Abstract
Polycomb group (PcG) proteins are transcriptional repressors of numerous genes, many of which regulate cell cycle progression or developmental processes. We used zebrafish to study Enhancer of zeste homolog 2 (Ezh2), the PcG protein responsible for placing the transcriptional repressive H3K27me3 mark. We identified a nonsense mutant of ezh2 and generated maternal zygotic (MZ) ezh2 mutant embryos. In contrast to knockout mice for PcG proteins, MZezh2 mutant embryos gastrulate seemingly normal, but die around 2 days post fertilization displaying pleiotropic phenotypes. Expression analyses indicated that genes important for early development are not turned off properly, revealing a regulatory role for Ezh2 during zygotic gene expression. In addition, we suggest that Ezh2 regulates maternal mRNA loading of zygotes. Analyses of tissues arising later in development, such as heart, liver and pancreas, indicated that Ezh2 is required for maintenance of differentiated cell fates. Our data imply that the primary role of Ezh2 is to maintain tissues after tissue specification. Furthermore, our work indicates that Ezh2 is essential to sustain tissue integrity and to set up proper maternal mRNA contribution and presents a novel and powerful tool to study how PcG proteins contribute to early vertebrate development.
Similar content being viewed by others
Introduction
Early development of multi-cellular organisms is a highly dynamic process requiring an exquisite and tight control over establishment and maintenance of cellular identity. Deregulation of these processes can lead to malformations or disease. Hence, a proper understanding of both cellular differentiation and maintenance of cell fate is relevant in many different settings.
To enable proper cellular specification, expression profiles have to become spatially and temporally restricted during development. Because every cell in theory has the same DNA content gene expression has to be determined at a higher order of regulation. This is in part achieved by chromatin: the complex of DNA wrapped around an octamer of histones plus associated proteins. The histone-octamer contains histones H2A, H2B, H3 and H4, which can be post-translationally modified1. In addition, DNA itself can be modified by methylation2. The combination of modifications, sometimes also referred to as the epigenome, is thought to determine the accessibility and transcriptional activity of DNA.
One of the protein complexes affecting chromatin modifications is the well-conserved Polycomb group (PcG) complex that was first identified in Drosophila. PcG proteins repress gene expression by depositing repressive histone marks, H3K27me3 and H2AK119Ub3. Well-known targets of PcG proteins are Hox genes4. Pioneering work established that PcG proteins are essential for proper patterning during early embryogenesis. In addition, it is proposed that PcG proteins are essential to balance pluripotency and differentiation potential of stem cells5,6,7,8. Besides a role in early embryogenesis, PcG proteins are important for tissue-specific development9,10,11,12.
PcG proteins are basically found in two complexes, Polycomb Repressive Complex 1 (PRC1) and PRC2. PRC2 contains Enhancer of Zeste Homolog 2 or 1 (EZH2/EZH1), Embryonic Ectoderm Development (EED) and Suppressor of Zeste 12 (SUZ12). In the canonical Polycomb pathway PRC2 is recruited to chromatin before PRC1. EZH2 has a catalytically active SET domain that places the repressive H3K27me3 mark. EZH1 also has methyltransferase activity, although less than EZH213 and is postulated to complement the function of EZH214. In addition, EZH2 is thought to act during proliferation, whereas EZH1 operates more in differentiated cells15. Following H3K27 tri-methylation, PRC1 is recruited, allowing the PRC1 component RING1 to ubiquitylate lysine 119 of histone H2A, stabilizing the repressive mark3. However, recent studies implicate that PRC1 is also active in the absence of PRC216. In addition, it was shown that PRC1 can promote H3K27 methylation via a positive feedback loop17.
Most PcG mouse mutants display pre-gastrulation embryonic lethality5,18,19. In mice, both homologs of RING1, Ring1 and Rnf2, are essential for development of primordial germ cells. During oogenesis Ring1 and Rnf2 serve redundant transcriptional functions, which are essential for proper zygotic genome activation (ZGA). Mutant embryos fail to activate gene transcription and loss of Ring1 and Rnf2 has an effect on development-associated genes20,21.
Although it is clear from published work that PcG proteins are involved in conserved processes that are essential for organismal functioning, many critical questions remain unanswered. For instance, it is not known what their role is during early development of a vertebrate system, a question that can be well addressed in zebrafish. PcG proteins are conserved in zebrafish as well as their accompanying epigenetic marks. Before ZGA, which starts around mid-blastula transition (MBT, 3.3 hours post fertilization) and is accompanied by degradation of maternal transcripts22,23,24,25, levels of H3K4me3 (a mark associated with active gene transcription) and H3K27me3 are low. From MBT onwards, the number of genes harboring H3K4me3 increases, which is followed by an increase of RNA Polymerase II occupancy. At the same time the number of genes marked with H3K27me3 slowly increases, suggesting a balance between gene activation and gene repression25,26,27. This also implies that H3K4me3 and H3K27me3 are important during early embryonic development, presumably for cell fate specification or maintenance. A hint for this comes from rnf2 mutant zebrafish embryos that die around 4–5 days post fertilization (dpf), a time at which organogenesis is normally completed, displaying defects in pectoral fin development28.
