be caused by relative stability of the luciferase reporter as compared with the fast degradation of the cps mRNA as indicated in our previous Northern blotting analysis12. Taken together, the transcriptional reporter and qRT-PCR experiments showed that certain inter-strain/type sequence variations in the cps promoter lead to significant changes in the transcription of the capsule locus.
Inter-type sequence polymorphisms in the cps promoter of the major IPD types alter the level of the pneumococcal encapsulation
Significant differences in the transcriptional capacity among the cps promoter sequences promoted us to determine whether these inter-strain/type sequence variations affect pneumococcal capsule production. The CPS level of the promoter replacement strains was determined by immunoblotting with an antibody against type-2 pneumococcal CPS. This experiment showed dramatic differences among most of the promoter replacement strains in the level of the capsule production (Fig. 4A). Consistent with its stronger transcriptional capacity (Fig. 3), the type-3 promoter produced the highest level of CPS, which was 71% higher than that of strain D39s (Fig. 4B). The 18C promoter also yielded 50% more CPS than the parent strain although no significant difference in the transcriptional capacity was detected between the two strains (Fig. 3). In contrast, significantly lower levels of the CPS were detected in the promoter replacement strains of types 6B (32%), 8 (20%), 11A (23%), 14 (55%), 19F (45%), 22F (33%), 23F (23%), and 33F (13%) (Fig. 4B). Importantly, each of these promoter sequences yielding the lower CPS production is also carried by many other strains from multiple capsular serotypes. For example, the sequence of the type-33F cps promoter used in this work shares the same modular structure and highly similar sequence with those of the strains representing at least 23 different capsular types (e.g., 6B, 7B, 10B, 10F, 12B, 12F, 14, 15A, 18B, 18C, 19F, 19B, 19F, 23A, 23F, 27, 29, 44, 46, 28F, 33A, 35A, and 35B).
The CPS of the pneumococci was assessed by immunoblotting using an antiserum specific for the type-2 CPS signal (A). The signal intensity on each spot was digitized with ImageJ and normalized to the corresponding value of D39s (B). The value of each bar was the mean ± standard error of the result from all of the detectable spots (>3) for each sample after considering dilution factors and being compared with the type-2 parental strain. *p < 0.05, **<0.01 and ***< 0.001.
The reduced CPS production in these reporter strains could be explained by the relatively lower transcription capacity of the corresponding cps promoters in the luciferase reporter and/or qRT-PCR experiments. Overall, the immunoblotting results were more consistent with the data obtained by the luciferase reporters (Fig. 3A), suggesting this is a more robust predictor of the capsule production. Together with the transcriptional detection results, the immunoblotting data indicated that the sequence polymorphisms in the cps promoter are capable of generating remarkable diversity in the transcription of the capsule genes and pneumococcal capsule production.
Inter-type sequence polymorphisms in the cps promoter of the major IPD types modulate pneumococcal pathogenic properties
The pneumococcal capsule hinders bacterial attachment to mammalian cells17. We reasoned that pneumococcal adhesion to epithelial cells may be affected by variations in the level of encapsulation. We first compared the levels of epithelial adhesion among the isogenic promoter replacement strains. As a control, the cps promoter-null strain showed a 60-fold more adhesion than the encapsulated parent strain (D39s) (Fig. 5A), confirming the blocking effect of the capsule on pneumococcal binding to host cells likely through masking the adhesive molecules on the cell surface. Consistent with the relatively thicker capsule (Fig. 4), the strains carrying the cps promoters of types 14 (by 4-fold) and 19F (by 3.6-fold) showed significantly higher levels of adhesion than the parent strain. To less extents, significant increase in adhesion was also observed with the strains carrying the promoters of types 4, 6B, 8, 19A, 22F, and 23F. Surprisingly, the strain with the lowest CPS production (33F) did not show the anticipated (highest) level of adhesion. Along the same line, the type-3 and -18C promoter strains did not show expected reduction in adhesion based on their CPS production levels. Nevertheless, this result demonstrated that certain inter-type sequence variations in the cps promoter lead to significant phenotypic impact on pneumococcal adhesion.
(A) Pneumococcal adhesion to human alveolar epithelial A549 cells. D39s and its promoter replacement derivatives were incubated with the confluent monolayers of A549 cells for 1 h before the monolayers were lysed to enumerate the number of viable pneumococci on TSA blood plates. The adhesion levels of the promoter replacement strain are displayed as the percentage values relative to the value in D39s. (B) Phagocytosis of the cps promoter derivatives by RAW264.7 murine macrophages. The RAW264.7 cell monolayers were infected with the pneumococci, treated with antibiotics, and lysed to quantify intracellular (phagocytosed) bacteria as in A. The phagocytosis level of each promoter replacement strain was shown as the percentage value relative to the value of the parent strain D39s. (C) The virulence levels of the promoter replacement strains. CD1 mice were intraperitoneally infected with a mixture of D39 and one of the promoter replacement strains in a 1:1 ratio. The pneumococci in the bloodstream of the mice were enumerated 21 h post infection as in A. Each filled circle represents a relative bacteremia level or competitive index (CI) value of a single mouse. The open circles indicate the mice, from which no unencapsulated pneumococci were recovered. The horizontal bar indicated the mean in each group of mice. The parental strain (type-2) was used as a reference. *p < 0.05, **<0.01 and ***< 0.001.
It is believed that inhibiting pneumococcal attachment to host phagocytes is a major mode of action for the capsule5. We thus determined potential impact of promoter sequence variations on pneumococcal phagocytosis in the RAW 264.7 murine macrophage. As a control, the encapsulated mutant showed substantial increase in phagocytosis (Fig. 5B, null), although the extent of the increase (18%) in phagocytosis was much less than in epithelial adhesion (Fig. 5A). In agreement with the capsule production (Fig. 4), the strains with the type-3 and -18C promoters showed relatively lower levels of phagocytosis, whereas those with the cps promoters of types 6B, 11A, and 19F displayed significantly enhanced uptake by the macrophages. The phagocytosis in the types 8, 14, and 22F strains could not be explained simply by their CPS levels (Fig. 4, Table S4).
