Abstract
Most common plant oils have little α-linolenic acid (C18:3Δ9,12,15, ALA) and an unhealthy ω6/ω3 ratio. Here, fatty acids (FAs) in the seeds of 11 species of Paeonia L., including 10 tree peony and one herbaceous species, were explored using gas chromatograph–mass spectrometer. Results indicated that all Paeonia had a ω6/ω3 ratio less than 1.0, and high amounts of ALA (26.7–50%), oleic acid (C18:1Δ9, OA) (20.8–46%) and linoleic acid (C18:2Δ9,12, LA) (10–38%). ALA was a dominant component in oils of seven subsection Vaginatae species, whereas OA was predominant in two subsection Delavayanae species. LA was a subdominant oil component in P. ostii and P. obovata. Moreover, the FA composition and distribution of embryo (22 FAs), endosperm (14 FAs) and seed coat (6 FAs) in P. ostii, P. rockii and P. ludlowii were first reported. Peony species, particularly P. decomposita and P. rockii, can be excellent plant resources for edible oil because they provide abundant ALA to balance the ω6/ω3 ratio. The differences in the ALA, LA and OA content proportion also make the peony species a good system for detailed investigation of FA biosynthesis pathway and ALA accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Tree peony symbolises happiness, wealth and power. It is renowned as Queen Wu Zetian’s favourite during the Tang Dynasty (AD 700), and has been crowned the ‘king of flower’ since that time. Now, tree peony seed oil has attracted considerable attention. The seeds are characterised by abundant unsaturated fatty acid (UFA, >90%) and high proportions of α-linolenic acid (C18:3Δ9,12,15, ALA), which accounts for 45%1,2.
The ω3 and ω6 fatty acids (FAs) are commonly in the form of ALA and linoleic acid (C18:2Δ9,12, LA), which are essential FAs for humans and must be obtained in food or dietary supplements3. The ω6 and ω3 FAs have reciprocal biological activities, and the ω6/ω3 ratio of consumption is associated with human health4. Healthy ratios of ω6 to ω3 FAs should be lower than 55; it has been recommended that human beings evolve on a diet with a ω6/ω3 FA ratio of ∼16. A lack of ω3 FA dietary intake causes a high ratio in our daily diet, such as sunflower (∼670), peanut (∼581.6), corn germ (∼100), olive oil (16.7) and soybean oil (∼3.9)7,8,9, and has been linked to blood lipid, cardiovascular, autoimmune and inflammatory diseases10,11. Owing to overharvesting and environmental pollution, fish oil can no longer serve as a source of ALA and fish cannot satisfy the vegetarian diet. Currently, plant seed oil, such as perilla (54%), flax (51%), rapeseed (9%), soybean (7%) and walnut (6%)7,12, has become the major source of ALA. However, as herbaceous plants, perilla and flax promotion is limited because of their low seed production and their competition with food crops7. As ALA-enriched resources, tree peony (45%), sacha inchi (50%), sea buckthorn (39%) and cypress (35%) are worthy of further exploration; the tree peony seed oil has a low ω6/ω3 ratio and a potential annual seed production of 57,855 tons1,13,14,15. In 2013, tree peony seeds were recommended as a new resource of ALA for seed oil production in China. At the beginning of 2015, the General Office of the State Council of China promulgated the opinions on accelerating the development of the woody oil industry, and tree peony seed oil was clearly proposed. The rediscovery of the value of this oil and the rapid development in recent years have brought unprecedented opportunities and challenges for studies on tree peony.
The genus Paeonia is divided into three sections: Moutan DC., Onaepia Lindl. and Paeonia16. In China, species in the sections of Moutan DC. and Paeonia are widely distributed. The former is shrub with a leathery disk that contains paeonol and a small amount of paeoniflorin. The latter is an herbaceous perennial, short and with a fleshy disk, with more paeoniflorin and paeonol-free. According to Hong’s taxonomic17, eight wild species, together with two subspecies, constitute section Moutan DC., which includes two subsections, namely, Delavayanae and Vaginatae. Compared with the progress made in Paeonia classification, protection, cultivation and origin of cultivated tree peoniy17,18,19,20,21, our understanding of tree peony oil is limited. To date, the FA compositions in the seeds of 60 tree peony cultivars has been reported, and another study compared the FA compositions in the seed kernel and coat of P. rockii1,2. Our present work aimed to examine the (i) FA composition of various species seeds from the genus Paeonia in China, and the (ii) FA distribution in embryo, endosperm and seed coat of P. ostii, P. rockii and P. ludlowii. Such information will be used to evaluate the potential germplasm with amounts of ALA as high-quality edible oil.
