Abstract
Rice (Oryza sativa L.) is an important staple crop. The exploitation of the great heterosis that exists in the inter-subspecific crosses between the indica and japonica rice has long been considered as a promising way to increase the yield potential. However, the male and female sterility frequently occurred in the inter-subspecific hybrids hampered the utilization of the heterosis. Here we report that the inter-subspecific hybrid sterility in rice is mainly affected by the genes at Sb, Sc, Sd and Se loci for F1 male sterility and the gene at S5 locus for F1 female sterility. The indica-compatible japonica lines (ICJLs) developed by pyramiding the indica allele (S-i) at Sb, Sc, Sd and Se loci and the neutral allele (S-n) at S5 locus in japonica genetic background through marker-assisted selection are compatible with indica rice in pollen fertility and in spikelet fertility. These results showed a great promise of overcoming the inter-subspecific hybrid sterility and exploiting the heterosis by developing ICJLs.
Similar content being viewed by others
Introduction
Asian cultivated rice (Oryza sativa L.) is the staple food for more than half of the world’s population. Continuing production of high-yielding rice is essential for maintaining global food security1,2. Asian cultivated rice is mainly distributed in Asia as well as some other areas in the world. During the course of evolution, the cultivated rice was differentiated into two distinct eco-geographic subspecies, indica and japonica3. The indica subspecies is generally adapted to the humid regions of tropical and subtropical Asia whereas japonica rice is mainly distributed in the temperate regions. Since China pioneered indica hybrid rice production in the 1970’s, a great success has been achieved in the hybrid rice development in China and around the world4,5. The indica hybrid rice usually has more about 20% yield increase compared with conventional varieties and now accounts for more than half of the annual rice planting area in China5,6. Currently used rice varieties with a close genetic relationship have limited heterosis, leading to a yield plateau for indica hybrid rice production7. Strong heterosis exists in the inter-subspecific hybrids and the exploitation of this heterosis has long been considered as a promising way to further increase rice yield potential4,5,8. However, the major obstacle of utilizing the heterosis between the subspecies is the strong hybrid sterility3,9,10,11,12.
The hybrid sterility occurs frequently in many remote crosses in rice9. The spikelet fertility of the hybrids varied widely among the crosses from almost completely sterile to fully fertile in a diallel set of 210 crosses involving 21 parents10. Spikelet fertility of F1 hybrids was found to be negatively related with the genetic divergence index of parental varieties13. The genetic basis of the inter-subspecific hybrid sterility has been extensively investigated in the last several decades. Approximately 50 loci for hybrid fertility have been identified in rice, including loci causing female gamete abortion and those inducing pollen sterility. Among the loci for hybrid fertility, some were identified in the inter-subspecific crosses of O. sativa while others were found in the crosses between O. sativa and other species of Oryza genus11. Among the loci causing female sterility in the inter-subspecific hybrids, S5 is a major locus14,15. The S5 locus was mapped onto chromosome 6 and three alleles, namely indica allele (S5-i), japonica allele (S5-j) and neutral allele (S5-n), were identified at the locus14,16. The S5 gene was subsequently isolated through map-based cloning approach, which encodes an aspartic protease to manipulate the female fertility of the hybrids17. The S5-i and S5-j alleles differed only at two nucleotides for the female sterility, while the S5-n allele has a loss-function mutation with 136 bp DNA-sequence deletion for the compatibility17,18,19. For F1 pollen sterility, five loci, i.e. Sa (S-E3), Sb (S-E2), Sc (S-E5), Sd and Se, were identified through a series of allelic test-crosses20,21,22,23,24. Among five identified F1 pollen sterility loci, the Sa locus was mapped to a region of 30 kb on chromosome 125,26 and was subsequently cloned27; the Sb locus was delimited to a region of 27 kb on chromosome 528,29; the Sc locus was narrowed down to a region of 46 kb on chromosome 330,31; the Sd locus was delimited to a region of 67 kb on chromosome 132; and the Se locus was mapped to an interval of 5 cM on chromosome 1233. Interestingly, some loci for hybrid sterility, such as S24 and S3134,35, S3536, S25 and S3637,38 were also identified in the same regions as the Sb, Sd and Se loci, respectively, by different research groups.