We next assessed the relative virulence of these strains by intraperitoneal co-infection of CD1 mice with a 1:1 mixture of D39 and one of the promoter replacement strains. The level of viable bacteria in the bloodstream of mice (bacteremia) 21 h postinfection was used as a marker for pneumococcal virulence. The wild type D39 (streptomycin sensitive) was used as a reference strain in the co-infection experiments for the purpose of differentiating the parent strain from its streptomycin-resistant derivative of D39s. The streptomycin-resistant derivative of D39 (D39s) was slightly attenuated in this mouse model because the ratio between the mutant and wild type bacteria recovered from each co-infected mouse was approximately 0.5:1 (~50% attenuation).
All of the promoter replacement strains displayed significant variations in virulence, except for those carrying the promoters of types 1, 4, 14, and 19A (Fig. 5C). In agreement with the CPS immunoblotting result, seven of the eight strains with reduced CPS (except for type-14) displayed significant attenuation in the bacteremia model (types 6B, 8, 11A, 19F, 22F, 23F, and 33F). Among these, the type-33F reporter exhibited the most severe attenuation (by 6,249-fold), which was also the poorest CPS producer (Fig. 4). The remaining six strains showed much less but significant attenuation (types 23F by 10.5-fold, 22F by 9-fold, 19F by 4-fold, 11A by 3.6-fold, 8 by 2-fold, and 6B by 1.3-fold). Consistent with the 50% increase in CPS production over the parent strain, the strain carrying the type-18C promoter showed a 4.3-fold enhancement in bacteremia level. This result suggested that pneumococcal growth in the bloodstream is proportionally enhanced by the CPS level. Contradictory to this notion, the type-3 reporter strain was dramatically attenuated by 451-fold although it synthesized 71% more CPS over the parent strain (Fig. 4), indicating that excessive production of the capsule is detrimental to pneumococcal fitness during the systemic infection. Consistent with our previous study12, D39s (the parent strain) showed a minor attenuation in virulence as compared with the wild type D39 (1-fold reduction), which was factored in our calculation of the final CI values. Finally, no viable bacteria were recovered from any of the 12 mice infected with the cps promoter-null strain TH4525 (Fig. 5C), confirming the essentiality of the capsule in pneumococcal virulence. These data demonstrated that many allelic variants of the cps promoter in the major IPD serotypes are able to generate profound phenotypic diversity in the capacity of pneumococcal virulence.
Inter-strain polymorphisms in the type-6B cps promoter alter the levels of pneumococcal encapsulation, epithelial adhesion, anti-phagocytosis and virulence in the D39 background
As exemplified in Fig. 1B, our earlier sequencing analysis revealed extensive inter-strain sequence variations in the cps promoter within many pneumococcal IPD serotypes, including type-6B. Based on the striking phenotypic impact of sequence variations in the cps promoter among the strains representing the major IPD serotypes, we further determined whether this functional influence operates among the cps promoter sequences within type-6B, because type-6B is a major international IPD serotype in both children and adults1, and was abundantly represented in the IPD strains that were sequenced in this study.
We initially generated reporter derivatives of strain D39s for the five cps promoter sequences of type-6B (represented in Fig. 1B, Fig. S2). These sequences were selected because they represented the diversity of the cps promoter, in terms of nucleotide sequence, size, and modular arrangement. The sequences between the dexB and cps6BA of type-6B strains were amplified from genomic DNA samples of five original 6B strains (Table 1), and used to generate the unmarked promoter replacement derivatives in D39s by counter selection as described in Fig. 3. The cps transcriptional activity and capsule production of each promoter replacement strain were assessed by qRT-PCR and immunoblotting, respectively. The strain carrying the longest type-6B cps promoter (Figs. 1B, 6B-1) was used as a reference for comparative analysis.
The cps promoter sequence from each of the five type-6B invasive isolates were amplified and used to replace the cps promoter of strain D39s. The resultant strains (from 6B-1 to 6B-5) were tested for their levels of transcription in cps2A (A), capsule production (B,C), epithelial adhesion (D), phagocytosis (E), and virulence (F). The values in each set of the experiments were normalized to those of strain 6B-1, and presented as in Figs 3, 4, 5. *p < 0.05, **<0.01 and ***< 0.001.
The qRT-PCR analysis of the cps2A (Fig. 6A) and cps2E (Fig. S4A) transcripts showed a comparable transcription activity for four of the five type-6B cps promoter sequences (6B-1, 6B-2, 6B-4, and 6B-5). The strain carrying the 6B-3 promoter sequence had significantly higher levels in the cps2A (by 41%) and cps2E (by 55%) transcription. However, the immunoblotting analysis revealed that this strain along with 6B-3 and 6B-5 produced substantially lower levels of CPS than strains 6B-2 and 6B-4; strain 6B-1 produced the least amount of CPS (Fig. 6B). The slightly more transcription of the cps genes in strain 6B-3 did not result in more CPS. The results obtained with the five type-6B cps promoter sequences thus confirmed the earlier immunoblotting analysis (Fig. 4) that inter-strain sequence variations in the cps promoter lead to significant functional impact on the capsule production of S. pneumoniae.
We further determined the effect of the type-6B cps promoter polymorphisms on pneumococcal adhesion, phagocytosis and virulence. Consistent with the immunoblotting results, strain 6B-1 with the lowest CPS showed a small but significantly higher level of epithelial adhesion to A549 cells than the rest of the five strains (by 30–50%) (Fig. 6D), verifying that the pneumococci with a thinner capsule are more capable of attaching to host cells. To our surprise, the 6B-2 and 6B-3 strains showed significantly enhanced phagocytosis in the RAW 264.7 murine macrophages (Fig. 6E and Table S4), suggesting that this phagocytosis model is not suitable for assessing capsular impact on pneumococcal phagocytosis. In full concordance with their encapsulation levels, the type-6B cps promoter replacement strains displayed two different levels of virulence in the mouse bacteremia model (Fig. 6F). The two strains producing thicker capsules (6B-2 and 6B-4) were much more virulent than the three counterparts with relatively lower levels of CPS (6B-1, 6B-3, and 6B-5). Specifically, the mice infected with strains 6B-2 and 6B-4 had 27.8- and 55.2-fold more pneumococci in the bloodstream than the 6B-1-infected animals, respectively. These results demonstrated that the sequence polymorphisms in the cps promoter among the strains of the same serotype significantly affect pneumococcal adhesion and virulence.