Results
FA composition in the seeds of various Paeonia species
In the seeds of sample species, 14 FAs were found, with a predominance of ALA (peak 13), LA (peak 11), oleic acid (C18:1Δ9, OA, peak 9), stearic acid (C18:0, SA, peak 8) and palmitic acid (C16:0, PA, peak 3) (Fig. 1). Myristic (C14:0, peak 1), pentadecanoic (C15:0, peak 2), cis-7-hexadecenoic (C16:1Δ7, peak 4), palmitoleic (C16:1Δ9, peak 5), margaric (C17:0, peak 6), heptadecenoic (C17:1Δ10, peak 7), vaccenic (C18:1Δ11, peak 10), arachidic (C20:0, peak 12) and eicosenoic acids (C20:1Δ11, peak 14) were minor FAs. The total FA (TFA) content, which ranged from 109.5 mg g−1 to 230.8 mg g−1, varied dramatically among the different species (Table 1). The percentage of TFA content was highest in P. decomposita (23.1%) and lowest in P. delavayi (11%). The saturated FA (SFA) and UFA content among species varied from 10.8 mg g−1 to 29.8 mg g−1 and from 98.6 mg g−1 to 210.8 mg g−1 respectively (Table 1). Table 1 also shows that the ratios of ω6/ω3 FA ranged from 0.3 to 0.83 in different species.
The FA compositions of 11 accessions are presented in Table 1. Among the Paeonia species, P. decomposita ssp. decomposita had the highest mean ALA concentration (114.7 mg g−1), whereas P. delavayi had the lowest (34.71 mg g−1). In nine other accessions, P. decomposita ssp. rotundiloba had the highest ALA concentration (97.54 mg g−1), followed by P. rockii ssp. rockii (90.57–94.47 mg g−1) and P. rockii ssp. atava (87.8 mg g−1), P. jishanensis (85.51 mg g−1), P. ostii (83.43 mg g−1), cultivars of Japanese tree peony ‘Daojin’ (59.26 mg g−1), P. obovata (51.99 mg g−1) and P. ludlowii (40.71 mg g−1). P. ostii and P. obovata had high LA contents at 59.41 and 45.03 mg g−1 respectively, whereas P. delavayi (11.03 mg g−1) had the lowest LA content, followed by P. ludlowii (26.93 mg g−1). Surprisingly, P. ludlowii and P. delavayi possessed a higher concentration of OA than ALA, a result that was significantly different from other species. Among used species, P. ludlowii (62.58 mg g−1) had the highest OA concentration, followed by P. decomposita (59.34 mg g−1), P. rockii (55.7 mg g−1) and P. delavayi (50.91 mg g−1). The lowest OA content was that of P. obovata (29.17 mg g−1). Furthermore, the minor FA content among tree peony species varied from 2.35 mg g−1 to 3.93 mg g−1. Unexpectedly, the minor FA content in P. obovata was 5.29 mg g−1 because its vaccenic acid content was 2.41 mg g−1, which was several times more than in other species.
Variations in the FA percentage content of various Paeonia species
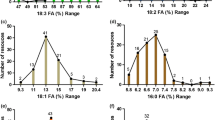
The results of FA compositions (Fig. 2) revealed significant differences among Paeonia species. Five major FAs comprised 97.5–98.7% of TFA content in tree peony species and about 96.2% in P. obovata (Fig. 2A). Hence, the minor FAs comprised 3.8% in P. obovata, a value higher than that in other tree peony species (2.5–1.3%) (Fig. 2B). On average, as the only major SFAs, the concentrations of PA and SA were basically identical. P. ludlowii (SFA, 13.6%; PA, 10.6%), P. delavayi (10.3%, 7.8%) and P. rockii ssp. atava (13.2%, 7.7%) showed much higher percentage contents of SFA and PA than those of other species (Table 1 and Fig. 2A). Particularly for SA, P. rockii ssp. rockii and P. rockii ssp. atava showed the highest percentage contents at 2.38% and 4.93% respectively (Fig. 2A). The major UFAs, namely, ALA, OA and LA, varied within 26.7–49.7%, 20.1–46.5% and 10.1–31.9%, respectively (Fig. 2A). Obviously, in most Paeonia species, with the exceptions of P. delavayi and P. ludlowii, ALA was the dominant compound (49.7% in P. decomposita), followed by LA and OA. The OA content (46.5%, 41%) was higher than ALA (31.7%, 26.7%) and LA (10.1%, 17.7%) in P. delavayi and P. ludlowii respectively. Margaric acid (C17:0, MA), which is a rare odd carbon number FA, was also found in all the investigated species. The MA content was higher in P. ludlowii (0.42%) than in other species (0.11–0.23%). P. obovata had high vaccenic acid (C18:1Δ11) content (1.72%), which was much higher than that in other species (0.19–0.78%).
