Abstract
As global warming and climate-change proceeds ever more rapidly, organisms depending on seasonal cues to synchronize reproduction face an unclear future. Reproduction in Diadema setosum in the Gulf of Aqaba (Red Sea) is seasonal, with mature individuals occurring from July to October. Gonad indexes (GI), in contrast, indicate that spawning occurs from August through December and suggests two main spawning events. Histological analysis, however, indicate that the second peak of GI values cannot be related to spawning, but rather correspond to recovering individuals. In Diadema, examination of GI values alone may thus lead to erroneous conclusions. GI was moderately-strong positively correlated with sea-surface temperatures, but not with chlorophyll-a concentrations or photoperiod. Spawning coincides with the onset of the annual chlorophyll-a increase, however, which might be advantageous for nutrition of the developing larvae. First significant GI increase coincides with the shortening of day-length, which may act as a cue for D. setosum gametogenesis. Gametogenesis is highly synchronised between sexes, although the mature phase of females exceeds that of males. The non-complete overlap may represent sampling bias or represent an adaptive strategy for enhancing fertilisation success. Skewed sex ratios (♀:♂ 1:0.59, n = 360) in the Gulf of Aqaba may be related to pollution.
Similar content being viewed by others
Introduction
Sea urchins from the genus Diadema are some of the most widespread, abundant and ecologically important echinoids in tropical regions1. Eight named extant species of Diadema are currently recognised2,3. Some of these contain distinct mitochondrial lineages4, which may imply the existence of additional, yet unnamed, species. They inhabit tropical waters from the intertidal zone to a depth of 360 m5. Species of Diadema are conspicuous members of benthic communities1 and are often regarded as keystone species in coral-reef environments6.
Diadema, like most echinoids, are broadcast spawners, releasing gametes into the seawater1. Successful reproduction, therefore, necessitates synchronous spawning of a certain number of individuals within a population to achieve the sperm concentrations needed for fertilisation. As such, population densities as well as reproductive behaviour, such as adult aggregations during spawning, play a vital role in fertilisation success7. There are, however, vast spatial and temporal variations in spawning synchrony and aggregative behaviour between different populations, even within a single species – Diadema antillarum for example was observed to form tight spawning aggregations in some areas8, but not in others9. In Diadema, as in most other sea urchins, gametogenesis typically follows a set of sequential maturation stages that are characterised by seasonal changes in gonad development and mass10. As fertilisation success is highly dependent on intra-population synchronisation, both gamete synthesis and spawning activation are believed to be mediated by external environmental cues11. Multiple environmental factors may be involved in regulation of this process and some have been suggested to also play an important role in regulating Diadema reproduction; among these are temperature12, photoperiod13, tides14, food availability15 and lunar cycles16. In addition, the length of the reproductive period has been observed to vary within the geographic range of wide-spread echinoderm species, with populations near the equator spawning more or less continuously and seasonally at the edges of their ranges12.
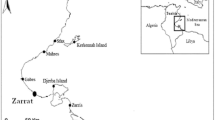
Diadema setosum inhabits a vast geographic range4, ranging from east Africa and the Red Sea, throughout the entire tropical Indian Ocean to Japan and the South Pacific Islands (Fig. 1). However, in contrast to the extensively studied D. antillarum, which has been studied across most of its geographic range, comparatively fewer detailed publications are available on the reproductive cycle of D. setosum. Most of the available information on the reproduction of D. setosum derives from a major study by John Pearse12, who reviewed reproductive patterns of four Info-Pacific echinoderm species based on published and novel fieldwork data. Since then additional accounts on the reproductive cycles of D. setosum from selected localities have been published14,17,18. The broad latitudinal range of Diadema setosum facilitates an examination of the ‘equatorial model’, one of the most prevalent paradigms in marine invertebrate reproduction. This model predicts that for species with a broad latitudinal distribution, continuous reproduction is expected in the tropics, while a restricted breeding season is expected at higher latitudes19. In the Gulf of Aqaba (GOA) D. setosum is a major component of the benthic fauna in both coral-reef and rocky environments20,21, being one of the most abundant echinoderms in these habitats and an important ecosystem engineer.
Distribution map and spawning periodicities of Diadema setosum.
Locations of known spawning times are indicated by dots. Radial plots indicate estimated spawning periods (black portions) for each location. The plots are constructed of 12 equally sized slices representing the monthly annual cycle from January to December (clockwise). In sites of continuous year-round spawning, peak spawning months are indicated by asterisk (if such distinction was mentioned in the literature). Distribution estimates were based on: Pearse12,57; Clark and Rowe58; Marsh and Marshall59; Rowe and Gates60; Shin61; Lessios et al.4; James62; Sastry63; Yokes and Galil64; Nader and El Indary65 as well as data available from the OBIS website (http://www.iobis.org/home). Lowercase letters indicate the underlying publication of spawning periodicities for the particular location (only publications specifically referring to spawning periodicities were considered for the construction of this map). a: Kenya - Muthiga18; b: Al-Ghardaqa - Pearse10; c: Wadi-el-Dom (Gulf of Suez)- Pearse10; d: Port Tawtiq (Gulf of Suez) - Fox46; e: Eilat (Gulf of Aqaba)- this study; f: Kuwait - Alsaffar and Lone17; g: Sichang (Thailand)- Kobayashi45 (report of spawning in a two month study during February and March) ; h: Seto (Japan)- Onoda66, Kobayashi and Nakamura67; i: Misaki (Japan)- Yoshida30; j: Philippines - Tuason and Gomez44; k: Singapore - Hori et al.31; l: Low Isles (Great Barrier Reef) - Stephenson68; m: Rabaul (New Britain Island) - Pearse12 (report of spawning by field observations on 24.02.1965 based on pers. comm. from J. J. Gonor); n: Fiji - Coppard and Campbell14. The map was created based on the Wikimedia Commons public domain map file BlankMap-World6.svg (https://commons.wikimedia.org/wiki/File:BlankMap-World6.svg) and manually edited using CorelDRAW x4.