Inter-strain polymorphisms in the type-6B cps promoter diversify pneumococcal encapsulation, epithelial adhesion, anti-phagocytosis and virulence in the ST858 (type-6B) background
To rule out potentially strain-specific effect in the experiments conducted in the D39 background (Fig. 6), we placed the same sets of the type-6B cps promoters in the dexB-cps6BA intergenic region of strain ST858, a virulent type-6B blood isolate and the natural carrier of the 6B-5 cps promoter (Fig. 1B). The cps promoter replacement derivatives were generated in strain ST858 as in D39. The resulting strains were used to characterize their activities in the cps gene transcription, type-6B capsule production, epithelial adhesion, anti-phagocytosis and virulence as described above. The strain carrying the 6B-1 cps promoter was used as a reference for comparison. The qRT-PCR analysis revealed similar levels of the cps6BA and cps6BE transcripts among the five promoter replacement strains (Fig. 7A and Fig. S4B). This result was comparable with what was observed with the D39 derivatives of the same cps promoter sequences, with the exception of the 6B-3 construct (Fig. 6A and Fig. S4A). However, the immunoblotting test showed that the 6B-1 and 6B-2 strains were more abundantly encapsulated than the other three promoter replacement strains (6B-3, 6B-4, and 6B-5). The profile of epithelial adhesion was similar to that of the CPS production, except for the 6B-4 strain (Fig. 7D). The 6B-4 promoter yielded 3.7-fold more adhesion than the reference strain. In agreement with epithelial adhesion, the 6B-4 and 6B-5 strains displayed substantially changed phagocytosis in the RAW 264.7 murine macrophage (Fig. 7E and Table S4), but the phagocytosis result of strain 6B-3 was opposite to the epithelial adhesion data (Fig. 7D–E), which could be caused by potential bacterial killing in the RAW 264.7 cells during the assay. Because the 6B-4 promoter resulted in comparable adhesion with the other four promoters in the D39 background (Fig. 6D), this result suggested that this sequence may be involved in background-specific pneumococcal interactions with host cells.
The cps promoter replacement derivatives were constructed in strain ST858 with the promoter sequences of 6B-1-5, and tested for cps transcription (A), capsule production (B,C), epithelial adhesion (D), phagocytosis (E), and virulence (F) as in Fig. 6. *p < 0.05, **<0.01 and ***< 0.001.
We finally assessed the functional impact of the type-6B cps promoter polymorphisms on the virulence of strain ST858 in the mouse bacteremia model. The streptomycin-resistant derivative of ST858 (ST858s) was slightly attenuated in this mouse model because the ratio between the mutant and wild type bacteria recovered from each co-infected mouse was approximately 0.5:1 (~50% attenuation). As in the D39 background (Fig. 6F), the same type-6B promoter sequences yielded distinct levels of bacteremia in ST858 (Fig. 7F). While the 6B-1 and 6B-2 strains showed a similar levels of bacteremia, significantly lower levels of bacteremia were observed with the counterparts carrying the other three promoters. As compared with strain 6B-1, 6B-3 (by 16-fold), 6B-4 (by 4.3-fold), and 6B-5 (by 2.3-fold) were substantially attenuated in the capacity of growth in the bloodstream. As a control, the promoterless mutant of ST858 was totally avirulent (without any detectable pneumococci from the blood of the infected mice). The virulence level of each ST858 derivative was in concordance with the amount of the CPS that was detected in the in vitro cultured pneumococci. These results demonstrated that the promoter sequence polymorphisms in the cps locus of type-6B strains are capable of diversifying the amount of capsule, epithelial adhesion, anti-phagocytosis and virulence in the type-6B genetic background of S. pneumoniae.
Discussion
The polysaccharide capsule is an outer layer of S. pneumoniae, which is essential for immune evasion and virulence of this pathogen. However, due to selection pressure by host immunity, the capsule locus is subjective to extensive sequence variation and antigenic variation through horizontal gene transfer-mediated sequence shuffling9,18. While it is well recognized that sequence diversity in the coding region of the capsule locus defines serotypes5,8, the inter-strain sequence variations in the cps promoter have been largely ignored thus far. This work represents the first comprehensive investigation into the functional impact of this region. Our sequencing analysis of 225 invasive pneumococcal isolates uncovered several intriguing features in the promoter region of the capsule locus: extensive sequence polymorphisms, differential arrangements of the sequence modules, promiscuous assortments of the promoter with the coding region, and selective preservation of the sequence polymorphisms. Subsequent experiments with unmarked promoter replacement strains in two genetic backgrounds showed that the allelic variations in the cps promoter generate profound phenotypic diversity in pneumococcal pathobiology.
Capsule is a common structure of many pathogenic bacteria19. At the cellular level, the amount of capsule may be controlled by transcriptional regulators in response to environmental conditions, as exemplified in Bacillus anthracis20, E. coli21,22,23, Pseudomonas aeruginosa24, and Streptococcus pyogenes25. At the population level, capsule production can be modulated by reversible sequence insertion/deletion in the capsule genes in N. meningitidis group B strains26,27,28, reversible amplification of the capsule locus in the type-b Haemophilus influenzae29,30,31, or insertion of the JUMPstart sequence in the capsule promoter of E. coli32,33. The capsule level of pneumococcal serotypes/strains is reversibly affected by spontaneous mutations in the capsule genes34,35,36,37, but it remains unknown if the capsule promoter of S. pneumoniae is subjective to transcriptional regulation at the cellular level. In this context, our finding in this study represents a unique mechanism for generating strain-to-strain variations in capsule production at the species level by diversifying the sequence and thereby transcriptional capacity of the cps promoter.