Variations in percentage content of main fatty acids (A) and minor fatty acids (B) among Paeonia species. I: Paeonia jishanensis; II: Paeonia ostii; III: cultivated Paeonia rockii ssp. rockii; IV: wild Paeonia rockii ssp. rockii; V: Paeonia rockii ssp. atava; VI: Paeonia decomposita ssp. decomposita; VII: Paeonia decomposita ssp. rotundiloba; VIII: Paeonia delavayi; IX: Paeonia ludlowii; X: Japanese tree peony ‘Daojin’; XI: Paeonia obovata.
Wet and dry matter contents of seed tissues
The wet and dry matter contents of 100 seeds and separation tissues (embryo, endosperm and seed coat) of the three species are shown in Table 2. The wet and dry contents of 100 seeds were 26.44 and 22.45 g, 29.57 and 23.58 g and 85.2 and 81.16 g in P. ostii, P. rockii and P. ludlowii, respectively; correspondingly, their seed moisture contents were 15.01%, 20.28% and 4.73%. For the dry matter content, the seeds of P. ludlowii exceeded that of P. ostii and P. rockii, at an average of 3.62- and 3.44-fold respectively. For the dry matter content, the endosperm and seed coat comprised 66.02%, 68.36% and 76.55% and 33.66%, 31.34% and 23.37% of the whole seed weight in P. ostii, P. rockii and P. ludlowii, respectively; correspondingly, the embryo only represented 0.33%, 0.3% and 0.08% of the mature seed dry mass.
FA composition in the different seed tissues among three species
Figure 3 and Table 3 show that the tissues displayed high variation for FA composition in tree peony seeds. For P. ludlowii, a total of 22, 14 and 7 FAs were found respectively in embryo, endosperm and seed coat. Identical results were obtained for P. ostii and P. rockii, except that only 6 FAs were observed in their seed coat and 19 FAs were found in P. rockii embryo. As the minor FAs, heneicosanoic (C21:0, peak 15), eicosadienoic (C20:2Δ11,14, peak 16), behenic (C22:0, peak 17), erucic (C22:1Δ13, peak 18), tricosanoic (C23:0, peak 19), tetracosanoic (C24:0, peak 20), tetracosaenoic (C24:1Δ15, peak 21) and pentacosanoic acids (C25:0, peak 22) were only detected in embryo, but not in endosperm, and only one (C21:0) in the P. ludlowii seed coat. The TFA content varied dramatically among different tissues of P. ostii, P. rockii and P. ludlowii at 264.3, 199.5 and 238.4 mg g−1 in embryo, 342.1, 271.1 and 174.1 mg g−1 in endosperm and 1.61, 3.46 and 4.13 mg g−1 in seed coat, respectively (Table 3).
1: C14:0; 2: C15:0; 3: C16:0; 4: C16:1Δ7; 5: C16:1Δ9; 6: C17:0; 7: C17:1Δ10; 8, C18:0; 9: C18:1Δ9; 10: C18:1Δ11; 11: C18:2Δ9,12; 12: C20:0; 13: 18:3Δ9,12,15; 14: C20:1Δ11; 15: C21:0; 16: C20:2Δ11,14; 17: C22:0; 18: C22:1Δ13; 19: C23:0; 20: C24:0; 21: C24:1Δ15; 22: C25:0; IS: internal standard, nonadecanoic acid.
In P. ostii, LA (137.7 mg g−1) predominated in embryo oil, and OA (51.67 mg g−1) and ALA (50.98 mg g−1) were subdominant. The major FAs of endosperm oil were ALA (144.1 mg g−1), LA (107.7 mg g−1) and OA (66.21 mg g−1). However, the oil and FA in seed coat were present in small quantities, and PA predominated FA at 0.57 mg g−1. In P. rockii, LA (63.2 mg g−1) still predominated in embryo oil, followed by ALA (58.19 mg g−1) and OA (56.72 mg g−1). ALA was present at the largest level in endosperm (148.2 mg g−1), followed by OA (59.61 mg g−1) and LA (45.73 mg g−1). For seed coat, FA had a trace amount, and PA predominated at 1.25 mg g−1. However, in P. ludlowii, OA was predominant not only in embryo (122.1 mg g−1) but also in endosperm (70.64 mg g−1). PA (2.06 mg g−1) was predominant in seed coat. The ω6/ω3 ratios also varied in different tissues, ranging within 1.1–2.7 in embryo, 0.3–0.7 in endosperm and 2–2.3 in seed coat (Table 3).