To overcome the inter-specific hybrid sterility, a strategy of developing indica-compatible japonica lines (ICJLs) which carry S-i alleles at the loci for hybrid sterility in japonica genetic background, was proposed23,39. The pyramiding effect of the genes for hybrid sterility was evaluated by developing pyramiding lines with S-i alleles at multiple loci of Sa, Sb and Sc24. Here, we report that the inter-subspecific hybrid sterility can be overcome by using ICJLs. The ICJLs carrying the S-i alleles at Sb, Sc, Sd and Se loci for F1 pollen fertility and the S-n allele at S5 locus for F1 female fertility in japonica genetic background are compatible with indica while incompatible with japonica, reversing the compatibility of general japonica rice. Our results showed that the ICJLs would be an important genetic stock for overcoming the inter-specific hybrid sterility in rice.
Results
F1 pollen sterility caused by allelic interaction at the Sb, Sc, Sd and Se loci
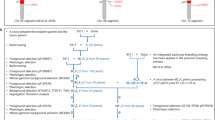
To assess F1 pollen sterility caused by the loci for F1 pollen sterility, a set of Taichung65 (T65) isogenic F1-sterile lines (TISLs) with the indica allele at the locus for F1 pollen sterility in the genetic background of T65 (a japonica variety) were developed by marker-facilitated backcrossing (Supplementary information). Each TISL carried only one chromosomal substituted segment from a donor indica cultivar detected by a survey of the genome with molecular markers (Fig. 1, Supplementary Fig. 1). Of the 11 TISLs, 3, 4, 3 and 1 TISLs carried S-i at the loci Sb, Sc, Sd and Se, respectively (Fig. 1, Supplementary Table 1). All the TISLs were test-crossed with T65 which has S-j allele at the all loci to generate heterozygote S-i/S-j at the corresponding locus. The F1 hybrids in the crosses showed partial pollen fertility, ranging from 36.59% to 79.00%. In all the crosses, distorted segregation from the Mendelian segregation ratio was observed in the F2 populations, in which the ratio of genotype JJ (S-j/S-j) was significantly lower than that of genotype II (S-i/S-i) (Fig. 1, Supplementary Table 2). The results indicated that the interaction between the S-i allele from the TISLs and the S-j allele from T65 at the loci caused pollen with the S-j allele aborted in varying degrees in the crosses. Similar results were obtained from the test-crosses of TISLs with other japonica testers (Supplementary Table 3).
Location and genetic effect of the genes at the Sb, Sc, Sd and Se loci on the chromosomal substituted segments in the TISLs.
(a) The Sb locus in TISL-Ob, TISL-Db and TISL-Xb. (b) The Sc locus in TISL-Gc, TISL-Zc, TISL-Pc and TISL-Dc. (c) The Sd locus in TISL-Gd, TISL-Zd and TISL-Dd. (d) The Se locus in TISL-Ge. Left: Chromosome locations of the Sb, Sc, Sd and Se loci on the substituted segments in the TISLs. The black horizontal bars represent substituted segments in the TISLs. The positions of the Sb, Sc, Sd and Se loci are indicated in the chromosome maps. Middle: Pollen fertility of F1 hybrids from the crosses of TISLs with T65. Error bars represent the SD. Right: Ratios of genotypes at the Sb, Sc, Sd and Se loci in the F2 populations from the crosses of TISLs with T65. JJ, Genotype of T65 (S-j/S-j). IJ, Heterozygous genotypes (S-i/S-j). II, Genotypes of TISLs (S-i/S-i). **Significantly different at the 0.01 probability level.
Pyramiding effect of the genes for F1 pollen sterility at the Sb, Sc, Sd and Se loci
To analyse the pyramiding effect of the genes for F1 pollen sterility at the Sb, Sc, Sd and Se loci, a set of 7 TISLs with different numbers of S-i allele at the Sb, Sc and Sd loci were developed using the indica variety Dijiaowujian as the S-i allele donor. In the test-crosses of the TISLs with T65, the F1 pollen fertility was 43.5%, 81.3% and 62.0% in the genotypes of S-i/S-j at the single locus of Sb, Sc and Sd, respectively. The pollen fertility was 42.4%, 19.7% and 51.7% in the double heterozygotes at Sb and Sc, Sb and Sd and Sc and Sd, respectively and the fertility was only 14.0% in the trihybrid at the Sb, Sc and Sd loci (Fig. 2a). These results indicated that the pollen fertility of heterozygotes decreased with the increase of the number of heterozygous loci for the F1 pollen sterility and the pollen fertility of heterozygotes at multiple loci was approximate to the product of pollen fertility in heterozygotes at each of the loci. Similar results were observed in a BC4F2 population derived from the backcross between Guangluai4 (a donor of the S-i allele at the Sb, Sd and Se loci) and T65. The F1 pollen fertility was 33.1%, 85.4% and 46.8% for the single heterozygous locus, respectively, whereas it was 21.3%, 15.0% and 31.3% for the two heterozygous loci of Sb and Sd, Sb and Se and Sd and Se, respectively and the fertility was only 7.0% for the line with three heterozygous alleles at Sb, Sd and Se loci (Fig. 2b).