Our immunoblotting analysis detected significant variations in the level of CPS among the isogenic promoter replacement derivatives in two different strain backgrounds (D39 and ST858). As examples, the promoter variants of types 8, 11A, 23F, and 33F produced up to 7-fold less CPS than the counterparts of other promoter variants (e.g., types 1, 2, 4 and 19A) in the D39 background. This pattern of phenotypic fluctuation was also observed among other promoter variants from different capsular types (inter-type). Further analysis of the type-6B promoter variants in both D39 (type-2) and ST858 (type-6B) also confirmed that the strain-to-strain sequence variations in the cps promoter of the same serotype (6B) generate significant diversity in the level of encapsulation. Our finding thus strongly suggests that the variability in the promoter sequence among pneumococcal strains serves as a novel mechanism for quantitative diversification of the capsule at the species level.
As a cover of the pneumococcal cells, the capsule directly interacts with various host cells and molecules for many fitness purposes, such as acquisition of nutrients, adhesion to mucosal epithelium, evasion of molecule- and cell-based bacterial clearance mechanisms, and recruitment of foreign DNA for natural genetic transformation. Electrostatic repulsion conferred by negative charge of the polysaccharide capsule is the critical for proper dispersion of pneumococcal cells and resistance to nonopsonic killing by human neutrophils for optimal colonization and immune evasion38,39. It is known that different types of the capsule significantly vary in magnitude of negative charge and other properties39,40,41,42, which is consistent with uneven prevalence of various pneumococcal serotypes39. Multiple studies have also shown that the level of encapsulation significantly affects pneumococcal capability of nasopharyngeal colonization and virulence38,43,44. In this literature context and the profound effect of the promoter sequence variations on the level of encapsulation in this study, we hypothesize that the pneumococci within the same serotypes can behave differently in humans when the same type-specific genes of the capsule locus are naturally driven by different promoter variants. This hypothesis is also supported by our observation that the isogenic cps promoter variants of S. pneumoniae differed in epithelial adhesion, anti-phagocytosis and virulence. This notion explains why the strains within the same serotypes can differ remarkably in epithelial adhesion, anti-phagocytosis and virulence17,45,46.
The variations among the cps promoter variants in the amount of capsule are mostly consistent with their differences in transcriptional capacity as best exemplified by the type-3 and -8 promoter sequences. These two sequences consistently yielded the highest (type-3) and lowest (type-8) values in both the transcription activity and capsule amount. We thus conclude that the differences among the promoter variants in the capsule production are caused by their variations in the transcription of the capsule genes. Our previous study revealed that all of the four sequence modules (IE, RUP, SS, and core promoter) in the cps promoter are necessary for the full encapsulation and virulence of strain D39, particularly the SS and core promoter modules12. Therefore, sequence variations in any of the sequence modules may alter the transcriptional capacity of the promoter. As illustrated in Fig. 1, there are many nucleotide insertions/deletions and/or multiple arrangements of the sequence modules among the cps promoter variants. Although it remains to be defined whether and how each of the sequence changes in the cps promoter contributes to the diversification of the cps gene transcription and pneumococcal encapsulation, our data have provided a functional foundation for future investigation into pathobiological implications of these promoter variants in pneumococcal fitness and evolution in the different environmental contexts.
Some of the isogenic promoter variants in two genetic backgrounds showed striking differences in virulence in the mouse bacteremia model. As manifested by the type-33F promoter, the bacteremia levels between the isogenic promoter variants can be different by several orders of magnitude. Similar inter-promoter differences in virulence were also observed in the type-6B promoter variants in both the D39 and ST858 backgrounds. These results indicated that the pneumococcal strains within the same serotypes are able to display various levels of virulence and likely other pathobiological traits if their type-specific genes are driven by different promoter variants. Consistent with the importance of the capsule in virulence, the extent of heterogeneity in virulence among the isogenic promoter variants largely parallels with the degree of encapsulation with certain exception. The type-33F promoter replacement strain produced the thinnest capsule and displayed the weakest virulence phenotype among the promoter variants tested thus far. Similarly, other relatively weaker promoters (e.g., types 6B, 8, 11A, 19F, 22F, and 23F) also yielded remarkably low levels of virulence. Because similar promoter sequences are widely shared by the strains of different serotypes, it is reasonable to predict that the pneumococcal strains with these types of promoters belong to the low virulence category. While the capsular types have been associated with the virulence levels of the pneumococci, this work suggests that the promoter type is a new determinant of the capsule-associated virulence phenotype.
The sequence variations in the cps promoter consist of minor polymorphisms (e.g., nucleotide replacements, insertions and deletions) and major modular insertions/deletions. While the former is likely to be caused by spontaneous sequence drift, the modular insertions/deletions appears to be originally achieved by acquisition/loss of multiple mobile elements (the RUP and IE modules). The highly mosaic but nonrandom nature of the sequence polymorphisms in the cps promoter indicate that most, if not all, of the variations have been maintained and shared by the pneumococcal strains as a function of selective pressure in humans.
Many selective factors may have contributed to the heterogeneity of the cps promoter sequence. For example, mucosal epithelial environment may favor the pneumococci with weak cps promoter variants, and thereby a relatively thin capsule for efficient colonization. Similarly, it is possible that adaptive immunity specific for the CPS preferentially targets the abundantly encapsulated pneumococci, thus selecting for those with relatively weak cps promoter variants. It is thus tempting to postulate the pneumococci with a thin capsule may be better equipped for evasion of the vaccine-induced adaptive immunity and/or vaccine replacement47,48. Conversely, the pneumococci with the stronger cps promoter variants may have advantages in cellular dispersion and evasion of non-opsonic phagocytic killing. Magee et al. show that type-3 pneumococcal mutants with a thin capsule (approximately 20% of the parent strain) are able to achieve adequate nasopharyngeal colonization but unable to cause systemic infection43. Other studies have shown that quantitative differences in the amount of capsule significantly contribute to the fitness advantages and disadvantages of the opacity phase variants of S. pneumoniae in colonization and systemic infection models38,44,49.