Characteristics of major FA content in embryo, endosperm and seed coat
Figure 4 presents the range and distribution of PA, MA, SA, OA, LA and ALA percentage contents in embryo, endosperm and seed coat of the three species. The content of six dominant FAs varied significantly in different tissues. The major SFAs, namely, PA, MA and SA, comprised 60.5% of the TFA content of seed coat in P. ludlowii, 46% in P. ostii and 42.2% in P. rockii. However, the SFA content of embryo and endosperm was lower than that of seed coat. For P. ludlowii, P. ostii and P. rockii, the SFA content was only 13.4%, 7.0% and 8.2% of embryo, and 13.4%, 6.2% and 5.5% of endosperm, respectively. Correspondingly, the contents of major UFAs, namely, OA, LA and ALA, were higher in embryo and endosperm than that in the seed coat of the three species. ALA was the dominant compound in P. ostii and P. rockii endosperm at 42.1% and 54.7% respectively, but P. ludlowii was a distinct species with higher OA (40.6%) content than ALA (27.8%) in endosperm. Furthermore, the OA (51.3%) was still a dominant compound in P. ludlowii embryo, and LA was subdominant. In P. ostii and P. rockii embryos, LA predominated at 52.2% and 31.7, and OA and ALA were the subdominant compounds at 19.5% and 19.3%, and 28.4% and 29.1% respectively. Obviously, PA was an identical dominant compound in the seed coat of P. ostii, P. rockii and P. ludlowii at 35.4%, 36.1% and 49.2%, respectively.
Discussion
In the present study, 14 FAs were found in the seeds of all examined species. A total of 22 kinds of FA were also detected in the embryos of P. ludlowii, P. ostii and P. rockii. However, Li et al.1 reported only nine kinds of FA in the seeds of 60 tree peony cultivars. In their study, the five dominant FAs were identical to our present research, but high variability was observed in the quantitative results. In addition, there are many significant minor FAs detected in our study. MA is one of the most important FAs found in all detected Paeonia species, specifically in the seed coat of three species (Table 3 and Fig. 4). In the previous study, MA was not detected maybe because methyl heptadecanoate was employed as the internal standard1,22. However, Li et al.2 reported the presence of MA in the seed kernel and coat of P. rockii. Some differences were also observed in the FA content in various studies. The differences could be attributed to the quantitative analysis method, different cultivars, growing conditions, harvest time and storage method. For the measurement of FA content, FA (mg)/seed dry weight (DW, g) is a typical unit of measure, which was the first option used in our study and in other research1,22. Even in the same research group, the FA compositions and contents achieved in the two studies were not completely identical1,22. Trace amounts of minor FA were nearly equal in all examined species, and only vaccenic acid (C18:1Δ11) was detected at a high level in herbaceous P. obovata. The seed of P. obovata had more than 8.6-, 6.1- and 3.6-fold the amount of vaccenic acid than in the seed of P. rockii ssp. atava, P. ludlowii and P. ostii, respectively (Fig. 2B). This result was a significant characteristic between herbaceous and tree peonies.
P. ludlowii and P. delavayi belong to subsection Delavayanae, whereas P. jishanensis, P. ostii, P. rockii and P. decomposita belong to subsection Vaginatae. Evidently, the FA composition and content of the two subsections were significantly different, particularly for major UFA. The seed oils of all examined species from subsection Vaginatae were richer in ALA than in LA and OA. Surprisingly, the subsection Delavayanae species possessed a higher OA concentration than ALA and LA. Thus, Delavayanae members could be a Vaginatae control study system for the FA desaturase (FAD), specifically for Δ15-desaturase. In subsection Vaginatae, the species also exhibited different FA composition characteristics. LA was present as a subdominant compound in P. ostii and P. jishanensis, and OA was subdominant in P. rockii and P. decomposita. Therefore, the two major UFA proportions in the seed oil of subsection Vaginatae species were not identical. On the contrary, for the seed oil of subsection Delavayanae species, OA was dominant, ALA was subdominant and LA was a third compound, findings that were consistent within P. ludlowii and P. delavayi. ALA was also the dominant compound, and LA was subdominant in P. obovata seed oil, which belonged to section Paeonia. This major FA composition proportion in P. obovata was identical to that in P. ostii and P. jishanensis.