The GOA is a narrow, semi-enclosed gulf located at the northeast tip of the Red Sea. It is of special research interest since it differs in its environmental parameters from both the Red Sea Main Basin (RSMB) and the Gulf of Suez (GOS) and has been suggested to have acted as a refugium for the Red Sea fauna during Pleistocene sea-level low-stands and salinity crises22. The GOA comprises a wide range of different habitats including lagoons, sea grass meadows, mangrove stands and some of the northern-most tropical coral reefs23. In contrast to the long and shallow GOS (250 km long and ca. 35 m in average depth), the GOA is shorter and deeper (160 km long and ca. 650 m average depth) and shows different environmental conditions24. Despite of their latitudinal similarity and in contrast to the relatively stable conditions of the GOA, the shallow depth of the GOS causes it to be temperate in character, reflecting seasonal changes of the same magnitude as the eastern Mediterranean25,26. Furthermore, based on species distribution and abundance, James and Pearse20 concluded that the environmental conditions within the GOA are more similar to those in the RSMB than to those in the GOS and that the major faunistic differences between these two gulfs are most likely related to their differing depths. Likewise, the reproductive cycle of some marine invertebrates, including some echinoid species, seem to differ between the two gulfs and the RSMB despite their relative geographical proximity (e.g., Echinometra sp.27). However, while the reproductive cycle of D. setosum is well studied for the GOS10,12 and the RSMB28, no data are so far available for the GOA.
Here we provide the first report on the reproductive biology of D. setosum from the GOA with the aim of evaluating the environmental cues that control and synchronise gamete maturation and identify spawning periodicities in this species. In addition, we provide information on sex ratios in naturally occurring Diadema populations from two contrasting environments with (a) high (GOA) and (b) low (Zanzibar) levels of anthropogenic interaction and evaluate sexual differences in resource allocation between males and females in these environments.
Results
Sex ratios and body size
The sex ratios of Diadema setosum from the GOA deviated significantly from a ratio of 1:1 indicating that females are more abundant than males (96 males, 164 females; Chi-square test, x2 = 17.785, df = 1, p < 0.0001), in contrast to the equal ratio recorded in Zanzibar (199 males, 215 females; Chi-square test, x2 = 0.62, df = 1, p = 0.432). No hermaphrodites were observed in any of the samples.
The weights and diameters of D. setosum from the GOA and Zanzibar were positively and highly correlated (r2 = 0.85, p < 2.2e-16 for the GOA and r2 = 0.83, p < 2.2e-16 for Zanzibar), demonstrating allometric weight increase with growth; the linear regression lines of the log transformed data are shown in Fig. 2a–c. The best fit of test diameter to the model was superior to that of test height (r2 = 0.82 and r2 = 0.73, respectively). No significant differences were observed between the regressions of males and females at all sites.
Size and sex relationships in populations of Diadema setosum from the Red Sea (Eilat) and Western Indian Ocean (Zanzibar).
Linear regression models of diameter and weight (log transformed) in two populations of D. setosum: (a) Eilat, (b) Zanzibar and (c) the pooled data from both locations. Females are denoted by red circles; males by green triangles. Regression lines with 95% confidence interval are fitted for each plot and the corresponding equations and respective r2 and p-values are provided. The number of samples used, for both females (nf) and males (nm), is given below the corresponding equations.
Gametogenic cycle
Histological inference
Four different gonad maturation stages have been recognised for male and female D. setosum (Fig. 3). These stages, termed Spent, Recovering, Growing and Mature, (corresponding to stage numbers I to IV, respectively; see Fig. 3 for details), appeared successively throughout the duration of the study from January through December. Despite being generally consistent, some variation among individuals from the same monthly sample was occasionally observed. Gonads maturation stages developed from a gamete and nutritive phagocyte (NP) depleted phase (Spent) to a stage of energy replenishment through NPs renewal (Recovering), followed by the synthesis of new germinal cells (Growing) and the accumulation of ripe gametes (Mature) ultimately leading to spawning.
The reproductive stages of Diadema setosum from the Gulf of Aqaba.
Histological photomicrographs of ovaries (a,c,e,g) and testes (b,d,f,h). Cross-sections through acini representing reproductive stages I–IV. Stage I (spent): Gonads are largely devoid of contents showing ova-free lumen in females (a) and spermatozoan-free lumen in males (b) and may contain unspawned ova and spermatozoa undergoing lysis. A thin layer of NPs is present along the ascinal walls in both sexes and may form a pale meshwork across the ascinus. Strongly basophilic previtellogenetic oocytes or primary spermatocytes, staining dark purple with Hematoxylin and eosin, may be present along the ascinal wall. Stage II (recovering): NPs proliferate throughout the gonads from the ascinal wall to the centre, gradually filling the lumen of ovaries (c) and testis (d). Limited groups of primary spermatocytes and clusters of previtellogenetic oocytes start appearing in the testicular and ovarian germinal epithelia, respectively and may occasionally project centrally. Stage III (growing): With the onset of vitellogenesis oocytes grow in size and become decreasingly basophilic. Both early and late vitellogenetic oocytes may be present along the ovarian wall and gradually migrate to the ovarian lumen as they mature (indicated by arrow) (e). All stages of germ cells are evident in the male germinal epithelium and continuously increase in number as new spermatogonia develop basally while spermatocytes migrate to the testicular lumen, where they accumulate as mature spermatozoa, forming visible columns of darkly stained cells (f). NP deplete and progressively occupy less space in both males and females. Stage IV (mature): By the end of this stage the NP layer in both ovaries and testes is largely exhausted. Ovaries are packed with mature ova, while oocytes at different maturation stages may still be evident in the germinal epithelium (g). The testicular lumen is densely packed with spermatozoa. Occasionally some ova and spermatozoa may be evident in the coelom (h). Scale bars represent 100 μm. Ge germinal epithelium; C coelom; Po previtellogenetic oocyte; EVo early vitellogenetic oocyte; LVo late vitellogenetic oocyte; NP nutritive phagocytes; Ov ova; L lumen; Sc spermatocytes; Sz spermatozoa; Ps primary spermatocytes.
The relative monthly frequencies of the four maturation stages are shown in Fig. 4. Males and females appeared to be highly synchronised with females showing a somewhat extended mature stage in comparison to males. Spent males and females were present in low proportions from late summer through winter (end of August to February). Males at the Recovering stage were only evident during winter (November–April), while recovering females were present year-round (although more common in winter). Gametogenesis initiated in May in both males and females and high proportions of growing individuals were present through August in females and as late as October in males. Maturation and spawning occurred from midsummer to early winter (July to October). Mature males and females were first observed in July, however, the duration of the mature female stage was longer and lasted to October, reaching a peak in September.
The annual gametogenetic cycle of Diadema setosum from Eilat (Gulf of Aqaba).