S. pneumoniae is famous for its ability to modify the genetic content by acquiring foreign DNA through natural genetic transformation18,50,51. The diverse but conserved nature of the cps promoter among the invasive isolates in modular arrangements and sequence polymorphisms indicates that the allelic variations in this region is enriched by shuffling the entire or partial sequences across the strains of this species by natural genetic transformation. The lack of strict association between the promoter variants and serotypes also implies that the promoter and coding regions of the capsule locus are able to undergo independent recombination during natural transformation events. It is thus reasonable to envision that sampling of an exogenous DNA molecule containing the complete sequence of the capsule locus may lead to at least three different recombination outcomes (Fig. 8). First, homologous recombination(s) mediated by the common sequences in the dexB and aliA regions may lead to complete replacement of the original capsule locus with the incoming sequence, and thereby gain a new type of capsule (serotype replacement). Second, the conserved sequences in the dexB and common cps genes (e.g., cpsABCD) may facilitate alternative recombination(s) only in the promoter region. This type of recombination would only alter the thickness but not antigenic/chemical properties of the original capsule. Lastly, homologous recombination(s) may also occur in the type-specific genes, which would retain the original promoter sequence but switch the capsule type. This type of promiscuous assortment between the promoter and coding regions of the capsule locus can generate additional level of phenotypic diversity in different populations (or strains), in addition to the well-know capsule type switch in this species. In the evolutionary perspective, our finding has provided exciting evidence to ultimately uncovering the mystery of pneumococcal resilience in the era of antibiotic and vaccine.
The abundantly encapsulated pneumococci (represented by the thick rectangle in the promoter region) may lyse and release their chromosomal DNA by autolysis or fratricide. The free DNA can be taken up by the nearby pneumococcal cells through natural genetic transformation, and recombined into the capsule locus of the recipient cells by one of the three modular homologous recombination schemes as indicated by the dashed arrows in each of the scenarios. The double-crossover mediated by the conserved sequences in the up (e.g., dexB)- and down (e.g., aliA)-stream regions of the locus would result in replacement of the entire capsule locus with the incoming DNA and generate a pneumococcal variant with a new capsule (left panel). Alternative recombination driven by the homologous sequences in the dexB region and common capsule genes (e.g., cpsABCD) may lead to the replacement of the promoter region but not the type-specific genes, altering the transcription of the capsule genes and level of encapsulation depending on the transcription capacity of the incoming cps promoter (middle panel). The last recombination potential occurs in the common genes and the aliA region, allowing the recipient cell to capture only the type-specific genes and produce a new capsule from the original promoter (right panel).
Materials and Methods
Bacterial strains, cultivation, and chemical reagents
The bacterial strains used for studying the cps promoter variants in this study are listed in Table 1 or Fig. S1. The 225 clinical isolates of S. pneumoniae used for sequence analysis of the cps promoter variants were obtained from the existing collections of the anonymized bacterial strains in the US CDS and individual Chinese hospitals. All of the isolates were originally isolated from normally sterile sites (e.g. blood, cerebrospinal fluid - CSF) of human patients. The US isolates (n = 47) were provided by the Active Bacterial Core surveillance (ABCs)/Emerging Infections Programs (EIP) Network of the US CDC, Atlanta, USA. The Chinese isolates (n = 178) were prospectively collected by the Fourth Military Medical University affiliated Xijin Hospital, Beijing Children’s Hospital, and China CDC, Beijing. The experiments associated with the use of these strains were carried out in accordance with the principles in the Chinese law for scientific experimentations with human materials and approved by the Institutional Review Board for human studies at Tsinghua University. The serotypes of the isolates were either previously characterized (for the US isolates) or determined by a multiplex PCR-based approach (for the Chinese isolates)52. S. pneumoniae was cultured in Todd-Hewitt broth with 0.5% yeast extract (THY) or on tryptic soy agar (TSA) plates with 3% defibrinated sheep blood at 37 °C with 5% CO2 as described53. Growth feature of D39 derivatives were determined in the THY broth as described previously12. Escherichia coli DH5α was used for constructing the reporter plasmids. All restriction enzymes and chemicals were purchased from New England Biolabs (NEB, Beijing, China), and Sigma-Aldrich (Shanghai, China) respectively unless otherwise stated.
DNA sequencing analysis
The cps promoter region, or the intergenic region between the 3′ dexB and 5′ cpsA, of all the isolates except for the type-3 strains, was amplified with primers Pr7524 and Pr75259. The promoter region of the type-3 isolates was amplified with primers Pr7662 and Pr8138. The sequences of the PCR products were determined by the ABI sequencer and analyzed by BLAST and relevant components of the DNAstar’s Lasergene package (version 10). The GenBank accession numbers for the cps promoter sequences used in the promoter replacement experiments are listed in Table 1.
Construction of transcriptional reporter constructs and luciferase assay
Luciferase reporter constructs of the cps promoter were generated in pIB166 shuttle plasmid as described12. The sequence between the stop codon of dexB and ribosomal binding site of cpsA was amplified from the genomic DNA of each target pneumococcal strain, and cloned in the ApaI/BamHI site of pTH393212, a derivative of pIB16654 containing the promoterless firefly luciferase gene. The insert sequence in each recombinant plasmid was verified by DNA sequencing. The resulting plasmids and primers/source strains used to prepare them are listed in Table 1. Luciferase assay was performed as described previously12. Briefly, a pellet from 100 μl original culture was suspended in 400 μl phosphate-buffered saline (PBS), pH 7.4; a fraction out of the suspension (20 μl) was placed into a Corning NBS 96-well white plate with clear bottom. Subsequently, luciferase substrate (100 μl; Promega, Madison, WI) was added to each well to allow the monitoring of luminescence. Each reporter construct was tested at least three separate times. The results of representative experiments are presented as means of three replicates ± standard errors.