In this study we generated maternal zygotic mutants for ezh2 to determine the role of Ezh2 during embryonic development. This unique model system makes it possible to obtain detailed information about the function of Ezh2 during early development. Our data show that Ezh2 is dispensable for gastrulation and tissue specification in zebrafish, despite major overall changes in gene expression, a finding that contrasts phenotypes observed in mice. Furthermore, our data indicate that Ezh2 is required for tissue maintenance in at least three different organs.
Results
Ezh2 is conserved in zebrafish
The Polycomb group protein Ezh2 is conserved between many species (Fig. 1a and Supplementary Fig. S1). In vertebrates, Ezh2 has a WD repeat domain at the N-terminus, which is implicated in binding Eed and Suz12 (Fig. 1a). In addition, the protein contains a SET domain at the C-terminus, which has histone methyltransferase activity. In contrast to the WD repeat domain, the SET domain is also present in invertebrate species.
The Polycomb group protein Ezh2 is conserved in zebrafish and ezh2 mRNA is maternally provided in zebrafish embryos.
(a) Schematic representation of Ezh2 orthologs in zebrafish, human, mouse and Drosophila. Detailed alignments (Supplementary Fig. S1) show high conservation between the different species. This is 85% and 86% between zebrafish and human and mouse, respectively. Black boxes indicate the location of the SET domain. Grey boxes indicate the location of the WD domain. (b) In situ hybridization for ezh2 at 2 cells, 30% epiboly, 1, 2 and 3 dpf. ezh2 mRNA is maternally provided and at 2 and 3 dpf it is expressed in the pectoral fins, gut, tectum, eye, mid-hindbrain region and the branchial arches (arrow heads). Scale bar is 200 μm.
When analyzing the mRNA expression profile of ezh2 in zebrafish we found that ezh2 mRNA is maternally loaded into the embryo, as we can detect it already at the two-cell stage (Fig. 1b), however Ezh2 protein does not seem to be maternally provided and is only visible after zygotic genome activation (Supplementary Fig. S2). During further early stages of development ezh2 mRNA is expressed ubiquitously, but becomes more restricted later during development. At 3 dpf ezh2 expression is restricted to the tectum, mid-hindbrain region, eyes, branchial arches and gut (Fig. 1b).
Generation of maternal zygotic ezh2 mutants
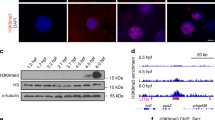
From an ENU-mutagenized library, a pre-mature stop mutation in ezh2 (hu5670) was identified (Supplementary Fig. S1)29. As shown in Fig. 1b, ezh2 mRNA is maternally provided. To study the effect of a complete loss of Ezh2 function on early development, we additionally eliminated the maternally provided ezh2 mRNA. In zebrafish, this can be achieved through germ cell transplantations (Fig. 2a)30. Surprisingly, we were able to generate fertile males and females carrying ezh2 mutant germ cells. In situ hybridization for ezh2 showed that maternal contribution as well as zygotic expression of ezh2 was indeed lost in maternal zygotic ezh2 (MZezh2) mutants (Fig. 2b). In heterozygous siblings maternal transcripts are also absent, while zygotic expression of ezh2 mRNA is present at around 50% epiboly (Fig. 2b). We subsequently investigated the presence of Ezh2 and H3K27me3 by immunohistochemistry. At 1 dpf Ezh2 and H3K27me3 are clearly detectable in wildtype embryos, while both are undetectable in MZezh2 mutants (Fig. 2c,d). Together these data indicate that ezh2(hu5670) is a strong loss of function allele.
Maternal zygotic ezh2 mutant embryos lack Ezh2 and H3K27me3 and show aberrant hox, pax, and shh gene expression.
(a) Schematic representation of germline transplantation at sphere stage to obtain germline mutant zebrafish. The progeny are maternal zygotic ezh2 mutant embryos (MZezh2hu5670/hu5670). (b) In situ hybridization for ezh2 mRNA shows maternal contribution of ezh2 as well as zygotic expression in wildtype embryos. Maternal contribution of ezh2 is lost (3 hpf) in MZezh2hu5670/+ and MZezh2hu5670/hu5670 embryos. Zygotic ezh2 expression (30% epiboly) is also lost in MZezh2hu5670/hu5670. Scale bar is 200 μm. (c) Immunostaining for Ezh2 in wildtype and MZezh2hu5670/hu5670 embryos at 1 dpf. Ezh2 shows representative nuclear localization in the forebrain of wildtype embryos and is lost in MZezh2hu5670/hu5670 embryos. Scale bar is 10 μm. (d) Immunostaining for H3K27me3 in wildtype and MZezh2hu5670/hu5670 embryos at 1 dpf. H3K27me3 shows representative nuclear localization in the tail of wildtype embryos and is lost in MZezh2hu5670/hu5670 embryos. Scale bar is 10 μm. (e) In situ hybridization for hoxa9b, pax2 and shh mRNA in MZezh2hu5670/+ and MZezh2hu5670/hu5670 embryos at 1 and 2 dpf. In MZezh2hu5670/+ embryos a clear boundary of hoxa9b expression is visible (arrow head) as well as expression in the pectoral fin buds (arrows). Expression is shifted to anterior in MZezh2hu5670/hu5670 embryos (arrow head). The expression pattern of hoxa9b in MZezh2hu5670/+ resembles that of wildtype embryos54. Scale bar is 200 μm. In MZezh2hu5670/+ embryos expression of pax2 is normal and amongst others restricted to the optic stalk, mid-hindbrain boundary and the spinal cord neurons32. Expression in the optic stalk is spread throughout the eye in MZezh2hu5670/hu5670 embryos. Expression of shh is comparable to wildtype embryos in MZezh2hu5670/+ embryos at 1 and 2 dpf31. In MZezh2hu5670/hu5670 embryos, expression of shh is outside the regular boundaries in the head region (arrow head) at 1 dpf and is still present at 2 dpf in the notochord, in contrast to MZezh2hu5670/+ embryos (arrow head). Scale bar is 500 μm. The numbers indicate the number of embryos with the displayed phenotype compared to the total number of embryos analyzed.