The relative frequencies (%) of gonad developmental stages in monthly samples of (a) females and (b) males as defined by histological cross-sections. Frequencies are based on histological analysis of 20 specimen per month studied from January 2010 to February 2011. Colours indicate reproductive stages I–IV (corresponding to stages: Spent, Recovering, Growing and Mature, respectively) as defined in the text (see Fig. 3 for detail). ND corresponds to no data for that sampling month. Radial schematic plots provide a graphical representation of the transition and overlap of the different reproductive stages based on a monthly annual cycle.
Inference of gonadosomatic indexes
The gonado-somatic indexes of D. setosum from the GOA illustrate an annual cycle (Fig. 5 and Supplementary Fig. S1). As no significant differences in individual mean GIs between males and females were found in any of the sampled months (Mann-Whitney U test, all FDR corrected p > 0.05), their data were pooled monthly. Although some differences were observed between the different indexes (Supplementary Fig. S1), as expected from the differences in their underlying allometric measurements, they presented similar trends. The observed monthly differences were confirmed by conducting analyses of covariance (ANCOVA) for all 13 monthly samples for both independent variables (test diameter and total wet body weight) to test for monthly differences in gonad weights. Monthly changes of the allometric exponent β (the regression slopes) of both variables were highly comparable and reflect an annual cycle with similar patterns when using either test diameters or total wet weights (Supplementary Fig. S2). GIs reached an annual peak from mid-summer to early winter showing two peaks (August-September and November-December) interrupted by a sharp drop in October (Fig. 5, Supplementary Fig. S1). However, while the first annual maxima in GI index coincided with the presence of mature gametes, no ripe individuals were observed in histological examinations during the second index maximum in November to December. GI values for all indexes were comparably lower during winter from January to May, after which GI build-up was observed. The GI values in February 2011 were similar to those of February 2010.
Temporal patterns of environmental gradients during the reproductive cycle Diadema setosum.
Monthly gonad index (GIW) calculated as wet gonad weights/total wet body weight × 100 of Diadema setosum from the GOA and monthly gradients of selected environmental variables. Boxes represent monthly GIW; centre black lines show the medians; box limits indicate the 25th and 75th percentiles; the 95% confidence interval of each median is represented by the notches and is defined as +/−1.58 × IQR/sqrt(n) (with n representing the monthly number of samples); whiskers extend to minimum and maximum values with open circles representing outliers; width of the boxes is proportional to the square root of the sample size. Indexes calculated based on 20 specimens per month. No data are available for January 2011. The colour of the boxplots corresponds to the dominant reproductive stage of that month (see Fig. 4 for details). Grey zone illustrates photoperiod. Red line illustrates daily measured sea surface temperatures (°C) and green line illustrates daily measured chlorophyll-a concentrations (μg/l) fitted as a smooth curve (solid lines) and standard errors (shaded margins). The smooth was calculated by local polynominal regressions.
Mean GI values varied significantly between months (Kruskal-Wallis test, GIW: H = 93.27, df = 12, p < 1.15e-14; GID: H = 99.77, df = 12, p < 6.19e-16; SGI: H = 74.8, df = 12, p < 4.01e-11). Monthly mean GI levels had a moderate-strong positive correlation with SST (Spearman rank order coefficient, GIW: rs = 0.82, p < 0.01; GID: rs = 0.63, p < 0.02). The gametogenetic cycle followed the increase in seawater temperatures and reached peak values during the warmest months of the year (Fig. 5). Mean chlorophyll-a concentrations and photoperiod had no correlation with GI (Spearman rank order coefficient, GIW: rs = −0.21, p = 0.48 and rs = −0.08, p = 0.81; GID: rs = 0.13, p = 0.67 and rs = −0.43, p = 0.14, respectively). Nonetheless, the annual fluctuations in chlorophyll-a concentrations showed continuous increase in chlorophyll-a starting by the end of October, following the spawning event (Fig. 5), reaching its annual high in the following months. In turn, the annual peak of the photoperiod cycle in June coincided with the first significant increase in gonad index of the annual reproductive cycle, immediately followed by the first appearance of mature gonads (in July) in both sexes (Fig. 4).
Oocyte growth and maturation
The monthly diameters of oocytes and ova are given in Fig. 6 and Supplementary Fig. S3 (illustrated as size frequency distributions). Oocytes were visible at basal levels throughout the year while ova were recorded from July through October. Oocyte mean diameters varied significantly between months (permutation ANOVA, df = 12, p < 2.2e-16) as they gradually increased in size from April to October, followed by a sharp decrease in oocyte diameter in November (Fig. 6). Ova mean diameters also varied significantly between months (permutation ANOVA, df = 3, p < 2.2e-16), reaching their annual maximum in September to October and were absent from all samples in the consecutive month. As ova did not increase in size after reaching maturation, monthly differences in mean ova diameters were the result of oocytes growing larger later in the season. This pattern coincided with the observed gametogenetic cycle and subsequent spawning.
Temporal variation in ova and oocyte diameters.
Diameters (μm) of ova (red boxes) and oocytes (gold boxes) from female Diadema setosum from the GOA. Measurements conducted from January 2010 through February 2011. Boxes represent monthly average oocyte diameters; centre black lines show the medians; box limits indicate the 25th and 75th percentiles; the 95% confidence interval of each median is represented by the notches and is defined as ±1.58 × IQR/sqrt(n) (with n representing the number of samples as indicated under the boxes); whiskers extend to minimum and maximum values with open circles representing outliers; width of the boxes is proportional to the square root of the sample size.
Discussion
Body size and sex ratios
Although some sea urchins show external sexual dimorphism29, no external sexual characteristics have been reported in Diadema1. Similarly, in the current study, no external sexual dimorphism or variation in texture and colouration of the gonads between males and females were evident in more than 670 specimens examined. Furthermore, no size differences were observed between males and females D. setosum in allometric comparisons of populations from the GOA (Eilat) and WIO (Zanzibar) (Fig. 2).
