Abstract
TERT is the catalytic subunit of telomerase which plays an essential part in cellular immortality by maintaining telomere integrity. TERT is commonly over-expressed in human malignancies, indicating its key role in cell transformation. The chromosome 5p15.33 TERT-CLPTM1L region has been associated with susceptibility of multiple cancers via a genome-wide association approach. However, the involvement of this locus in papillary thyroid carcinoma (PTC) etiology is still largely unknown. We analyzed 15 haplotype-tagging single nucleotide polymorphisms (htSNPs) of the TERT-CLPTM1L region in a two stage case-control design. After genotyping 2300 PTC patients and frequency-matched 2300 unaffected controls, we found that TERT rs2736100 genetic variant is significantly associated with elevated PTC risk. Ex vivo reporter gene assays indicated that the PTC susceptibility rs2736100 polymorphism locating in a potential TERT intronic enhancer has a genotype-specific effect on TERT expression. Correlations between rs2736100 genotypes and tissue-specific TERT expression supported the regulatory function of this genetic variant in vivo. Our data demonstrated that the functional TERT rs2736100 SNP as a novel genetic component of PTC etiology. This study, together with recent studies in other cancers, unequivocally establishes an essential role of TERT in cancers.
Similar content being viewed by others
Introduction
Thyroid carcinoma is the most common endocrine malignancy and showed quickly increased incidence over last two decades. According to the Chinese Cancer Registry, the incidence of thyroid carcinoma is 6.6 per 100,000 individuals in China1,2. Papillary thyroid carcinoma (PTC), named for their papillary histological architecture, accounts for about eighty percent of all thyroid carcinomas. Ionizing radiation, nodular disease of the thyroid and family history account for known risk factors of PTC currently3. However, only a portion of exposed individuals develop PTC, suggesting that genetic factors may also impact thyroid malignant transformation4.
Accumulated evidences demonstrated that the chromosome 5p15.33 region (TERT-CLPTM1L) is a common susceptibility locus of multiple cancers. Genome-wide association studies (GWAS) declared that independent susceptibility single nucleotide polymorphisms (SNPs) in 5p15.33 were identified in different malignancies, including lung cancer5,6,7,8,9,10, melanoma11, nonmelanoma skin cancer11,12, glioma13, bladder cancer14, pancreatic cancer15, testicular germ cell cancer16, estrogen-negative breast cancer17, ovarian cancer18 and prostate cancer19. Therefore, it is plausible that several functional DNA elements might exist in the region and influence cancer etiology. There are two known oncogenes, TERT and CLPTM1L, in the locus. Activated TERT (telomerase reverse transcriptase) transcription enhances telomerase activities and accelerates malignant transformation20,21. In lung cancer, oncogene CLPTM1L (cleft lip and palate-associated transmembrane 1 like protein) plays an a protumorigenic role and is critical for Ras-driven lung cancers22,23,24. In pancreatic cancer, CLPTM1L functions as a growth-promoting gene and its overexpression may lead to an abrogation of normal cytokinesis and enhance aneuploidy in pancreatic cancer cells22,23,24.
Considering the impacts of the 5p15.33 TERT-CLPTM1L locus on PTC susceptibility is still largely unknown, we examined the associations between 15 haplotype-tagging SNPs (htSNP) covering the entire TERT-CLPTM1L locus and PTC risk in three large independent case-control studies. To investigate the biological function of the PTC susceptibility TERT rs2736100 SNP, we examined impacts of its genotypes on TERT expression ex vivo and in vivo.
Material and Methods
Study subjects
A total of three case-control sets were included in the current study. (i) Zhejiang set: 500 PTC cases from Zhejiang Province Cancer Hospital (Hangzhou, Zhejiang Province, China) and sex- and age-matched 500 controls. (ii) Jiangsu set: 1000 cases with PTC from Huaian No. 2 Hospital (Huaian, Jiangsu Province, China) and sex- and age-matched (±5 years) 1000 healthy controls. (iii) Jilin set: 800 PTC patients from The First Affiliated Hospital of Jilin University (Changchun, Jilin Province, China) and 800 sex- and age-matched healthy controls. Sixty pairs of PTC specimens and thyroid normal tissues adjacent to the tumors were obtained from surgically removed specimens of patients in Zhejiang Province Cancer Hospital and Huaian No. 2 Hospital. All individuals were ethnic Han Chinese. The detailed information on subject recruitments can be found in Table 1. This study was approved by the institutional Review Boards of Zhejiang Province Cancer Hospital, Huaian No. 2 Hospital and The First Affiliated Hospital of Jilin University. At recruitment, the written informed consent was obtained from each subject. The methods were carried out in accordance with the approved guidelines.