F1 pollen sterility caused by allelic interactions at different combinations of the Sb, Sc, Sd and Se loci.
(a) F1 pollen fertility in the crosses of TISLs developed from the Dijiaowujian donor with T65 in the first cropping season in 2002. (b) F1 pollen fertility in the crosses of TISLs developed from the Guangluai4 donor with T65 in the second cropping season in 2005. Error bars represent the SD.
Overcoming the pollen sterility of the inter-subspecific hybrids by developing of the TISLs with S-i alleles at the Sb, Sc, Sd and Se loci
TISL-Dbcd with S-i at the Sb, Sc and Sd loci from Dijiaowujian and TISL-Gde with S-i at the Sd and Se loci from Guangluai4 were selected to develop pyramiding line with S-i at the Sb, Sc, Sd and Se loci. The pyramiding line TISL-Dbc-Gde carried the S-i allele at the Sb and Sc loci from Dijiaowujian and the S-i allele at the Sd and Se loci from Guangluai4 (Fig. 3a–c, Supplementary Table 4). TISL-Dbc-Gde carried 62.1 cM of substituted segments with the S-i allele from the indica donors that accounted for only 4.06% of the rice genome (Fig. 3c, Supplementary Table 4). TISL-Dbc-Gde was similar to T65 in terms of plant type, panicle type and grain type (Fig. 3a,b). No significant difference between TISL-Dbc-Gde and T65 was detected on the traits of days from sowing to heading, plant height, thousand grain weight, grain length, grain width and panicle number per plant (Supplementary Table 5). These results indicated that TISL-Dbc-Gde was a japonica line with a genotype and phenotype similar to T65. To evaluate the compatibility, TISL-Dbc-Gde was test-crossed with a set of testers consisting of typical indica and japonica varieties for two years. In the F1 hybrids from the crosses of TISL-Dbc-Gde with the japonica testers, the pollen fertility was 0.97% and 2.40% and the spikelet fertility was close to complete sterility in the two experiments. In the F1 hybrids from the crosses of TISL-Dbc-Gde with the indica testers, the pollen fertility was 88.22% and 89.17% and the spikelet fertility was 61.25% and 57.15%. As a control, the pollen fertility was 91.40% and 17.83% and the spikelet fertility was 94.60% and 6.96% in the F1 hybrids from the crosses of T65 with the japonica and indica testers, respectively (Fig. 3d–g, Supplementary Tables 6 and 7). These results indicated that TISL-Dbc-Gde showed reverse compatibility with T65. In other words, TISL-Dbc-Gde was compatible with the indica testers but incompatible with the japonica testers in terms of pollen fertility. Thus, the pollen sterility of the inter-subspecific hybrids was effectively overcome in the crosses of TISL-Dbc-Gde with the indica testers.
Phenotypes and genotypes of TISL-Dbc-Gde with S-i at the Sb, Sc, Sd and Se loci.
(a) Plant morphology of TISL-Dbc-Gde (left) and T65 (right). Scale bar, 10 cm. (b) Panicle-type and grain-type of TISL-Dbc-Gde (left) and T65 (right). Scale bar, 2 cm. (c) Positions of the substituted segments in the genome of TISL-Dbc-Gde. Vertical bars are a graphical representation of chromosomes. Deep parts are substitution segments from donors and Light parts are the genetic background from T65. (d) Pollen grains stained by I2-KI solution in the F1 hybrids of four crosses. Scale bar, 30 μm. (e) Spikelet fertility in the panicles of the F1 hybrids of four crosses. Scale bar, 2 cm. (f) Pollen fertility of the F1 hybrids from the crosses between TISL-Dbc-Gde and the testers in the second cropping season of 2012 (n = 15). (g) Spikelet fertility of the F1 hybrids from the crosses between TISL-Dbc-Gde and the testers in the second cropping season of 2012 (n = 15). H1, TISL-Dbc-Gde/Ballila; H2, T65/Ballila; H3, TISL-Dbc-Gde/Aijiaonante; H4, T65/Aijiaonante; C1, TISL-Dbc-Gde/japonica testers; C2, TISL-Dbc-Gde/indica testers; C3, T65/japonica testers; C4, T65/indica testers. Error bars represent the SD.