Diadema are gonochoric with only rare reports of hermaphroditism29. In line with previous observations, all specimens in the current study had separate sexes. Sex ratios in echinoids have been predominantly reported to be 1:111, although a few reports showed deviation from this ratio. When deviations occur, however, they rarely stray vastly from the 1:1 ratio. D. setosum is no exception1 and most reported populations have a 1:1 ratio1,17,30, although reports of slight deviations do exist (Hori et al.31 found more males than females (1:0.7) in 459 D. setosum individuals from Singapore). D. setosum sex ratios in the current study deviated significantly from a 1:1 ratio in the GOA (1:0.59 females to males, in 360 specimens sampled), whereas D. setosum populations from Zanzibar did not (n = 414), matching the situation in adjacent populations from Kenya (Muthiga, unpubl. in Muthiga and McClanahan1). The high female proportion observed in the GOA throughout the duration of this study is unusual. Sex determination mechanisms in echinoids (as well as all other echinoderms) are, however, largely unknown1,29 and the causes for unequal sex ratios are currently unidentified. Nonetheless, extreme environmental conditions such as unusually cold winter temperatures29 and parasitic infection by ascothoracid crustaceans have previously been suggested as possible explanations (J.S. Pearse and P. Newell, personal communication in Coppard and Campbell14). In Fiji, high levels of tributyltin (TBT) in the seawater have been suggested as the cause of extremely femininely biased D. setosum populations14. As no parasitic infections have been observed in specimens from the current study this explanations seem unlikely for the GOA populations. High TBT concentrations, on the other hand, have been measured in the past from sites adjacent to the current sampling locality32. Interestingly, sex ratios in Echinometra sampled in the same locality on the exact same dates, showed equal proportions of males and females33 – possibly indicating differential susceptibility to pollutants. Specimens of Tripneustes gratilla elatensis, again from the same sampling site, showed mass skeletal deformations that are thought to have been caused by chemical pollutants34, while other echinoid species in the area, including Diadema and Echinometra, showed no signs of deformations. Evidently, different echinoid species are susceptible to different extents by varying environmental perturbations, most likely owing to their different life histories, nutrition and microhabitat distribution. Although more studies are needed to elucidate the effect of environmental conditions on echinoid sex determination, instances of skewed sex ratios may serve as indication for potential detrimental processes that may prevail in the environment. The abundance of stressors to the marine environment in the GOA24 calls for special attention for any deviation from expected values or violation of equilibriums.
Gametogenesis – evidence from histology and gonad indexes
Histological analysis of the gonads of Diadema from the GOA revealed that gametogenesis in both sexes was highly synchronised with mature individuals appearing from July to October. Interestingly, the mature phase of female Diadema in the GOA (July to October) seemed to be twice as long as that of males (July to August) (Fig. 4). Although sufficient to indicate synchronous spawning, these non-completely overlapping spawning periodicities are perplexing. It is possible that male spawning periodicities are underestimated in the current study as the number of sampled females always exceeded the number of males (up to 2.5:1), consequently better representing intra-population diversity in females. Alternatively, an extended spawning period by only one of the sexes have also been claimed to be an adaptive strategy to enhance fertilisation success by ensuring fertilisation of individuals (in this case males) spawning late or out of season25,35.
Gonad indexes (GI), the ratio between gonad and body size, have long been used as a tool for studying the reproductive cycle of a large range of species19 including many echinoids12,36. In principle, the GI is applied to depict temporal variations in gonad size that reflect the phases of the reproductive cycle19. However, many concerns and criticisms have been stressed regarding the validity of GI and its implication in reproductive studies37,38. Two fundamental drawbacks are associated with the use of gonad indexes: 1) they provide no information on the cellular level within the gonads, thus, when nutritive phagocytes within the gonads are used for synthesis of gametes, the index may remain the same despite the progress in gametogenesis19,39. 2) Most indexes implicitly assume an isometric relationship between gonad and body size that is, in most cases, not verified and in some instances utterly wrong37. In the current study, we have used histological analyses as well as several gonadal indexes and comparisons with ANCOVA to mitigate the risk of drawing erroneous conclusions. Indeed, our GI analyses (in all of the indexes used) demonstrated an increase in the index during November and December (Supplementary Fig. S1). However, our histological data clearly shows that no mature individuals and no ripe gonads were present past October, refuting the possibility of a second spawning event in the observed population. In fact, the high index values in November and December were predominantly recorded in recovering individuals (Fig. 4). Other causes than reproduction can potentially lead to changes in gonadal reserves and manifest as peaks in the GI40. King et al.41 for example, noticed that the gonads of Centrostephanus rodgersii returned to the recovering stage within a month of spawning with the GI returning to near pre-spawning levels. Similarly, post-spawning growth in Diadema from the GOA also appears to occur rapidly as the spent phase was encountered in relatively low frequencies. Rapid post-spawning growth was also reported in other echinoid species such as C. rodgersii41, Strongylocentrotus franciscans42 and Paracentrotus lividus36. Guillou and Michel43 attributed the occurrence of such GI peaks to abnormally low seawater temperatures in Sphaerechinus granularis off south Brittany. Seawater temperatures abnormalities may also be driving the currently observed post-spawning increase in GI for D. setosum from the GOA, as 2010 was significantly warmer than other years and in fact had the warmest winter on record since measurements began by the Monitoring Program at the Gulf of Eilat (Israel National Monitoring Program at the Gulf of Eilat, 2014 annual report; Fig. E3).
Small, pre-vitellogenetic oocytes were present in the ovaries throughout the year (Fig. 6, Supplementary Fig. S3). Ovaries began accumulating mature ova as early as July and had attained high ova content by September, prior to spawning, when oogenesis was completed. However, similar to other echinoid species, not all oocytes mature to ova and excess oocytes undergo phagocytosis, facilitating the reallocation of nutrients to the remaining growing oocytes10,27. Production of a large number of excess oocytes consequently leads to phagocytosis of the unspawned portion, producing a bimodal oocytes size frequency distribution25. In contrast, when only a low number of excess oocytes are being produced, the continuous progression of small to large oocytes is manifested as a unimodal distribution33,35. In this respect, Diadema from the GOA seem to produce few excess oocytes per gametogenetic cycle and their spawning is most likely exhaustive.
Geographic patterns of Diadema reproduction
The currently observed pattern of D. setosum summer restricted reproduction in the GOA, at the north-western edge of its range, lends support to the ‘equatorial model’ of Giese and Pearse19. D. setosum continuous reproduction around the tropics was recorded from as widely distant localities as: Kenya18, Singapore31, the Philippines44 and Fiji14 (Fig. 1). Reports from other locations such as Thailand45 and Rabaul in the New Britain Islands12, where spawning has been reported to occur in February and March, did not cover the entire annual cycle and reproduction in these areas may in fact still reflect a continuous pattern.