Construction of pneumococcal mutants
All unmarked deletions and sequence replacements in S. pneumoniae strains were carried out with the Janus cassette (JC)-mediated counter selection as described previously55. Strains D39s (an streptomycin-resistant derivative of D39), TH4702 (D39s-ΔPcps::JC, a cps-promoter JC-replacement derivative of D39s), TH4525 (TH4702-ΔPcps, a cps-promoter unmarked deletion derivative of TH4702) were previously constructed12. The unmarked cps promoter replacement strains were generated in D39s or ST858s, a streptomycin-resistant derivative of type-6B strain ST858. ST858 was originally isolated from the blood of a patient in the US and obtained from the US CDC from the Active Bacterial Core surveillance (ABCs)/Emerging Infections Programs (EIP) Network of the US CDC. ST858s was generated with an amplicon of the rpsL1-containing sequence from strain ST58855, and used to generate the JC knock-in mutant TH7180 (ST858s-ΔPcps::JC) by replacement of the cps promoter region (sequence between the dexB stop codon and cps6BA start codon) in ST858s with JC through an amplicon from genomic DNA of TH4702 with primers Pr7339/Pr7344. The unmarked promoter replacement strains were prepared in the D39 and ST858 backgrounds by amplifying the flanking sequences of the cps promoter region from genomic DNA of each strain with the same primer set: Pr7339/Pr7340 and Pr7343/Pr7344, linking the PCR products to each exogenous cps promoter amplicon by fusion PCR using Primers Pr7339 and Pr7344, transforming TH4702 or TH7180 with target fusion PCR products, and selected/screened for streptomycin-resistant and kanamycin-sensitive transformants. The exogenous cps promoter sequences were individually amplified from genomic DNA samples of the source strains with strain-specific primers as described in Table S3. All of the mutated loci in the resulting pneumococcal mutants were verified by DNA sequencing.
RNA extraction and quantitative real-time reverse transcriptase PCR (qRT-PCR)
Total pneumococcal RNA was purified using an RNAprep pure Cell/Bacteria Kit (TianGen, Beijing, China) according to the supplier’s instructions. The mRNA level of the cps genes was quantified by quantitative RT-PCR (qRT-PCR) as described12. The pneumococcal rplI gene encoding the ribosomal protein RplI was amplified with primers Pr7709 and Pr7710 as an internal control. The transcripts of the cps2A/6BA and cps2E/6BE genes were detected with primer pairs Pr7705/Pr7706 and Pr7707/Pr7708, respectively. Each qRT-PCR experiment was repeated at least three times. The results of representative experiments are presented as means of three replicates ± standard errors.
Anti-CPS antibodies and immunoblotting
Pneumococcal CPS was detected by immunoblotting as described previously12. The type-2 or -6B capsular polysaccharide in D39 and ST858 derivatives was semi-quantified with a rabbit antiserum against the type-2 CPS (1:20,000 dilution) from Statens Serum Institut (Copenhagen, Denmark) or a self-prepared rabbit antiserum against the CPSs (types 4, 6B, 9V, 14, 18C, 19F, and 23F) (1:100 dilution) in the pneumococcal PCV7 vaccine. Anti-PCV7 serum was prepared in New Zealand white rabbits (Vital River, Beijing, China) by subcutaneous immunization with the PCV vaccine according to the standard protocol56. The animal works associated with the antibody production in rabbits were in accordance with the principles in the Chinese law on the humane use of animals for scientific experimentations, and were approved by the Institutional Animal Care and Use Committee in Tsinghua University. The bacterial cultures were pelleted by centrifugation and resuspended to an OD620 of 0.4 in phosphate-buffered saline (PBS) and 2-fold dilution before being used for CPS immunoblotting. The CPS-binding activities of the primary antibodies were detected by peroxidase-conjugated goat anti-rabbit IgG (H+L) (Bio-Rad, 1:10,000 dilution) and the ClarityTM Western ECL reagent (Bio-Rad). The ImageJ software (ImageJ 1.47v; National Institutes of Health) was used to digitize the amount of pneumococcal CPS on 2X, 4X, 8X and 16X dilutions spots on the basis of its chemiluminescence intensity level. The results of representative experiments are presented as means of three replicates ± standard errors. The error bars are means of the detectable spots for each sample.
Bacterial adhesion to epithelial cells and phagocytosis
Pneumococcal adhesion to host cells was determined with the confluent monolayers of human lung alveolar epithelial cell line A549 in 24-well plates as described in our previous study12. Each monolayer was infected with 107 colony-forming units (CFUs) of pneumococci. Phagocytosis of pneumococcal was determined with RAW264.7 murine macrophage cells57. Bacteria were incubated with RAW264.7 cells for 2 h, followed by incubating 1 h to kill extracellular bacteria with Penicillin (20 μg/ml) and gentamycin (400 μg/ml), extensive washing and lysis of the cells. The adherent or invasion bacteria were quantified by plating the cell lysates on TSA blood plates and counting the CFU. Each adhesion or phagocytosis experiment was repeated at least three times. The results of representative experiments are presented as means of four replicates ± standard errors.
Pneumococcal virulence test
The virulence capacity of pneumococcal strains was assessed in a murine bacteremia model as previously described12. The animal works associated with pneumococcal infection in mice were conducted in accordance with the principles in the Chinese law on the humane use of animals for scientific experimentations, and were approved by the Institutional Animal Care and Use Committee in Tsinghua University. Briefly, groups of 10 female CD1 mice (6–8 weeks old, Vital River) were infected by intraperitoneal inoculation with approximately 500 CFUs per mouse for each co-infection of D39 and its derivative (~250 CFUs each) or 106 CFUs per mouse for the corresponding experiment with ST858 and its derivative (~5 × 105 CFUs each). The viable pneumococci in the bloodstream of each mouse were quantified by plating the blood sample on TSA agar dishes with (mutant) or without (mutant plus wild type) streptomycin selection 21 h post inoculation for enumeration of colonies. The level of attenuation was expressed as a competitive index (CI), which was defined as the output CFUs ratio (mutant/wild type) divided by the input CFU ratio (mutant/wild type) as described12. This factor was considered in calculation of the final CI values. When only the wild-type pneumococci (no mutant) were recovered from a mouse in the co-infection experiments, a value of 1 was given for the mutant output for the convenience of data presentation in common logarithms.