Overcoming the spikelet sterility of the inter-subspecific hybrids by developing ICJLs
Although the pollen fertility was almost normal, the spikelet fertility was still low in the F1 hybrids from the crosses of TISL-Dbc-Gde with the indica testers (Supplementary Tables 6 and 7). To survey the problem, the genetic behaviour of six loci for hybrid sterility was investigated in all available crosses of TISL-Dbc-Gde with the indica testers. No distorted segregation was found at the Sa, Sb, Sc, Sd and Se loci for F1 pollen sterility in eighteen F2 populations, except for two populations at the Sa locus and one population at the Sd locus. However, distorted segregation was found in all five F2 populations at the S5 locus for F1 embryo sac sterility (Supplementary Table 8). These results implied that the partial spikelet sterility in the crosses of TISL-Dbc-Gde with the indica testers was mainly controlled by the allelic interaction of S-i/S-j at the S5 locus. To identify the S5-n gene, 171 accessions of O. sativa collected throughout the world were used to identify the allele types at the S5 locus using functional molecular markers of the S5 gene. Seventeen of the accessions were identified as carrying the S5-n allele (Supplementary Table 9). To develop the ICJLs, seven japonica accessions with the S5-n allele were selected as donors and crossed with TISL-Dbc-Gde. Totally seven ICJLs from the seven crosses were developed through marker-assisted backcrossing (Supplementary information). The ICJLs carried homozygous S-i allele at the Sb, Sc, Sd and Se loci from TISL-Dbc-Gde and the S5-n allele from the S5-n donors (Fig. 4a,b, Supplementary Table 10). To evaluate the compatibility, the ICJLs were test-crossed with a set of testers consisting of typical indica and japonica varieties. The F1 pollen fertility of the ICJLs was similar to that of TISL-Dbc-Gde when test-crossed with the indica or japonica testers. All of the ICJLs were compatible with the indica testers but incompatible with the japonica testers in terms of pollen fertility (Fig. 4c,e,f, Supplementary Tables 11 and 12). The F1 spikelet fertility of the ICJLs was different from that of TISL-Dbc-Gde when test-crossed with the indica testers. All of the ICJLs were compatible with the indica testers and had high spikelet fertility in the crosses while they were incompatible with the japonica testers and had high spikelet sterility in the crosses (Fig. 4d–f, Supplementary Tables 11 and 13). These results indicated that the ICJLs showed reverse compatibility with T65 and other japonica varieties since they were compatible with indica while incompatible with japonica rice. Thus, the spikelet sterility of the inter-subspecific hybrids was effectively overcome.
Phenotypes and genotypes of ICJLs with S-i at the Sb, Sc, Sd and Se loci and S-n at the S5 locus.
(a) Plant morphologies of ICJL-T-W21, ICJL-T-W22 and ICJL-T-W23. Scale bar, 10 cm. (b) Positions of the substituted segments in the ICJL-T-W21 genome. Vertical bars are a graphical representation of the chromosomes. Deep parts are substitute segments from donors and light parts are the genetic background from japonica donors. (c) Pollen grains of the F1 hybrids from six crosses. Pollen grains were stained with the I2-KI solution. Scale bar, 30 μm. (d) Spikelet fertility in the panicles of the F1 hybrids from six crosses. Scale bar, 2 cm. (e) Pollen fertility and spikelet fertility of the F1 hybrids from the crosses of ICJLs (TISL-Dbc-Gde and T65 as controls) with the indica testers in the second cropping season in 2014 (n = 10). (f ) Pollen fertility and spikelet fertility of the F1 hybrids from the crosses of ICJLs (TISL-Dbc-Gde and T65 as controls) with the japonica testers in the second cropping season in 2014 (n = 10). H1, ICJL-T-W21/9311; H2, TISL-Dbc-Gde/9311; H3, T65/9311; H4, ICJL-T-W21/T65; H5, TISL-Dbc-Gde/T65; H6, T65. T1, ICJL-T-W6; T2, ICJL-T-W19; T3, ICJL-T-W21; T4, ICJL-T-W22; T5, ICJL-T-W23; T6, ICJL-T-W24; T7, ICJL-T-W27; T8, TISL-Dbc-Gde; T9, T65. Error bars represent the SD.