In contrast to Echinometra, where striking differences were observed between GOS and RSMB populations (the former showing seasonal reproduction while the latter continuous27), no seasonal reproductive differences between these basins were observed in Diadema10. In the GOA the spawning season of D. setosum is restricted to the warm summer months (Fig. 1e), similar to the spawning season reported for D. setosum from both the GOS and RSMB10,46, with peak spawning occurring between September and October. This is consistent with D. setosum reports from all but one locality throughout the northern hemisphere (Fig. 1). Alsaffar and Lone17 reported peak spawning between April and May off the coast of Kuwait at the northern tip of the Persian Gulf. This deviation in the spawning season despite similar latitudinal position with the GOA and GOS may be attributed to the unique environmental conditions in the Persian Gulf. The area shows extreme seasonal differences in seawater temperatures, varying from a minimum of 13.2 °C to a maximum of 31.5 °C47. More so, Alsaffar and Lone17 reported fluctuations from a minimum of 10.6 °C in January and a maximum 32.8 °C in August throughout the duration of their experiment. Such extreme high summer temperatures may be exceeding the maximum threshold for Diadema reproduction, shifting spawning periodicities to cooler months in the northern Persian Gulf. Indeed, the seawater temperatures during the spawning season of the Kuwaiti populations (24–30 °C, Apr-May) are comparable with the spawning season temperatures at the GOS (25–30 °C, Jun-Sep) and GOA (25–28 °C, Jul-Oct). Furthermore, Pearse10 notion of D. setosum being reproductively inactive at SST below about 25 °C seems to hold true also for the GOA population, as despite of the fact that growing stage individuals were already observed in May, a significant increase in gonad growth only occurred in June once SST crossed the 25 °C threshold (Fig. 4).
The latitudinal similarity of the latter three localities may also provide insights into the role of photoperiod in regulating Diadema reproduction. In contrast to temperature that varies considerably between these three localities for any given point in time, photoperiod, being governed solely by latitude, does not. Thus, although photoperiod has been noted as one of the main exogenous factors that control the reproductive cycle in echinoids48,49,50, it cannot account for the currently observed differences in spawning periodicities. Similarly, even within the GOA, no correlation was found between GI and photoperiod (Fig. 6). These results appear to be in agreement with Pearse27, who found no such correlation in Echinometra populations in the adjacent GOS. Nonetheless, the longest mean day length was reached in June, corresponding with a significant increase in GI (Fig. 6). Thus, although photoperiod does not seem to be the trigger for spawning, shortening days may still serve as an exogenous cue for gametogenesis. A similar tendency of gametogenesis initiation triggered by the onset of shortening day length was also reported in other echinoid species, such as Strongylocentrotus droebachiensis49, Eucidaris tribuloides51, as well as Echinometra sp. from the GOA33.
Similar to photoperiod, no direct correlation was found between D. setosum’s reproductive cycle and chlorophyll-a concentrations that is used as a proxy for phytoplankton abundance. Although some echinoid spawning periodicities have been associated with phytoplankton blooms52, these are unlikely to directly entrain reproductive cycles due to their huge spatial variability11. Food availability is nonetheless an important factor for the developing larvae and thus spawning periodicities timed during or just prior to annual peaks in phytoplankton are advantageous for the newly developing larvae, as the latter are ensured a constant supply of food throughout the early stages of their lives. In the GOA, D. setosum spawn at the onset of to the annual increase in chlorophyll-a concentrations. A similar pattern was also observed in Echinometra from the same localities33 as well as in Kenya35.
Indeed, the reproductive cycle of the two most abundant and ecologically significant echinoids in the GOA - Echinometra sp. and D. setosum21 – largely overlaps. Although gametogenesis in Diadema appears to be longer than in Echinometra, peak spawning of these two species occurs between September and October, with the reproductive season of Echinometra lasting almost twice as long as that of Diadema33. One possible explanation for this difference may be attributed to the different life histories and behaviour of these two species. As Diadema are considerably more mobile than Echinometra53 and often display aggregative reproductive behaviour8, an individual may improve its reproductive success by grouping with other conspecifics and tightly timing its reproductive effort to a short, exhaustive and highly synchronised spawning event. In contrast, Echinometra refrains from straying away from their crevices and has never been observed to aggregate prior to spawning. In populations of relatively low densities, such as in the GOA (Bronstein unpubl. data), Echinometra may benefit from an extended spawning season during which local groups in the population, defined by their spatial distribution, may spawn at different times during the season, triggered by the spawning of their nearby individuals.
The current study is the first to demonstrate the reproductive cycle of D. setosum in the Gulf of Aqaba. It facilitates a geographical comparison with other localities of reported D. setosum reproduction and enables evaluation of the prevailing environmental cues that control reproduction in echinoids. In addition, the current study highlights potential pitfalls in the use of gonad indexes and the risk of drawing erroneous conclusions when GI data is not supported by histological analyses. Further studies of these important reef structuring echinoids are needed to facilitate comparisons over multi-annual cycles and elucidate the forces that drive biased sex ratio in echinoids.
Methods
Twenty individuals of Diadema setosum were collected monthly along the coast of Eilat in the GOA (Red Sea, 29°32′48.1″N, 34°57′12.2″E; see Fig. 1) between January and December 2010, with an additional sampling conducted in February 2011. The samples were collected haphazardly by snorkelling at depths of 1 to 3 m and brought to the laboratory at the Inter-University Institute (IUI) in Eilat for further analysis.
The largest corona diameter (measured at the ambitus), corona height and wet weight of each specimen were recorded. Measurements to the nearest 0.5 mm were performed using thin-blade Vernier callipers to prevent interference by the spines. Weight was measured to the nearest 0.001 g after drying each specimen from excessive water for 5 min. After weighing and completion of external measurements, specimens were dissected and the gonads removed and weighed to the nearest 0.001 g (wet weight). Sex was determined by observing a small piece of gonad under a light microscope and later confirmed using the histological sections. Samples from Zanzibar (06°01′20.99″S, 39°25′30.21″E) in the western Indian Ocean were added to the analyses to facilitate comparison with other Diadema populations. In total 260 and 414 specimens were analysed from Eilat and Zanzibar, respectively.
Following external and gonadal measurements, gonad samples were prepared for histological examination. Preparation, documentation and analyses of the histological sections follow Bronstein and Loya33. Male and female gonads were classified into four gametogenetic stages, adopted from the staging methods of Yoshida30 and Pearse10, corresponding to fluctuations in both the nutritive phagocyte (NP) and germ cell populations. Temporal variations in oocyte and ova diameters were assessed by randomly measuring 50 oocytes and/or ova of selected female specimens. Only primary oocytes sectioned through the nucleolus and ova sectioned through the nucleus were considered for this analysis.
Sea surface temperatures (SST) and chlorophyll-a measurements were obtained from the Israel National Monitoring Program at the Gulf of Eilat (http://www.meteo-tech.co.il/eilat-yam/eilat_periodical_en.asp). Photoperiod data were obtained from the Earth System Research Laboratory of NOAA Global Monitoring Division (http://www.esrl.noaa.gov/gmd/grad/solcalc/calcdetails.html).