Statistical analysis
Statistical significance of the data from qRT-PCR, luciferase, and immunoblotting was determined by two-tailed unpaired Student’s t test. The data from animal infection and cell experiments were analyzed by Wilcoxon signed-rank and one-way analysis of variance (ANOVA) tests, respectively. Significant differences are defined by p values <0.05 (*), <0.01 (**), and <0.001(***).
Additional Information
How to cite this article: Wen, Z. et al. Allelic Variation of the Capsule Promoter Diversifies Encapsulation and Virulence In Streptococcus pneumoniae. Sci. Rep. 6, 30176; doi: 10.1038/srep30176 (2016).
References
Musher, D. M. In Principles and Practice of Infectious Diseases Vol. 2 (eds Mandell, G. L., Bennett, J. E. & Dolin, R. D. ) 2623–2642 (Elsevier Churchill Livingstone, 2010).
O’Brien, K. L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902, 10.1016/S0140-6736(09)61204-6 (2009).
Walker, C. L. et al. Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416, 10.1016/S0140-6736(13)60222-6 (2013).
Avery, O. T., MacLeod, C. M. & McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79, 137–158 (1944).
Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol 65, 563–581, 10.1146/annurev.micro.62.081307.162944 (2011).
Nahm, M. H. & Katz, J. In Fundamental Immunology (ed William E. P. ) Ch. 41, 1001–1015 (Lippincott-Raven Publishers, 2012).
Lee, C. J., Banks, S. D. & Li, J. P. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol 18, 89–114, 10.3109/10408419109113510 (1991).
Geno, K. A. et al. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev 28, 871–899, 10.1128/CMR.00024-15 (2015).
Bentley, S. D. et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet 2, e31, 10.1371/journal.pgen.0020031 (2006).
Llull, D., Munoz, R., Lopez, R. & Garcia, E. A single gene (tts) located outside the cap locus directs the formation of Streptococcus pneumoniae type 37 capsular polysaccharide. Type 37 pneumococci are natural, genetically binary strains. J Exp Med 190, 241–251, 10.1084/jem.190.2.241 (1999).
Iannelli, F., Pearce, B. J. & Pozzi, G. The type 2 capsule locus of Streptococcus pneumoniae. J Bacteriol 181, 2652–2654 (1999).
Wen, Z. et al. Sequence elements upstream of the core promoter are necessary for full transcription of the capsule gene operon in Streptococcus pneumoniae strain D39. Infect Immun 83, 1957–1972, 10.1128/IAI.02944-14 (2015).
Moscoso, M. & Garcia, E. Transcriptional regulation of the capsular polysaccharide biosynthesis locus of streptococcus pneumoniae: a bioinformatic analysis. DNA Res 16, 177–186, 10.1093/dnares/dsp007 (2009).
Shainheit, M. G., Mule, M. & Camilli, A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun 82, 694–705, 10.1128/IAI.01289-13 (2014).
Feldman, C. & Anderson, R. Review: Current and new generation pneumococcal vaccines. J Infect 69, 309–325, 10.1016/j.jinf.2014.06.006 (2014).
Lanie, J. A. et al. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 189, 38–51, 10.1128/JB.01148-06 (2007).
Ring, A., Weiser, J. N. & Tuomanen, E. I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest 102, 347–360, 10.1172/JCI2406 (1998).
Chewapreecha, C. et al. Dense genomic sampling identifies highways of pneumococcal recombination. Nature genetics 46, 305–309, 10.1038/ng.2895 (2014).
Wen, Z. & Zhang, J.-R. In Molecular Medical Microbiology Vol. 1 (eds Tang, Y.-W. et al. ) Ch. 3, 33–52 (Academic Press, 2015).
Drysdale, M., Bourgogne, A. & Koehler, T. M. Transcriptional analysis of the Bacillus anthracis capsule regulators. J Bacteriol 187, 5108–5114, 10.1128/JB.187.15.5108-5114 (2005).
Gottesman, S. & Stout, V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Molecular microbiology 5, 1599–1606, 10.1111/j.1365-2958.1991.tb01906.x (1991).
Torres-Cabassa, A. S. & Gottesman, S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol 169, 981–989 (1987).
Majdalani, N. & Gottesman, S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol 59, 379–405, 10.1146/annurev.micro.59.050405.101230 (2005).
Bayer, A. S., Eftekhar, F., Tu, J., Nast, C. C. & Speert, D. P. Oxygen-dependent up-regulation of mucoid exopolysaccharide (alginate) production in Pseudomonas aeruginosa. Infection and immunity 58, 1344–1349 (1990).
Wessels, M. R. In Gram-Positive Pathogens (eds Fischetti, V. A. et al. ) 37–46 (ASM Press, 2006).
Hilse, R., Hammerschmidt, S., Bautsch, W. & Frosch, M. Site-specific insertion of IS1301 and distribution in Neisseria meningitidis strains. J Bacteriol 178, 2527–2532 (1996).
Hammerschmidt, S. et al. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J 15, 192–198 (1996).
Hammerschmidt, S. et al. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol 20, 1211–1220, 10.1111/j.1365-2958.1996.tb02641.x (1996).
Kroll, J. S., Loynds, B. M. & Moxon, E. R. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol 5, 1549–1560, 10.1111/j.1365-2958.1991.tb00802.x (1991).
Corn, P. G., Anders, J., Takala, A. K., Kayhty, H. & Hoiseth, S. K. Genes involved in Haemophilus influenzae type b capsule expression are frequently amplified. J Infect Dis 167, 356–364, 10.1093/infdis/167.2.356 (1993).
Noel, G. J., Brittingham, A., Granato, A. A. & Mosser, D. M. Effect of amplification of the Cap b locus on complement-mediated bacteriolysis and opsonization of type b Haemophilus influenzae. Infection and immunity 64, 4769–4775 (1996).