SNP selection and genotyping
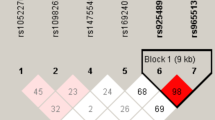
An htSNP approach was used to investigate genetic polymorphisms in the TERT-CLPTM1L locus globally (a 91716 bp region of chromosome 5p15.33)25,26,27. HapMap SNPs which have been genotyped among Han Chinese and Japanese populations (HapMap Rel 21, NCBI B36) with a minor allele frequency >5% were included in htSNP selection. A total of 15 htSNPs were chosen in a 95716 bp region (the 91716 bp TERT-CLPTM1L locus and 2 kb up-stream plus 2 kb down-stream regions of the locus). The selection criteria included the sample size inflation factor, Rh2, of ≥0.8 and a block-by-block method using Haploview 4.2 software (Supplementary Table 1). All htSNPs were genotyped through the MassArray system (Sequenom Inc., San Diego, California, USA). A 5% blind, random DNA samples was analyzed in duplicates and the reproducibility was 99%. To reduce the costs of the study, we genotyped the TERT rs2736100 T > G SNP in two validation sets using the PCR-based restriction fragment length polymorphism (RFLP) as described in Supplementary Table 2. A 5% samples were genotyped by two investigators and the reproducibility was 98.5%.
Dual-luciferase reporter gene assays
The intron 2 region of TERT (including the rs2736100 flanking region) was amplified with human genomic DNA from healthy control individuals carrying either TERT rs2736100 TT genotype or rs2736100 GG genotype. Specific PCR primer pairs with the KpnI and XhoI restriction sites were showed in Supplementary Table 3. The PCR products were digested and ligated into an appropriately digested pGL3-Basic vector. The resultant TERT reporter gene plasmids were designated pTERT-T or pTERT-G, which were only different at the rs2736100 polymorphic site. Sanger sequencing of the insertions confirmed the orientation and integrity of the two constructs.
Both reporter gene constructs (pGL3-Basic, pTERT-T, or pTERT-G) and pRL-SV40 (Luciferase Assay System; Promega) were transfected into PTC cell line BCPAP cells or HEK293 cells. As previously described, dual luciferase activities were determined at 48 h after transfection28. For each plasmid construct, three independent transfection experiments were performed, and each was done in triplicates.
Real-time qPCR of TERT mRNA
Total cellular RNA was isolated from sixty pairs of PTC specimens and normal tissues adjacent to the tumors with TRIzol Reagent (Invitrogen) and converted to cDNA using the PrimeScript RT Master Mix (TaKaRa). TERT mRNA expression in tissues was analyzed using the TaqMan real-time qPCR method. Relative gene expression quantization for TERT (ABI, Assay ID Hs00972656_m1) was calculated using β-actin (ABI, Assay ID 4333762T) as an internal reference gene was carried out using the ABI 7500 real-time PCR system in triplicates.
Statistics
The Pearson chi-square test was used to examine selected characteristics between PTC cases and controls for categorical variables. The associations between TERT-CLPTM1L genotypes and PTC risk were estimated by odds ratios (ORs) and their 95% confidence intervals (CIs) computed by logistic regression models. All ORs were adjusted for age or sex, where it was appropriate. One-way ANOVA was used for the correlations between genotypes of rs2736100 and TERT mRNA expression. A P value of less than 0.05 was used as the criterion of statistical significance. All statistical tests were two-sided and performed using SPSS 16.0 (SPSS Inc.).