Data analyses were performed using R statistical software54. When data violated test assumptions of normal distribution and homoscedasticity, non-parametric tests or permutation analysis were performed. Permutations were performed using the packages lmPerm (Wheeler 2010; lmPerm: Permutation tests for linear models. http://www2.uaem.mx/r-mirror/web/packages/lmPerm/lmPerm.pdf), allowing all permutations of Y (i.e., Perm = “Exact”). Chi-squared tests were used to test possible differences in sex ratios. Due to the problems associated with gonad indexes37,55, several indexes have been generated and compared to explore monthly differences in gonad development. Variation in gonad weight was additionally examined by performing permutation analysis of co-variance (ANCOVA) (with gonad wet weight as the response variable and total wet weight or test diameter as the covariates). Initially, Mann-Whitney U tests were applied to test for between-sexes differences in every month (p-values corrected for multiple testing using the False Discovery Rate (FDR) correction). As no significant differences were found, both sexes were pooled for further analyses. Gonad indexes (GI) were created based on measurements of gonads wet weights, test diameters and total wet weights. Initially, GIW (gonad index wet weight) was calculated, as this is the most commonly used gonadosomatic index37. This index is based on gonad wet weight and the total wet weight (GIW = Gonad wet weight (g)/Total wet weight (g) × 100). However, as some sea urchin species may lose coelomic fluid once removed from the field in a process that varies among individuals56, test diameters have been used as an alternative to total wet weight, producing GID (GID = Gonad wet weight (g)/Test diameter (mm) × 100). Still, as both former indexes implicitly assume an isometric relationship between the two measurements (i.e., that both the gonad and body weight/diameter increase at the same rate), a third, standardised index (SGI) has been implemented following Ouréns et al.38: SGIi = logGWWi − logGWWi; where logGWWi represents the logarithm of the observed gonad wet weight in the ith individual and logGWWi is the predicted value from the regression for an individual of this body size. Kruskal-Wallis non-parametric analysis of variance (ANOVA), followed by Dunn’s post-hoc tests, were used to check for differences in the calculated indexes between months using the R package dunn.test (Dinno 2016; Dunn’s Test of Multiple Comparisons Using Rank Sums. https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf). Monthly mean GI values of the different indexes calculated were tested against monthly mean SST, chlorophyll-a and photoperiod using Spearman’s rank correlation. Pairwise Kolmogorov-Smirnov tests were applied to test for monthly differences in size frequency distributions between oocytes and ova and p-values were adjusted for multiple testing to minimise false discovery rate, using the Bonferroni correction. Monthly mean diameters of oocytes and ova were compared using permutation ANOVAs followed by Tukey’s honest significant difference (HSD) post-hoc test.
Additional Information
How to cite this article: Bronstein, O. et al. Reproduction of the long-spined sea urchin Diadema setosum in the Gulf of Aqaba - implications for the use of gonad-indexes. Sci. Rep. 6, 29569; doi: 10.1038/srep29569 (2016).
References
Muthiga, N. A. & McClanahan, T. R. Diadema. in Sea Urchins: Biology and Ecology Developments in aquaculture and fisheries science (ed Lawrence, J. M. ) Ch. 18, 257–269 (Elsevier, 2013).
Kroh, A. Diadema Gray, 1825. In World Echinoidea Database. Accessed at http://www.marinespecies.org/echinoidea/alpha.php?p=taxdetails&id=123395 on 2016-03-03 (eds Kroh, A. & Mooi, R. ) (2015).
Chow, S. et al. DNA barcoding and morphological analyses revealed validity of Diadema clarki Ikeda, 1939 (Echinodermata, Echinoidea, Diadematidae). ZooKeys 585, 1–16 (2016).
Lessios, H. A., Kessing, B. D. & Pearse, J. S. Population structure and speciation in tropical seas: global phylogeography of the sea urchin Diadema. Evolution 55, 955–975 (2001).
Pawson, D. L. The Echinoderm Fauna of Ascension Island, South Atlantic Ocean. Smithsonian Contributions to the Marine Sciences, 1–31 (1978).
Sammarco, P. W. Echinoid grazing as a structuring force in coral communities: Whole reef manipulations. Journal of Experimental Marine Biology and Ecology 61, 31–55 (1982).
Pennington, T. J. The ecology of fertilization of echinoid eggs: The consequences of sperm dilution, adult aggregation and synchronous spawning. Biological Bulletin 169, 417–430 (1985).
Randall, J. E., Schroeder, R. E. & Starck, W. A. Notes on the biology of the echinoid Diadema antillarum. Caribbean Journal of Science 4, 421–433 (1964).
Levitan, D. R. Asynchronous spawning and aggregative behaviour in the sea urchin Diadema antillarum (Philippi). In Echinoderm Biology: Proceedings of the Sixth International Echinoderm Conference, Victoria, Canada, 23–27 August 1987 (eds Burke, R. D., Mladenov, P. V., Lambert, P. & Parsley, R. L. ) 181–186 (Balkema, A. A. 1988).
Pearse, J. S. Reproductive periodicities of Indo-Pacific invertebrates in the Gulf of Suez. III. The echinoid Diadema setosum (Leske). Bulletin of Marine Science 20, 697–720 (1970).
Mercier, A. & Hamel, J. F. Advances in Marine Biology Vol. 55 (eds Mercier, Annie & Hamel, Jean-Francois ) 302 pp. (Elsevier, 2009).
Pearse, J. S. Patterns of reproductive periodicities in four species of indo-pacific echinoderms. Proceedings of the Indian Academy of Sciences - Section B 67, 247–279 (1968).
Hernández, J. C., Clemente, S. & Brito, A. Effects of seasonality on the reproductive cycle of Diadema aff. antillarum in two contrasting habitats: implications for the establishment of a sea urchin fishery. Marine Biology 158, 2603–2615 (2011).
Coppard, S. E. & Campbell, A. C. Lunar periodicities of diadematid echinoids breeding in Fiji. Coral Reefs 24, 324–332 (2005).
Garrido, M. J., Haroun, R. J. & Lessios, H. A. Annual reproductive periodicity of the sea urchin Diadema antillarum Philippi in the Canary Islands Bulletin of Marine Science 67, 989–996 (2000).
Iliffe, T. M. & Pearse, J. S. Annual and lunar reproductive rhythms of the sea urchin, Diadema antillarum (Philippi) in Bermuda. International Journal of Invertebrate Reproduction 5, 139–148 (1982).