This research firstly reported the FA composition and content of seed in P. decomposita. The Danba population of P. decomposita ssp. decomposita, which is located within the altitude of 2050–3000 m, and Lixian population of P. decomposita ssp. rotundiloba, which is located within the altitude of 1700–2700 m, were sampled in this study. Obviously, the ALA and TFA contents in P. decomposita ssp. decomposita were significantly the highest, followed by those of P. decomposita ssp. rotundiloba for ALA concentration. Except for P. decomposita, P. rockii showed the second higher levels of TFA and ALA contents among investigated species. Wild-type P. rockii ssp. rockii from Longguliang population, cultivated P. rockii from Yuzhong population and P. rockii ssp. atava from Taibai population were sampled to evaluate the seed oil quality. Wild species had higher TFA and ALA contents than cultivated species. By contrast, the wild-type ssp. atava showed lower levels of TFA and ALA concentrations than the wild-type ssp. rockii and cultivated P. rockii. Among investigated species, P. jishanensis exhibited the third higher levels of TFA and ALA contents. The wild populations of P. decomposita, P. rockii and P. jishanensis were all grown within high altitude areas of 2050–3000, 1100–2800 and 900–700 m, respectively17,18. Therefore, the large amount of ALA accumulation was closely related to the ecological environment of the plant, particularly the high altitude. Recently, Connor et al.23 reported that FA composition may be too sensitive to environmental conditions to be a reliable signal of physiological maturity. Rapeseed oil content is a typical quantitative trait that can be easily influenced by environmental factors24. In a soybean oil study, the ALA concentration of wild soybean accessions was correlated with the growing environment25.
The FA compositions and distributions of embryo, endosperm and seed coat in P. ostii, P. rockii and P. ludlowii were reported in the present study for the first time. The FA composition and content in seed tissues were dramatically different. Because the low oil content of seed coat and the low dry weight of embryo, the endosperm had the highest oil yield. PA was the dominating compound in the seed coat, which was identical in the three species. For P. ostii and P. rockii, ALA was the dominant compound in endosperm, and LA was predominant in embryo. But for the embryo and endosperm in P. ludlowii, the FA profile was dominated by OA. Different seeds tissues also display high variation for FA composition, e.g. coffee seed, argan seed, and oil palm26,27,28. The tissue-specific gene expression or correlated transcription factor results in various FA distributions28. The sugar content in seed coat is correlated significantly and positively with rapeseed oil content, and seed coat may affect oil synthesis in embryo by regulating sucrose transport29. Thus, the difference in the FA composition of different tissues has many possible causes and is regulated by biochemical factor and genetic manipulation.
ALA is the shorter-chain ω3 FA from plant oil, and its deficiency could lead to abnormal function30,31. A high ω6/ω3 ratio in the diet can accentuate ω3 FA deficiency, e.g. edible oil rich in corn germ (∼100), peanut (∼581.6) and sunflower (∼670)7. The optimal ratio may vary with the disease under consideration: 5:1 ratio can suppress asthma symptoms, 4:1 is good for the prevention of cardiovascular disease and 2.5:1 suppresses colon cancer cell proliferation6. Another report suggested that the ideal ratio of ω6 to ω3 FA is 1:1–2:132. Surprisingly, the ω6/ω3 ratio in peony seed oil is less than 1.0 in all analysed species. The lowest ratio was nearly 0.3 in the seed oil of P. decomposita ssp. decomposita and P. delavayi, followed by 0.4 of P. decomposita ssp. rotundiloba, P. rockii ssp. rockii and P. rockii ssp. atava. The highest ratio was only 0.8 in the seed oil of P. obovata, followed by 0.7 of P. ostii and P. ludlowii. Interestingly, the ω6/ω3 ratio of endosperm (0.3, 0.6) was lower than that of whole seed (0.4, 0.7) in P. rockii and P. ludlowii, but the ratio was nearly 0.8 of endosperm in P. ostii, which was higher than that of whole seed (0.7). The endosperm is the main storage of tree peony seed oil, and thus had the highest ALA and LA content proportions.