Discussion
Hybrid sterility is the most common form of postzygotic reproductive isolation in plants. The best-known example is perhaps the hybrid sterility between indica and japonica subspecies of Asian cultivated rice40. The hybrid sterility between indica and japonica subspecies of Asian cultivated rice is a complex trait. It consists of male sterility or pollen sterility and female sterility or embryo-sac sterility and varies in different crosses and in different environments15,24,40,41. In some studies, the hybrid sterility was only detected by spikelet fertility. Thus, it is not known to what extent male and female gamete abortions influence the spikelet fertility15. Among approximate 50 loci for the hybrid sterility identified in rice, some loci were detected in inter-subspecific hybrids of O. sativa while others were found in inter-specific hybrids between O. sativa and other species of Oryza genus11. Thus, it is not clear how many loci involving in the inter-specific hybrid sterility. In this study, the ICJLs, carrying the S-i allele at the Sb, Sc, Sd and Se loci and the S-n allele at the S5 locus, were almost completely compatible with indica testers and incompatible with japonica testers in pollen fertility and in spikelet fertility. These results indicated that the hybrid sterility in inter-subspecific hybrids was mainly controlled by the genes at the Sb, Sc, Sd, Se and S5 loci.
Understanding the genetic basis of hybrid sterility in rice lays the foundation for overcoming the hybrid sterility in inter-subspecific hybrids. At least three strategies have been proposed for overcoming the hybrid sterility in indica-japonica crosses. First, the neutral alleles, or wide-compatibility genes (WCGs) can be introgressed from the wide-compatibility varieties (WCVs) into the parents whose hybrids exhibit strong yield heterosis. The second strategy is to breed ‘ICJLs’ by introgressing indica alleles of several hybrid sterility loci into japonica lines by backcrossing. The third is to produce artificial neutral alleles by suppressing expression of the genes causing hybrid sterility with RNAi or microRNA technology, if such gene silencing does not affect the plant growth or development40. The S5-n gene was identified as a WCG and the varieties carrying S5-n, named WCVs, were believed to be compatible with both indica and japonica14. However, it has been frequently found that the S5-n gene alone is not sufficient for producing indica-japonica hybrids with normal fertility. S5-n can only overcome the sterility caused by embryo sac abortion15. We proposed the strategy of development of ‘ICJLs’ which carry the S-i alleles at the loci for hybrid sterility in the japonica genetic background by backcrossing and MAS23,39. Following this strategy, we developed a set of the ICJLs which carry the S-i allele at the Sb, Sc, Sd and Se loci and the S-n allele at the S5 locus, as an outcome of two decades of continuing pursuit. The ICJLs are compatible with indica while incompatible to japonica rice. Thus, the hybrid sterility of indica-japonica rice was effectively overcome in the crosses of the ICJLs with the indica rice. It provides the first example of overcoming hybrid sterility in plants.
The breeding of indica-japonica rice has been practiced since the last decades. On the one hand, the utilization of inter-subspecific crosses has been considered as an efficient approach to develop traditional varieties with high yield potential1,2. On the other hand, the hybrids between indica and japonica varieties often show strong heterosis compared with intra-subspecific hybrids. For example, in China’s “super” rice breeding, the two-line or three-line method was used to develop F1 hybrid combinations by crossing an intermediate type between indica and japonica with an indica parent in order to use inter-subspecific heterosis1,5. The ICJLs will be an important genetic stock for breeding of indica-japonica rice. First, the ICJLs will be used to develop traditional varieties with higher yield potential. In the crosses of ICJLs with indica varieties, recombination of the genes from indica and japonica (ICJLs) will be no longer limited by hybrid sterility. Second, the ICJLs will be used to develop inter-subspecific hybrid rice. In the two-line or three-line rice hybrid system, ICJLs can be used to develop sterile line (A line), or restorer line (R line). Recently, a platform for breeding by design of CMS sterile lines based on an SSSL library in rice was developed42. By the combination of the strategy of the ICJLs with the platform for breeding by design, the three-lines of ICJLs for hybrid rice will be developed effectively. It will lead to open a new horizon in utilization of heterosis in the indica-japonica hybrid rice.
Methods
Plant materials and growth conditions
All of the TISLs, ICJLs, indica testers, japonica testers and other plant materials in this study (Supplementary information) were planted in an experimental station at South China Agricultural University, Guangzhou (23°07′N, 113°15′E). The materials were planted in two cropping seasons each year. The first cropping season was from late February to middle July and the second cropping season was from late July to middle November. The seeds were sowed on seed beds and the seedlings were transplanted to the fields. Field management, including irrigation, fertilizer application and pest control, followed essentially normal agricultural practices.