Xue, P., Corbett, D., Goldrick, M., Naylor, C. & Roberts, I. S. Regulation of expression of the region 3 promoter of the Escherichia coli K5 capsule gene cluster involves H-NS, SlyA, and a large 5′ untranslated region. J Bacteriol 191, 1838–1846, 10.1128/JB.01388-08 (2009).
Hobbs, M. & Reeves, P. R. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Molecular microbiology 12, 855–856, 10.1111/j.1365-2958.1994.tb01071.x (1994).
Waite, R. D., Struthers, J. K. & Dowson, C. G. Spontaneous sequence duplication within an open reading frame of the pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol Microbiol 42, 1223–1232, 10.1046/j.1365-2958.2001.02674.x (2001).
Waite, R. D., Penfold, D. W., Struthers, J. K. & Dowson, C. G. Spontaneous sequence duplications within capsule genes cap8E and tts control phase variation in Streptococcus pneumoniae serotypes 8 and 37. Microbiology 149, 497–504, 10.1099/mic.0.26011-0 (2003).
Schaffner, T. O. et al. A point mutation in cpsE renders Streptococcus pneumoniae nonencapsulated and enhances its growth, adherence and competence. BMC microbiology 14, 210, 10.1186/s12866-014-0210-x (2014).
Shainheit, M. G., Valentino, M. D., Gilmore, M. S. & Camilli, A. Mutations in pneumococcal cpsE generated via in vitro serial passaging reveal a potential mechanism of reduced encapsulation utilized by a conjunctival isolate. Journal of bacteriology 197, 1781–1791, 10.1128/JB.02602-14 (2015).
Nelson, A. L. et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 75, 83–90, 10.1128/IAI.01475-06 (2007).
Li, Y., Weinberger, D. M., Thompson, C. M., Trzcinski, K. & Lipsitch, M. Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infection and immunity 81, 4519–4524, 10.1128/IAI.00724-13 (2013).
Melin, M. et al. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun 77, 676–684, 10.1128/IAI.01186-08 (2009).
Melin, M. et al. Serotype-related variation in susceptibility to complement deposition and opsonophagocytosis among clinical isolates of Streptococcus pneumoniae. Infect Immun 78, 5252–5261, 10.1128/IAI.00739-10 (2010).
Melin, M., Trzcinski, K., Meri, S., Kayhty, H. & Vakevainen, M. The capsular serotype of Streptococcus pneumoniae is more important than the genetic background for resistance to complement. Infect Immun 78, 5262–5270, 10.1128/IAI.00740-10 (2010).
Magee, A. D. & Yother, J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun 69, 3755–3761, 10.1128/IAI.69.6.3755-3761.2001 (2001).
Kim, J. O. & Weiser, J. N. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis 177, 368–377, 10.1086/514205 (1998).
Frazao, N. et al. Virulence potential and genome-wide characterization of drug resistant Streptococcus pneumoniae clones selected in vivo by the 7-valent pneumococcal conjugate vaccine. PloS one 8, e74867, 10.1371/journal.pone.0074867 (2013).
Mizrachi Nebenzahl, Y. et al. Virulence of Streptococcus pneumoniae may be determined independently of capsular polysaccharide. FEMS Microbiol Lett 233, 147–152, doi: http://dx.doi.org/10.1016/j.femsle.2004.02.003 (2004).
Croucher, N. J. et al. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nature genetics 45, 656–663, 10.1038/ng.2625 (2013).
Weinberger, D. M., Malley, R. & Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 378, 1962–1973, 10.1016/S0140-6736(10)62225-8 (2011).
Weiser, J. N. In Gram-Positive Pathogens (eds Fischetti, V. A. et al. ) 268–274 (American Society for Microbiology Press, 2006).
Hanage, W. P., Fraser, C., Tang, J., Connor, T. R. & Corander, J. Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science 324, 1454–1457, 10.1126/science.1171908 (2009).
Croucher, N. J. et al. Rapid pneumococcal evolution in response to clinical interventions. Science 331, 430–434, 10.1126/science.1198545 (2011).
Pai, R., Gertz, R. E. & Beall, B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 44, 124–131, 10.1128/JCM.44.1.124-131.2006 (2006).
Chen, H. et al. Genetic requirement for pneumococcal ear infection. PLoS One 3, e2950, 10.1371/journal.pone.0002950 (2008).
Biswas, I., Jha, J. K. & Fromm, N. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology 154, 2275–2282, 10.1099/mic.0.2008/019265-0 (2008).
Lu, L., Ma, Y. & Zhang, J. R. Streptococcus pneumoniae recruits complement factor H through the amino terminus of CbpA. J Biol Chem. 281, 15464–15474, 10.1074/jbc.M602404200 (2006).
Harlow, E. & Lane, D. Using antibodies: A laboratory manual. (Cold Spring Harbor Laboratory Press, 1999).
Zhang, J. R. et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102, 827–837, 10.1016/S0092-8674(00)00071-4 (2000).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22, 4673–4680, 10.1093/nar/22.22.4673 (1994).
Acknowledgements
We thank Yonghong Yang, Zhujun Shao, Yueyun Ma, and the Active Bacterial Core surveillance (ABCs)/Emerging Infections Programs (EIP) Network of the US CDC for providing the clinical isolates. This study was supported by grants from the Ministry of Science and Technology of China (No. 2012CB518702), National Natural Science Foundation of China (No. 31530082), Tsinghua University Research Program (No. 20161080058), and the Grand Challenges Exploration of the Bill and Melinda Gates Foundation (No. OPP1021992). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
Z.W. and J.-R.Z. designed the experiments; Z.W. and Y.L. performed the experiments; Z.W., Y.L., F.Q. and J.-R.Z. analyzed the results; Z.W. and J.-R.Z. wrote the paper. All authors amended and commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wen, Z., Liu, Y., Qu, F. et al. Allelic Variation of the Capsule Promoter Diversifies Encapsulation and Virulence In Streptococcus pneumoniae. Sci Rep 6, 30176 (2016). https://doi.org/10.1038/srep30176
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30176
- Springer Nature Limited