In summary, this study evaluated the seed oil quality of 11 species of Paeonia L. All Paeonia species had less than 1.0 ω6/ω3 ratio. The species also had dramatically high ALA content, ranging from 26.7% (P. ludlowii) to 50% (P. decomposita ssp. decomposita); relatively high content of OA ranging from 20.8% (P. ostii) to 46% (P. ludlowii); and high content of LA ranging from 10% (P. delavayi) to 38% (P. obovate). The FA composition in the seed oil of P. decomposita, which had the highest ALA content, was firstly reported. Interestingly, a lower ALA was generally observed in the two subsection Delavayanae species of P. ludlowii and P. delavayi than in other subsection Vaginatae species. On the contrary, a higher OA was often found in Delavayanae than in Vaginatae. OA was a dominant compound in Delavayanae, whereas ALA was predominant in Vaginatae species. LA was a subdominant compound in the seed oil of P. ostii and P. obovata, which was different from OA being a subdominant compound in P. rockii and P. decomposita. Moreover, the FA composition and distribution of embryo, endosperm and seed coat in P. ostii, P. rockii and P. ludlowii were also reported for the first time. Embryo had 22 FAs, endosperm had 14 FAs and only 6 FAs were found in seed coat. Taken together, peony species, particularly P. decomposita and P. rockii, had high seed production, high ALA content and low ω6/ω3 ratio, and would thus be an excellent resource in providing abundant ALA edible oil. Furthermore, P. decomposita, P. ostii, P. obovata, P. delavayi and P. ludlowii differed in ALA, LA and OA content proportions. Hence, the peony species will be a good study system for the mechanism of FA metabolism and ALA accumulation. However, as a good source of ALA and other PUFAs, peony oil could be more hypersensitive to lipid peroxidation processes, especially in the industrial production. Lipid peroxidation process might be a more complicated problem, and this effect will be investigated in the future work step by step.
Methods
Seed materials
The Paeonia species used in this study were P. jishanensis, P. ostii, P. rockii ssp. rockii and ssp. atava, P. decomposita ssp. decomposita and ssp. rotundiloba, P. delavayi, P. ludlowii, P. obovata and one cultivar of Japanese tree peony ‘Daojin’. The plants were introduced into the garden and cultivated for three years at Shanghai Chenshan Botanical Garden (31°4′52″N, 121°10′14″E). In August 2015, the seeds were harvested when the plants had attained complete physiological maturity, and each seed sample was obtained from three to four plants of a given species. The mature seeds were used for FA analysis and stored in brown headspace vials filled with nitrogen. Independent samples were analysed in triplicate.
Separation of seed coat, endosperm and embryo
A total of 373, 200 and 210 matured seeds were directly hand-picked from P. ostii, P. rockii and P. ludlowii, respectively. Subsequently, the seed coat, endosperm and embryo of each seed were separated, weighed and flash-frozen in liquid nitrogen. All the separated embryos were used for the FA analysis. Seed coat and endosperm were randomly sampled from three to five seeds.
Extracting lipids
Stored seeds were dried to a constant weight at 60 °C. Dried seeds comprising three replicate samples were pulverised in liquid nitrogen using a mortar and pestle. The total lipid content was extracted from the biomass according to Folch et al.33 with slight modification. Firstly, approximately 0.2 g of dried powder was weighed and placed in 20 mL screw-cap glass tubes, in which 3 mL of 1:2 chloroform:methanol (v/v) mixture was added. The extraction was performed in a water bath at 35 °C for 1 h at a rotation speed of 120 rpm. After full extraction, 1.0 mL of supplementary chloroform was added to the solution, which was then re-vortexed. Next, 1.8 mL ddH2O was added so that the final volume ratio of chloroform:methanol:ddH2O was 1:1:0.9. This process was followed by centrifugation at 4000 g for 15 min. The chloroform layer was collected and transferred to another 20 mL screw-cap glass tube. The entire extraction process was repeated twice. After phase separation, the chloroform layer was withdrawn, dried with sample concentrators under a nitrogen evaporator and stored at −20 °C for further use.
FA methylation
FA methylation was prepared according to the procedures described by Bligh and Dyer34. The concentrated seed lipids prepared above were re-dissolved in 2 mL H2SO4 methanol solution (4% H2SO4). After charging with nitrogen gas, the bottle was vortexed for 1 min and placed in 90 °C water bath for 1 h. The bottle was mixed by vortexing after the addition of 1 mL ddH2O and 1 mL hexane, followed by centrifugation at 4000 g for 15 min. The supernatant was transferred to a new tube, concentrated by bubbling nitrogen and stored in 4 °C for GC-MS analysis. Additionally, 20 μL nonadecanoic acid (50 mg/mL in hexane) was used as the internal standard for each sample.