Alsaffar, A. H. & Lone, K. P. Reproductive cycles of Diadema setosum and Echinometra mathaei (Echinoidea: Echinodermata) from Kuwait (northern Arabian Gulf). Bulletin of Marine Science 67, 845–856 (2000).
Muthiga, N. A. Coexistence and reproductive isolation of the sympatric echinoids Diadema savignyi Michelin and Diadema setosum (Leske) on Kenyan coral reefs. Marine Biology 143, 669–677 (2003).
Giese, A. C. & Pearse, J. S. Reproduction of marine invertebrates Vol. 1 (eds Giese, A. C. & Pearse, J. S. ) 1–49 (Academic Press, 1974).
James, D. B. & Pearse, J. S. Echinoderms from the Gulf of Suez and the Northern Red sea. Biological Association of India 11, 78–125 (1969).
Benayahu, Y. & Loya, Y. Seasonal occurrence of benthic-algae communities and grazing regulation by sea urchins at the coral reefs of Eilat, Red Sea. In Third International Coral Reef Symposium. (Rosenstiel School of Marine and Atmospheric Science, University of Miami) 383–389 (1977).
DiBattista, J. D. et al. On the origin of endemic species in the Red Sea. Journal of Biogeography 43, 13–30 (2016).
Loya, Y. Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Marine Biology 13, 100–123 (1972).
Loya, Y. The coral reefs of Eilat – past, present and future: three decades of coral community structure studies. In Coral Health and Disease (eds Rosenberg, Eugene & Loya, Yossi ) Ch. 1, 1–34 (Springer Berlin Heidelberg, 2004).
Pearse, J. S. Reproductive periodicities of Indo-Pacific invertebrates in the Gulf of Suez. I. The echinoids Prionocidaris baculosa (Lamarck) and Lovenia elongata (Gray). Bulletin of Marine Science 19, 323–350 (1969).
Pearse, J. S. The Gulf of Suez: Signs of stress on a tropical biota. Bulletin of the Institute of Oceanography and Fisheries (Egypt), 9, 148–159 (1983).
Pearse, J. S. Reproductive periodicities of Indo-Pacific invertebrates in the Gulf of Suez. II. The echinoid Echinometra mathaei (de Blanville). Bulletin of Marine Science 19, 580–613 (1969).
Guirguis, A. N. The Early Development of the Sea Urchin Diadema setosum (Leske, 1778) in the region of North Jeddah, Red Sea. Journal of King Abdulaziz University: Medical Sciences 21, 3–14 (2010).
Pearse, J. S. & Cameron, R. A. Echinodermata: Echinoidea in Reproduction of marine invertebrates, echinoderms and lophophorates (eds Giese, A. C., Pearse, J. S. & Pearse, V. B. ) 513–662 (Boxwood Press, 1991).
Yoshida, M. Some observations on the maturation of the sea urchin, Diadema setosum. The Zoological Society of Japan 25, 265–272 (1952).
Hori, R., Phang, V. P. E. & Toong, J. L. Preliminary study on the pattern of gonadal development of the sea urchin, Diadema setosum, off the coast of Singapore. Zoological Science 4, 665–673 (1987).
Herut, B. & Halicz, L. Preliminary screening for organic and metal pollutants in the northern Gulf of Eilat. Haifa: Israel Oceanographic & Limnological Research Ltd. (2004).
Bronstein, O. & Loya, Y. Photoperiod, temperature and food availability as drivers of the annual reproductive cycle of the sea urchin Echinometra sp. from the Gulf of Aqaba (Red Sea). Coral Reefs 34, 275–289 (2015).
Dafni, J. Pollution induced mass-deformities in Tripneustes: Biomechanical aspects. in Echinoderms: Durham – Proceedings of the 12th International Echinoderm Conference, 7–11 August 2006, (eds Harris, Larry G., Boetger, S. Anne, Walker, Charles, W. & Lesser, Michael P. ) 601–607 (CRC Press, 2010).
Muthiga, N. A. & Jaccarini, V. Effects of seasonality and population density on the reproduction of the Indo-Pacific echinoid Echinometra mathaei in Kenyan coral reef lagoons. Marine Biology 146, 445–453 (2005).
Byrne, M. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Marine Biology 104, 275–289 (1990).
Ebert, T. A., Hernandez, J. C. & Russell, M. P. Problems of the gonad index and what can be done: analysis of the purple sea urchin Strongylocentrotus purpuratus. Marine Biology 158, 47–58 (2011).
Ouréns, R., Freire, J. & Fernández, L. Definition of a new unbiased gonad index for aquatic invertebrates and fish: its application to the sea urchin Paracentrotus lividus. Aquatic Biology 17, 145–152 (2012).
Nichols, D. & Barker, M. F. A comparative study of reproductive and nutritional periodicities in two populations of Asterias rubens (Echinodermata: Asteroidea) from the English Channel. Journal of the Marine Biological Association of the United Kingdom 64, 471–484 (1984).
Sellem, F. & Guillou, M. Reproductive biology of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats of northern Tunisia (south-east Mediterranean). Journal of the Marine Biological Association UK 87, 763–767 (2007).
King, C. K., Hoegh-Guldberg, O. & Byrne, M. Reproductive cycle of Centrostephanus rodgersii (Echinoidea), with recommendations for the establishment of a sea urchin fishery in New South Wales. Marine Biology 120, 95–106 (1994).
Bernard, F. R. Fishery and Reproductive Cycle of the Red Sea Urchin, Strongylocentrotus franciscanus, in British Columbia. Journal of the Fisheries Research Board of Canada 34, 604–610 (1977).
Guillou, M. & Michel, C. Reproduction and growth of Sphaerechinus granularis (Echinodermata: Echinoidea) in southern Brittany. Journal of the Marine Biological Association of the United Kingdom 73, 179–192 (1993).
Tuason, A. Y. & Gomez, E. D. The reproductive biology of Tripneustes gratilla Linnaeus (Echinoidea: Echinodermata) with some notes on Diadema setosum Leske. In Proceedings of the International Symposium on Marine Biogeography and Evolution in the Southern Hemisphere. 2, 707–716 (1979).
Kobayashi, N. Spawning periodicity of the sea urchin Diadema setosum in Thailand. Phuket Marine Biological Centre Research Bulletin 59, 95–98 (1994).
Fox, H. M. Lunar periodicity in reproduction. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character 95, 523–550 (1924).