Marker development and assay
DNA was extracted from fresh young leaves using the CTAB method43. The PCR profile used for amplification followed a previously described protocol44. The SSR markers used in this study were selected on rice microsatellite maps45,46. To identify the genotypes at the S5 locus, we selected five functional markers and designed several markers based on the DNA sequences of S5 locus12,19,47. To identify the genotypes at the Sa locus, we selected marker G02-14827. To conduct marker-assisted selection at other loci, SSR markers PSM8, PSM12 and PSM180 and the insertion/deletion (InDel) markers IND19 and ID5 were developed in our lab (Supplementary Table 14).
Examination of pollen and spikelet fertility
To examine pollen fertility, 6–9 mature flowers were collected from the upper one-third of the panicles of plants during the flowering time and fixed in FAA solution (ethanol, formaldehyde and acetic acid at a ratio of 89:6:5). The pollen was stained with a 1% I2-KI solution containing 0.1% (w/v) iodine and 1% (w/v) potassium iodide. More than 300 pollen grains were randomly scanned per plant. The pollen was divided into three types: normal pollen (normal size and fully stained), stained abortive pollen (small size and lightly stained) and empty abortive pollen (small size and empty)20. To examine spikelet fertility, three panicles per plant were harvested during the maturation time. Ten to twenty plants were recorded for each variation.
Statistical analysis
The statistical model yij = μ + Gi + εij was used to analyse the variance (ANOVA) of all data obtained from the experimental materials in one environment, where yij was the jth observed value of the target trait for the ith genotype and μ, G and ε were the population mean value, genotypic effects and residual error, respectively. For the estimation of genotypic effects on target traits, the significance of the difference between one genotype and the control selected at the α probability level was tested by the least significant difference (LSD) method. Data were given as the mean ± standard deviation (SD) and transferred by arcsin−1 prior to analysis if they were provided as a percentage. Statistical analysis and graphing were performed using SPSS version 1848 and SigmaPlot49.
Additional Information
How to cite this article: Guo, J. et al. Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 6, 26878; doi: 10.1038/srep26878 (2016).
References
Peng, S., Khush, G. S., Virk, P., Tang, Q. & Zou, Y. Progress in ideotype breeding to increase rice yield potential. Field Crops Res 108, 32–38 (2008).
Khush, G. S. Strategies for increasing the yield potential of cereals: case of rice as an example. Plant Breeding 132, 433–436 (2013).
Kato, S., Kosaka, H. & Hara, S. On the affinity of rice varieties as shown by fertility of hybrid plants. Bull. Sci. Fac. Agric. Kyushu. Univ. 3, 132–147 (1928).
Yuan, L. & Virmani, S. Status of hybrid rice research and development. In Hybrid rice (Internationa Rice Research Institute, Manila, Philippines), 7–24 (1988).
Cheng, S., Zhuang, J., Fan, Y., Du, J. & Cao, L. Progress in research and development on hybrid rice: a super-domesticate in China. Ann. Bot. 100, 959–966 (2007).
Huang, J. E. Z., Zhang, H. & Shu, Q. Workable male sterility systems for hybrid rice: Genetics, biochemistry, molecular biology and utilization. Rice 7, 13 (2014).
Peng, S., Laza, R. C., Visperas, R. M., Khush, G. S., Virk, P. & Zhu, D. Rice: progress in breaking the yield ceiling “New directions for a diverse planet”. In: Proceedings of the 4th international crop science congress. Brisbane, Australia. 26 (2004).
Xiong, Z. et al. Latitudinal distribution and differentiation of rice germplasm: its implications in breeding. Crop Sci. 51, 1050–1058 (2011).
Oka, H. Genic analysis for the sterility of hybrids between distantly related varieties of cultivated rice. J. Genet. 55, 397–409 (1957).
Liu, K., Zhou, Z., Xu, C., Zhang, Q. & Maroof, M. An analysis of hybrid sterility in rice using a diallel cross of 21 parents involving indica, japonica and wide compatibility varieties. Euphytica 90, 275–280 (1996).
Ouyang, Y., Chen, J., Ding, J. & Zhang, Q. Advances in the understanding of inter-subspecific hybrid sterility and wide-compatibility in rice. Chin. Sci. Bull. 54, 2332–2341 (2009).