FA analysis
The FA methyl esters were subjected to GC–MS (GC7890/MS5975, Agilent) on a HP-88 capillary column (60 m × 0.25 mm, 0.2 μm, Agilent). The column temperature was held at 70 °C for 1 min, increased to 210 °C at 10 °C/min for 0 min and then to 220 °C at 10 °C/min for 0 min, and subsequently to 235 °C at 10 °C/min for 8 min. The injector temperature was set at 250 °C for split injection at a split ratio of 5:1. The injection volume was 1 μL. Helium was used as the carrier gas at a flow rate of 1 mL/min, and the ionisation potential of the mass-selective detector was 70 eV.
FA identification was achieved through a mass spectrum database search (NIST MS Search 2.0) and co-eluted with the 37-component FAME Mix (Sigma, USA). A standard curve method with an internal standard was used as the quantitative approach to construct three calibration plots of internal standard peak–area ratio versus standard concentration, as determined by the least squares method. The five major FAs in each sample were quantified in absolute terms using the linear regression of their corresponding standard, while the minor FAs were measured using methyl nonadecanoic acid as the internal standard. FAMEs were expressed as milligrams per gram DW of sample. All samples were analysed in triplicate.
Statistical analysis
The statistical analysis method was used for data processing according to the procedures described by Dunn and Clark35. All experiments were performed with three replicates. The statistical analysis of the FA contents and percentages were tested by One-way ANOVA analysis (p < 0.05) and comparisons between means were performed with Tukey′s test.
Additional Information
How to cite this article: Yu, S. et al. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 6, 26944; doi: 10.1038/srep26944 (2016).
References
Li, S. S. et al. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 173, 133–140 (2015).
Li, Z. X., Qin, G. W., He, J. H. & Cao, X. Y. Comparative analysis of fatty acid composition in seed kernel and coat of Paeonia rockii seeds. Seed 29(1), 34–36 (2010).
Covingtion, M. B. Omega-3 fatty acids. Am. Fam. Physician. 70, 133–140 (2004).
Asif, M. Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils. J. Orient. Pharm. Exp. Med. 11, 51–59 (2010).
FAO & WHO Fats and oils in human nutrition: Report of a joint expert consultation. FAO Food and Nutrition Paper No. 57, Rome (1994).
Simopoulos, A. P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379 (2002).
Connor, W. E. Alpha-linolenic acid in health and disease. The American journal of clinical nutrition 69, 827–828 (1999).
Lee, D. S., Noh, B. S., Bae, S. Y. & Kim, K. Characterization of fatty acids composition in vegetable oils by gas chromatography and chemometrics. Analytica Chimica Acta. 358(2), 163–175 (1998).
Carvalho, I. S., Miranda, I. & Pereira, H. Evaluation of oil composition of some crops suitable for human nutrition. Industrial Crops and Products 24, 75–78 (2006).
Mozaffarian, D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Alternative Therapies in Health and Medicine 11(3), 24–30 (2004).
Stark, A. H., Crawford, M. A. & Reifen, R. Update on alpha-linolenic acid. Nutrition Reviews 66(6), 326–332 (2008).
Kris-Etherton, P. M. et al. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 71, 179–188 (2000).
Follegatti-Romero, L. A., Piantino, C. R., Grimaldi, R. & Cabral, F. A. Supercritical CO2 extraction of omega-3 rich oil from Sacha inchi (Plukenetia volubilis L.) seeds. J. Super. Fluids. 49(3), 323–329 (2009).
Yang, B. & Kallio, H. P. Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J. Agric. Food Chem. 49(4), 1939–1947 (2001).
Nehdi, I. A. Cupressus sempervirens var. horizentalis seed oil: Chemical composition, physicochemical characteristics, and utilizations. Ind. Crop Prod. 41, 381–385 (2013).
Stern, F. C. A study of the genus Paeonia. London, The Royal Horticultural Society. 1–146 (1946).
Hong, D. Y. Peonies of the world: Taxonomy and Phytogeography. Royal Botanical Gardens Kew, London, UK (2010).
Hong, D. Y. Peonies of the world: polymorphism and diversity. London, UK, Royal Botanic Gardens, Kew (2011).
Yuan, J. H., Cornille, A., Giraud, T., Cheng, F. Y. & Hu, Y. H. Independent domestications of cultivated tree peonies from different wild peony species. Mol. Ecol. 23, 82–95 (2014).