Results
Table 2 showed genotype distributions of 15 SNPs in the TERT-CLPTM1L loci in the Zhejiang discovery set. All observed genotype frequencies in both PTC patients and controls conform to Hardy-Weinberg equilibrium (all P > 0.05). Among the 15 SNPs, frequencies of rs2736100 genotypes among cases differed significantly from those among healthy controls (P < 0.05). In details, rs2736100 genetic variant was associated with significantly elevated PTC risk (allelic OR = 1.39, 95% CI = 1.16–1.66, P = 7.0 × 10−6) (Table 2). There were no statistically significant associations between other 14 SNPs and PTC risk (all P > 0.05) (Table 2), we did not examine these SNPs in the next analyses.
Logistic regression analyses showed that the rs2736100 G allele was a risk allele. Subjects having the TG genotype had an OR of 1.34 (95% CI = 1.01–1.79, P = 0.047) for developing PTC compared with subjects having the TT genotype. It was observed that the odds of having the rs2736100 GG genotype in cases was 1.36 (95% CI = 1.14–1.62, P = 7.4 × 10−4) compared with the TT genotype. In Jiangsu validation set, a significantly increased OR was also associated with the rs2736100 GT or GG genotype (OR = 1.44, 95% CI = 1.18–1.76, P = 0.003) or (OR = 1.43, 95% CI = 1.26–1.62, P = 3.8 × 10−6). Moreover, the significant association between rs2736100 SNP and PTC risk were also observed in Jilin validation set (Table 3). Individuals with rs2736100 GG genotype showed significantly increased PTC risk compared with those with rs2736100 TT genotype (OR = 1.18, 95% CI = 1.02–1.37, P = 0.025). However, rs2736100 GT genotype was not significantly associated with PTC risk (OR = 1.05, 95% CI = 0.82–1.34, P = 0.695) in Jilin set. The PTC risk associated with the rs2736100 genetic variant was further examined by stratifying for age and sex using the combined data of three case-control sets (Table 4). Significant associations between rs2736100 TG or GG genotype and PTC risk were observed in all stratified groups (all P < 0.05).
Since the rs2736100 variant locates in the TERT intron 2 region, we investigated the impacts of this polymorphism on TERT gene expression via dual-luciferase reporter gene assays (Fig. 1). We found that the intron 2 segment containing the rs2736100 flanking sequence showed enhancer activities in HEK293 cells or BCPAP PTC cells. Moreover, the TERT rs2736100G allelic reporter construct (pTERT-G) showed significantly higher luciferase activities compared to the rs2736100T allelic reporter construct (pTERT-T) in HEK293 cells (P < 0.01) or BCPAP PTC cells (P < 0.05) (Fig. 1).
Transient luciferase reporter gene expression assays with constructs containing different rs2736100 allele of the TERT intron 2 region in HEK293 cells (A) or BCPAP cells (B). pRL-SV40 were cotransfected with these contructs to standardize transfection efficiency. Fold-changes were detected by defining the luciferase activity of cells co-transfected with pGL3-basic as 1. All experiments were performed in triplicates in three independent transfection experiments and each value represents mean ± SD. Compared with pGL3-Basic transfected cells, *P < 0.05; **P < 0.01.
We next examined the allele-specific effect of rs2736100 polymorphism on TERT gene expression in thyroid tissue specimens. Interestingly, significant up-regulation of TERT in PTC tissues was observed compared with normal tissues (P = 0.0003). We found that subjects with the rs2736100 GG or GT genotype had significantly lower TERT mRNA levels (mean ± SE) than those with the TT genotype in normal thyroid tissues (0.538 ± 0.078 [the rs2736100 GG genotype, n = 16] or 0.322 ± 0.023 [the rs2736100 GT genotype, n = 27] vs. 0.164 ± 0.024 [the rs2736100 TT genotype, n = 17], both P < 0.05). As shown in Fig. 2B, similar results were found in PTC tissues (1.550 ± 0.188 [the rs2736100 GG genotype, n = 16] or 0.441 ± 0.036 [the rs2736100 GT genotype, n = 27] vs. 0.214 ± 0.026 [the rs2736100 TT genotype, n = 17], both P < 0.01).