Yang, J. et al. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337, 1336–1340 (2012).
Liu, P. et al. Predicting hybrid fertility from maker-based genetic divergence index of parental varieties: implications for utilizing inter-subspecies heterosis in hybrid rice breeding. Euphytica 203, 47–57 (2015).
Ikehashi, H. & Araki, H. Genetics of F1 sterility in remote crosses in rice. In Rice Genetics: Proceedings of the First Rice Genetics Symposium (International Rice Research Institute), 119–130 (1986).
Song, X., Qiu, S., Xu, C., Li, X. & Zhang, Q. Genetic dissection of embryo sac fertility, pollen fertility and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theor. Appl. Genet. 110, 205–211 (2005).
Yanagihara, S. et al. Molecular analysis of the inheritance of the S-5 locus, conferring wide compatibility in indica/japonica hybrids of rice (Oryza sativa L.). Theor. Appl. Genet. 90, 182–188 (1995).
Chen, J. et al. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc. Natl Acad. Sci. USA 105, 11436–11441 (2008).
Ji, Q. et al. Two sequence alterations, a 136 bp InDel and an A/C polymorphic site, in the S5 locus are associated with spikelet fertility of indica-japonica hybrid in rice. J. Genet. Genom. 37, 57–68 (2010).
Du, H., Ouyang, Y., Zhang, C. & Zhang, Q. Complex evolution of S5, a major reproductive barrier regulator, in the cultivated rice Oryza sativa and its wild relatives. New Phytol. 191, 275–287 (2011).
Zhang, G. & Lu, Y. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). I. Diallel analysis of the hybrid sterility among isogenic F1 sterile lines (in Chinese with English abstract). Chinese J. Rice Sci. 3, 97–101 (1989).
Zhang, G. & Lu, Y. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). II. A genic model for F1 pollen sterility (in Chinese with English abstract). Acta Genet. Sin. 20, 222–228 (1993).
Zhang, G., Lu, Y., Liu, G., Yang, J. & Zhang, H. Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). III. Allele differentiation of F1 pollen sterility in different types of varieties (in Chinese with English abstract). Acta Genet. Sin. 20, 541–551 (1993).
Zhang, G., Lu, Y., Zhang, H., Yang, J. & Liu, G. Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). IV. Genotypes for F1 pollen sterility (in Chinese with English abstract). Acta Genet. Sin. 21, 34–41 (1994).
Zhang, G. & Lu, Y. Genetics of F1 pollen sterility in Oryza sativa. In Rice Genetics III (International Rice Research Institute, Manila, Philippines), 418–422 (1996).
Zhuang, C., Zhang, G., Mei, M. & Lu, Y. Molecular mapping of the S-a locus for F1 pollen sterility in cultivated rice (Oryza sativa L.). Acta Genet. Sin. 26, 213–218 (1999).
Su, J. & Liu, Y. Fine mapping and cloning of the gene S-a for F1 pollen sterility in cultivated rice (Oryza sativa L.). Mol Plant Breed. 1, 757–758 (2003).
Long, Y. et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl Acad. Sci. USA 105, 18871–18876 (2008).
Zhuang, C., Mei, M., Zhang, G. & Lu, Y. Chromosome mapping of the S-b locus for F1 pollen sterility in cultivated rice (Oryza sativa L.) with RAPD markers (in Chinese with English abstract). Acta Genet. Sin. 29, 700–705 (2002).
Li, W., Zeng, R., Zhang, Z., Ding, X. & Zhang, G. Fine mapping of locus S-b for F1 pollen sterility in rice (Oryza sativa L.). Chin. Sci. Bull. 51, 675–680 (2006).
Zhang, Z. & Zhang, G. Fine mapping of the S-c locus and marker-assisted selection using PCR markers in rice (in Chinese with English abstract). Acta Agronomica Sinica 27, 704–709 (2001).
Yang, C., Chen, Z., Zhuang, C., Mei, M. & Liu, Y. Genetic and physical fine-mapping of the Sc locus conferring indica-japonica hybrid sterility in rice (Oryza sativa L.). Chin. Sci. Bull. 49, 1718–1721 (2004).
Li, W., Zeng, R., Zhang, Z., Ding, X. & Zhang, G. Identification and fine mapping of S-d, a new locus conferring the partial pollen sterility of intersubspecific F1 hybrids in rice (Oryza sativa L.). Theor. Appl. Genet. 116, 915–922 (2008).