Downing, N. Coral reef communities in an extreme environment: The northwest Arabian Gulf. In Proceedings of The Fifth International Coral Reef Congress. Tahiti, 27 May -1 June 1985. Vol. 6 (eds Gabrie, C. & Harmelin, M. ) 343–348 (1985).
Pearse, J. S., Pearse, V. B. & Davis, K. K. Photoperiodic regulation of gametogenesis and growth in the sea urchin Strongylocentrotus purpuratus. Journal of Experimental Zoology 237, 107–118 (1986).
Walker, C. W. & Lesser, M. P. Manipulation of food and photoperiod promotes out-of-season gametogenesis in the green sea urchin, Strongylocentrotus droebachiensis: implications for aquaculture. Marine Biology 132, 663–676 (1998).
Wangensteen, O. S., Turon, X., Casso, M. & Palacín, C. The reproductive cycle of the sea urchin Arbacia lixula in northwest Mediterranean: potential influence of temperature and photoperiod. Marine Biology 160, 3157–3168 (2013).
McClintock, J. B. & Watts, S. A. The effects of photoperiod on gametogenesis in the tropical sea urch in Eucidaris tribuloides (Lamarck) (Echinodermata: Echinoidea). Journal of Experimental Marine Biology and Ecology 139, 175–184 (1990).
Himmelman, J. H. Phytoplankton as a stimulus for spawning in three marine invertebrates. Journal of Experimental Marine Biology and Ecology 20, 199–214 (1975).
McClanahan, T. R. Coexistence in a sea urchin guild and its implications to coral reef diversity and degradation. Oecologia 77, 210–218 (1988).
R: A language and environment for statistical computing (R Foundation for Statistical Computing. Retrieved from http://www.R-project.org, Vienna, Austria, 2013).
Packard, G. C. & Boardman, T. J. The misuse of ratios, indices and percentages in ecophysiological research. Physiological Zoology 61, 1–9 (1988).
Régis, M. B. Étude des possibilités d’élevage des oursins réguliers en fonction de la valeur de certains indices physiologiques. Oceanologica Acta 3, 7–15 (1980).
Pearse, J. S. Distribution of Diadema savignyi and D. setosum in the tropical Pacific. In Echinoderms: San Francisco. Proceedings of the Ninth International Echinoderm Conference, San Francisco, California, USA, 5–9 August 1996 (eds Mooi, Rich & Telford, Malcolm ) 777–782 (Balkema, A. A. 1998).
Clark, A. M. & Rowe, F. W. E. Monograph of shallow-water Indo-west Pacific echinoderms. Trustees of the British museum (Natural History) i–vii, 1-238, pls 231–231 (1971).
Marsh, L. M. & Marshall, J. Some aspects of the zoogeography of north-western Australian echinoderms (other than holothurians). Bulletin of Marine Science 33, 671–687 (1983).
Rowe, F. E. W. & Gates, J. Echinodermata. Vol. 33 1–510 (CSIRO, 1995).
Shin, S. New record of two echinoids (Echinodermata, Echinoidea) in Korea. Korean Journal of Systematic Zoology 16, 219–226 (2000).
James, D. B. Echinoderms of the Maldives. Records of the Zoological Survey of India 102, 121–125 (2004).
Sastry, D. R. K. Echinodermata of Andaman and Nicobar Islands, Bay of Bengal: an annotated list. Records of the Zoological Survey of India Occasional Paper 233, 1–207 (2005).
Yokes, B. & Galil, B. S. The first record of the needle-spined urchin Diadema setosum (Leske, 1778) (Echinodermata: Echinoidea: Diadematidae) from the Mediterranean Sea. Aquatic Invasions 1, 188–190 (2006).
Nader, M. R. & El Indary, S. First record of Diadema setosum (Leske, 1778) (Echinodermata, Echinoidea, Diadematidae) from Lebanon, Eastern Mediterranean. Aquatic Invasions Records 6, S23–S25 (2011).
Onoda, K. Notes on the development of some Japanese echinoids with special reference to the structure of the larval body. Japanese Journal of Zoology 6, 637–654 (1936).
Kobayashi, N. & Nakamura, K. Spawning periodicity of sea urchins at Seto. II. Diadema setosum. Publications of the Seto Marine Biological Laboratory 15, 173–184 (1967).
Stephenson, A. The breeding of reef animals. II. Invertebrates other than corals. Great Barrier Reef Expedition 1928–29. Scientific Reports 3, 247–272 (1934).
Acknowledgements
We gratefully acknowledge A. Rankov and J. Larsson for their assistance in the field and laboratory. We are in debt to Dr. I. Brikner for assistance with the histological analysis and for fruitful discussions. We thank the Inter University Institute (IUI) at Eilat for providing laboratory and logistical support throughout this study. This research was supported by the Israel Science Foundation (ISF) to Y.L., O.B. was partially supported by a postdoctoral fellowship of the Israeli Council For Higher Education and the Steinhardt Museum of Natural History, Israel National Center for Biodiversity Studies, Tel Aviv University, Israel. Finally we thank the editor and three anonymous reviewers for their constructive comments that helped improve the manuscript.
Author information
Authors and Affiliations
Contributions
O.B. and Y.L. conceived the idea of the research. O.B. conducted the experiment. O.B. and A.K. analysed the results. O.B., A.K. and Y.L. wrote the text and prepared the figures.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bronstein, O., Kroh, A. & Loya, Y. Reproduction of the long-spined sea urchin Diadema setosum in the Gulf of Aqaba - implications for the use of gonad-indexes. Sci Rep 6, 29569 (2016). https://doi.org/10.1038/srep29569
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29569
- Springer Nature Limited
This article is cited by
-
Influences of habitat and seasonal changes on gonadal maturation of Echinometra mathaei (Echinodermata: Echinoidea) and Tridacna squamosa (Mollusca: Bivalvia) in the Red Sea, Egypt
Environmental Monitoring and Assessment (2023)
-
Effect of mercury upon sporadic variations of sea urchin, Echinometra mathaei in the marine environment
International Journal of Environmental Science and Technology (2022)
-
Abundance and population characteristics of the invasive sea urchin Diadema setosum (Leske, 1778) in the south Aegean Sea (eastern Mediterranean)
Journal of Biological Research-Thessaloniki (2021)
-
Multiyear trend in reproduction underpins interannual variation in gametogenic development of an Antarctic urchin
Scientific Reports (2021)
-
Reproduction of the Sea Urchin Echinometra mathaei (Echinoidea: Echinodermata) Found on Buleji, Rocky Coast, Pakistan, North Arabian Sea
Thalassas: An International Journal of Marine Sciences (2019)