Discussion
In this study, we systematically evaluated PTC susceptibility genetic variants in the TERT-CLPTM1L locus and their regulatory role in TERT gene expression ex vivo and in vivo. In the discovery case-control set, we identified one PTC susceptibility genetic variant (rs2736100) after genotyping 15 TERT-CLPTM1L htSNPs. The significant association between TERT rs2736100 and PTC was validated in two validation case-control sets. Ex vivo luciferase gene assays demonstrated that the PTC susceptibility rs2736100 polymorphism locates in a potential TERT intronic enhancer and has a genotype-specific impact on TERT expression. Additionally, correlations between rs2736100 genotypes and tissue-specific TERT gene expression levels supported the regulatory function of this genetic variant in vivo.
TERT is the catalytic subunit of telomerase which that plays a essential part in cellular immortality by maintaining telomere length at the end of chromosomes29,30. TERT is well-known to be over-expressed in many human malignancies, indicating its key role in transformation of human normal cells31. In line with this, transgenic mice with induced TERT expression showed significantly increased development of tumors32,33. High TERT expression and telomerase activity have been found in thyroid cancers, particularly in the advanced forms of the disease34,35. Additionally, highly prevalent TERT promoter mutations have been repeatedly found in PTC, which highlighting the importance of in etiology of PTC36,37,38.
Although the TERT rs2736100 SNP were repeatedly identified as a susceptibility polymorphisms in many cancers4,5,6,7,8,9,10,11,12,13,14,15,16, its role in PTC etiology is still largely unknown even after several GWAS of thyroid cancer publised39,40,41. To the best of our knowledge, this is the first study to examine the association between the TERT rs2736100 polymorphism and PTC risk. We believe that the association between the rs2736100 SNP and increased PTC risk is biologically plausible since the PTC susceptibility rs2736100 G allele showed consistently higher oncogene TERT gene expression than T allele.
In all, we identified the functional TERT rs2736100 genetic polymorphism as a novel genetic component of the PTC etiology in Chinese populations. This study, together with recent studies in other cancers, unequivocally establishes an important role of TERT SNPs in cancer development, especially human thyroid cancer. However, further investigations in additional ethnic populations are desirable to validate our observations.
Additional Information
How to cite this article: Ge, M. et al. Functional evaluation of TERT-CLPTM1L genetic variants associated with susceptibility of papillary thyroid carcinoma. Sci. Rep. 6, 26037; doi: 10.1038/srep26037 (2016).
References
Chen, W. et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 27, 2–12 (2015).
Schneider, A. B. & Sarne, D. H. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab 1, 82–91 (2005).
Goldgar, D. E., Easton, D. F., Cannon-Albright, L. A. & Skolnick, M. H. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 86, 1600–1608 (1994).
Wang, Y. et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet 40, 1407–1409 (2008).
McKay, J. D. et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet 40, 1404–1406 (2008).
Broderick, P. et al. Deciphering the impact of common genetic variation on lung cancer risk: a genome-wide association study. Cancer Res 69, 6633–6641 (2009).
Landi, M. T. et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet 85, 679–691 (2009).
Wang, Z. et al. Imputation and subset-based association analysis across different cancer types identifies multiple independent risk loci in the TERT-CLPTM1L region on chromosome 5p15.33. Hum Mol Genet 23, 6616–6633 (2014).
Rafnar, T. et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 41, 221–227 (2009).
Stacey, S. N. et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet 41, 909–914 (2009).
Yang, X., Yang, B., Li, B. & Liu, Y. Association between TERT-CLPTM1L rs401681[C] allele and NMSC cancer risk: a meta-analysis including 45,184 subjects. Arch Dermatol Res 305, 49–52 (2013).
Shete, S. et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41, 899–904 (2009).
Rothman, N. et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet 42, 978–984 (2010).
Petersen, G. M. et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 42, 224–228 (2010).
Turnbull, C. et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet 42, 604–607 (2010).
Haiman, C. A. et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet 43, 1210–1214 (2011).
Beesley, J. et al. Functional polymorphisms in the TERT promoter are associated with risk of serous epithelial ovarian and breast cancers. PLoS One 6, e24987 (2011).
Kote-Jarai, Z. et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 43, 785–791 (2011).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Kolquist, K. A. et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet 19, 182–186 (1998).
James M. A. et al. Functional characterization of CLPTM1L as a lung cancer risk candidate gene in the 5p15.33 locus. PLoS One 7, e36116 (2012).