Yuan, J. h., Cheng, F. Y. & Zhou, S. L. The phylogeographic structure and conservation genetics of the endangered tree peony, Paeonia rockii (Paeoniaceae), inferred from chloroplast gene sequences. Conserva. Geneti. 12, 1539–1549 (2011).
Zhou, S. L. et al. Multiple species of wild tree peonies gave rise to the ‘king of flowers’, Paeonia suffruticosa Andrews. Proc. R. Soc. B. 281, 1687–1694 (2014).
Li, S. S. et al. Fatty acid composition of developing tree peony (Paeonia section Moutan DC.) seeds and transcriptome analysis during seed development. BMC Genomi. 10.1186/s12864-015-1429-0 (2015).
Connor, K. et al. Development, fatty acid composition, and storage of drupes and seeds from the endangered pondberry (Lindera melissifolia). Biologi. Conserva. 37, 489–496 (2007).
Si, P. et al. Influence of genotype and environmention oil and protein concentrations of canola (Brassica napus L.) grown across southern Australia. Aust. J. Agric. Res. 54, 397–407 (2003).
Chae, J. H. et al. Identification of environmentally stable wild soybean genotypes with high alpha-linolenic acid concentration. Crop Sci. 55, 1629–1636 (2015).
Joët, T. et al. Metabolic pathways in tropical dicotyledonous albuminous seeds: Coffea arabica as a case study. New Phytolo. 182, 146–162 (2009).
Errouane, K. et al. The embryo and the endosperm contribute equally to argan seed oil yield but confer distinct lipid features to argan oil. Food Chem. 181, 270–276 (2015).
Dussert, S. et al. Comparative transcriptome analysis of three oil palm fruit and seed tissues that differ in oil content and fatty acid composition. Plant Physiol. 162, 1337–1358 (2013).
Liu, J. et al. Effects of specific organs on seed oil accumulation in Brassica napus L. Plant Sci. 227, 60–68 (2014).
Neuringer, M., Connor, W. E., Van Petten, C. & Barstad, L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin. Invest. 73, 272–276 (1984).
Neuringer, M., Connor, W. E., Lin, D. S., Barstad, L. & Luck, S. Biochemical and functional effects of prenatal and postnatal omega-3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. 83, 4021–4025 (1986).
Simopoulos, A. P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Bio. Med. 233, 674–688 (2008).
Folch. J., Lees, M. & Sloane, G. M. A. Simple method for the isolation and purification of total lipids from animal tissues. J Biol. Chem. 226, 497–509 (1957).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J Biochem. Physiol. 37, 911–917 (1959).
Dunn, O. J. & Clark, V. A. Applied statistics: analysis of variance and regression. J. Educa. Statis. 15(2), 175–178 (1990).
Acknowledgements
We give much thanks to Prof. Xiao-Ya Chen for revising manuscript, and Mr. Jia-Jue Li, Master sudent of Yun-Peng Wang, and Dr. Xiu-Li Zeng for their help in the sample collection. This work was funded by the Chenshan Key Scientific Research Projects of Shanghai Municipal Administration of Landscaping and City Appearance (G142435, G152424, and G162419), the National Natural Science Foundation of China (31470328), and the scientific project of Shanghai Municipal Science and Technology Commission (14DZ2260400).
Author information
Authors and Affiliations
Contributions
S.Y. performed the data analysis and the most experiments, drafted and revised the manuscript. S.D. contented the wet and dry matter in seed tissues. J.Y. designed the project, supervised the experiments, data analysis and sample collection, and prepared the manuscript and critically revised the manuscript. Y.H. co-designed the project, and participated in interpreting results and manuscript preparation. All authors carefully read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, S., Du, S., Yuan, J. et al. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci Rep 6, 26944 (2016). https://doi.org/10.1038/srep26944
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26944
- Springer Nature Limited
This article is cited by
-
Study on chemical differences between the seeds of Paeonia ludlowii and oil-producing peony based on UPLC-Q-TOF–MS/MS and GC–MS/MS
Journal of Food Measurement and Characterization (2024)
-
Transcriptome sequencing and gene expression analysis revealed early ovule abortion of Paeonia ludlowii
BMC Genomics (2023)
-
Efficient plant regeneration via meristematic nodule culture in Paeonia ostii ‘Feng Dan’
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Histological and cytological study on meristematic nodule induction and shoot organogenesis in Paeonia ostii ‘Feng Dan’
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Transcriptomic analysis of α-linolenic acid content and biosynthesis in Paeonia ostii fruits and seeds
BMC Genomics (2021)