Zhu, W. et al. Preliminary identification of F1 pollen sterility gene S-e in Oryza sativa (in Chinese with English abstract). J. South. China Agricult. Univ. 29, 1–5 (2008).
Zhao, Z. et al. Molecular analysis of an additional case of hybrid sterility in rice (Oryza sativa L.). Planta 233, 485–494 (2011).
Zhao, Z. et al. Fine mapping of S31, a gene responsible for hybrid embryo-sac abortion in rice (Oryza sativa L.). Planta 226, 1087–1096 (2007).
Kubo, T., Yamagata, Y., Eguchi, M. & Yoshimura, A. A novel epistatic interaction at two loci causing hybrid male sterility in an inter-subspecific cross of rice (Oryza sativa L.). Genes Genet. Syst. 83, 443–453 (2008).
Kubo, T. & Yoshimura, A. Linkage analysis of an F1 sterility gene in japonica/indica cross of rice. Rice Genet Newslett 18, 52–54 (2001).
Wen, J., Zhang, W., Jiang, L., Chen, L., Zhai, H. & Wan, J. Two novel loci for pollen sterility in hybrids between the weedy strain Ludao and the japonica variety Akihikari of rice (Oryza sativa L.). Theor. Appl. Genet. 114, 915–925 (2007).
Zhang, G. & Lu, Y. Breeding of the indica-compatible japonica lines and their use in the breeding of super-high-yield hybrid rice (in Chinese with English abstract). Hybrid Rice 14, 3–5 (1999).
Ouyang, Y., Liu, Y. & Zhang, Q. Hybrid sterility in plant: stories from rice. Curr. Opin. Plant Biol. 13, 186–192 (2010).
Oka, H. I. Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genet. 77, 521–534 (1974).
Dai, Z. et al. Development of a platform for breeding by design of CMS lines based on an SSSL library in rice (Oryza sativa L.). Euphytica 205, 63–72 (2015).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325 (1980).
Panaud, O., Chen, X. & McCouch, S. R. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol. Gen. Genet. 252, 597–607 (1996).
McCouch, S. R. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9, 199–207 (2002).
Zhang, Z. et al. A genome-wide microsatellite polymorphism database for the indica and japonica Rice. DNA Res. 14, 37–45 (2007).
Sundaram, R. M. et al. Development and validation of a PCR-based functional marker system for the major wide-compatible gene locus S5 in rice. Mol. Breeding 26, 719–727 (2010).
Coakes, S. J. & Ong, C. SPSS Version 18.0 for Windows Analysis without anguish (John Wiley & Sons) (2011).
Charland, M. B. SigmaPlot for Scientists (William C Brown Press) (1995).
Acknowledgements
We would like to thank Dr. H. I. Oka for providing two TISLs (TISL-Ob and TISL-Pc) in 1988, which were named E2 and E5, respectively. We would also like to thank Prof. Xiaohua Ding for conducting some experiments prior to pass away in 2012 and Prof. Yonggen Lu for consulting. This work was supported by grants from the National Natural Science Foundation of China (91435207 and U1031002).
Author information
Authors and Affiliations
Contributions
J.G. and X.X. developed the pyramiding lines and ICJLs and tested their compatibility; W.L. and W.Z. developed the TISLs and pyramiding lines and tested their F1 fertility; H.Z. performed field experiments; X.L., Z.D. and G.T. performed some of the experiments; Z.L., Z.Z., R.Z., X.F. and S.W. performed some of the lab experiments; J.G. prepared the data; G.L. performed the biometry; and G.Z. designed the experiments, supervised this study and wrote the manuscript. All the authors have discussed the results and contributed to the drafting of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, J., Xu, X., Li, W. et al. Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci Rep 6, 26878 (2016). https://doi.org/10.1038/srep26878
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26878
- Springer Nature Limited
This article is cited by
-
Pyramiding of Low Chalkiness QTLs Is an Effective Way to Reduce Rice Chalkiness
Rice (2024)
-
Two complementary genes in a presence-absence variation contribute to indica-japonica reproductive isolation in rice
Nature Communications (2023)
-
Improving bridge effect to overcome interspecific hybrid sterility by pyramiding hybrid sterile loci from Oryza glaberrima
Scientific Reports (2023)
-
Characterization and fine-mapping of a new Asian rice selfish genetic locus S58 in Asian–African rice hybrids
Theoretical and Applied Genetics (2023)
-
Rice functional genomics: decades’ efforts and roads ahead
Science China Life Sciences (2022)