James, M. A. et al. CRR9/CLPTM1L regulates cell survival signaling and is required for Ras transformation and lung tumorigenesis. Cancer Res 74, 1116–1127 (2014).
Jia, J. et al. CLPTM1L promotes growth and enhances aneuploidy in pancreatic cancer cells. Cancer Res 74, 2785–2795 (2014).
Zhang, X. et al. The identification of an ESCC susceptibility SNP rs920778 that regulates the expression of lncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis 35, 2062–2067 (2014).
Wang, H. et al. The ALDH7A1 genetic polymorphisms contribute to development of papillary thyroid carcinoma. Tumour Biol 35, 12665–12670 (2014).
Yang, M. et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 29, 2565–2573 (2011).
Zhang, X. et al. A functional BRCA1 coding sequence genetic variant contributes to risk of papillary thyroid carcinoma. Carcinogenesis 34, 2309–2313 (2013).
Smekalova, E. M. et al. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc) 77, 1120–1128 (2012).
Mocellin, S., Pooley, K. A. & Nitti, D. Telomerase and the search for the end of cancer. Trends Mol Med 19, 125–133 (2013).
Blasco, M. A. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6, 611–622 (2005).
González-Suárez, E. et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J 20, 2619–2630 (2001).
González-Suárez, E., Flores, J. M. & Blasco, M. A. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol 22, 7291–7301 (2002).
Saji, M. et al. Human telomerase reverse transcriptase (hTERT) gene expression in thyroid neoplasms. Clin Cancer Res 5, 1483–1489 (1999).
Brousset, P. et al. Telomerase activity in human thyroid carcinomas originating from the follicular cells. J Clin Endocrinol Metab 82, 4214–4216 (1997).
Umbricht, C. B. et al. Telomerase activity: a marker to distinguish follicular thyroid adenoma from carcinoma. Cancer Res 57, 2144–2147 (1997).
Liu, X. et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 20, 603–610 (2013).
Landa, I. et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab 98, E1562–E1566 (2013).
Vinagre, J. et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 4, 2185 (2013).
Gudmundsson, J. et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet 41, 460–464 (2009).
Gudmundsson, J. et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet 44, 319–322 (2012).
Figlioli, G. et al. Novel genome-wide association study-based candidate loci for differentiated thyroid cancer risk. J Clin Endocrinol Metab 99, E2084–E2092 (2014).
Acknowledgements
This study was financially supported by the National High-Tech Research and Development Program of China (2015AA020950); National Natural Science Foundation of China (31271382); the Fundamental Research Funds for the Central Universities (YS1407); the open project of State Key Laboratory of Molecular Oncology (SKL-KF-2015-05).
Author information
Authors and Affiliations
Contributions
M.Y. and M.G. conceived and designed the experiments; M.S. performed the experiments; M.S. and C.A. analyzed the data; M.G., C.A., W.Y., X.N., Z.W., J.Z., Z.L., J.L., Z.D. and L.Z. contributed materials/analysis tools; M.Y. and M.S. wrote the manuscript. All authors reviewed and approved the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ge, M., Shi, M., An, C. et al. Functional evaluation of TERT-CLPTM1L genetic variants associated with susceptibility of papillary thyroid carcinoma. Sci Rep 6, 26037 (2016). https://doi.org/10.1038/srep26037
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26037
- Springer Nature Limited
This article is cited by
-
TERT Gene rs2736100 and rs2736098 Polymorphisms are Associated with Increased Cancer Risk: A Meta-Analysis
Biochemical Genetics (2022)
-
CLPTM1L induces estrogen receptor β signaling-mediated radioresistance in non-small cell lung cancer cells
Cell Communication and Signaling (2020)
-
Targeted next-generation sequencing in papillary thyroid carcinoma patients looking for germline variants predisposing to the disease
Endocrine (2019)
-
The correlations between DNA methylation and polymorphisms in the promoter region of the human telomerase reverse transcriptase (hTERT) gene with postoperative recurrence in patients with thyroid carcinoma (TC)
World Journal of Surgical Oncology (2017)
-
TERT promoter Mutation and Its Association with Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-analysis
Scientific Reports (2016)