Since hox, pax, and shh genes are well-known targets of PcG proteins, we investigated whether these transcripts were differentially expressed in MZezh2 mutants. Indeed, the clear boundary of hoxa9b expression is shifted anteriorly in MZezh2 mutant embryos at 1 dpf (Fig. 2e, Supplementary Fig. S2). In addition, expression of pax2 was no longer restricted to the optic stalk, but was present in the entire eye. Expression of shh was also observed outside the regular boundaries of expression at 1 dpf. At 2 dpf shh expression was prolonged and still visible in the notochord in MZezh2 mutants, while this is not observed in heterozygous siblings. Interestingly, zebrafish embryos that lack maternal ezh2, but do express zygotic ezh2, display normal spatiotemporal expression patterns for hoxa9b, pax2, and shh (Fig. 2e)28,31,32, indicating that zygotic ezh2 expression can rescue the loss of maternal ezh2 during embryonic patterning. Consistent with this, animals lacking only maternally provided ezh2 are viable and fertile (data not shown).
MZezh2 mutant embryos complete gastrulation and appear to have a normal gross body plan at 1 dpf (Fig. 3a). However, these embryos seem to lack a clear mid-hindbrain boundary, even though pax2 expression is present at this region (Fig 2e). At 2 dpf MZezh2 mutant embryos display a pleiotropic phenotype, including small eyes, accumulation of blood near the yolk extension, a stringy heart, heart edema and absence of pectoral fins (Fig. 3a). To determine whether these phenotypes are caused by the loss of ezh2, ezh2 mRNA was injected into one-cell-stage MZezh2 mutants and heterozygous siblings. At 2 dpf, ezh2 mRNA-injected MZezh2 mutants were phenotypically indistinguishable from the heterozygous siblings, evidenced by normally sized eyes and normal circulation of the blood (Fig. 3b). This indicates that the observed pleiotropic phenotype is a specific result from the loss of ezh2.
Maternal zygotic ezh2 mutants form a normal body plan and display a pleiotropic phenotype at 2 dpf.
(a) MZezh2hu5670/hu5670 appear relatively normal at 1 dpf, although a clear mid-hindbrain boundary appears to be absent (arrow head). They display a pleiotropic phenotype at 2 dpf, having small eyes, a stringy heart and blood accumulation (arrow heads). MZezh2hu5670/+ show normal development. (b) The pleiotropic phenotypes of MZezh2hu5670/hu5670 can be rescued by injection of full-length ezh2 mRNA (300 pg). The numbers indicate the number of embryos with the displayed phenotype compared to the total number of embryos injected in two experiments. (c) Expression analysis of ezh1 and ezh2 in wildtype and MZezh2hu5670/hu5670 embryos at 0 hpf, 3.3 hpf and 1 dpf. Expression of ezh1 is not detectable in MZezh2hu5670/hu5670 embryos and wildtype embryos at 0 hpf, 3.3 hpf and 1 dpf. ezh1 is expressed in wildtype control embryos at 5 dpf. ezh2 is expressed in wildtype embryos at 0 hpf, 3.3 hpf, 1 dpf and 5 dpf, showing a decrease in expression over time. ezh2 expression cannot be detected in MZezh2hu5670/hu5670 embryos. Relative expression was calculated based on expression of housekeeping genes β-actin and ef1α. Error bars represent standard deviation. n.d. is not done. (d) In situ hybridization for eng1 (muscle pioneer marker) and myoD (somite marker) at 1 dpf in MZezh2hu5670/hu5670 embryos and MZezh2hu5670/+. Both eng1 and myoD are normally expressed in MZezh2hu5670/hu5670 and MZezh2hu5670/+. Scale bar is 500 μm. (e) In situ hybridization for ntl at 1 dpf shows no difference in spatiotemporal expression between MZezh2hu5670/hu5670 embryos and the heterozygous siblings. At 2 dpf in situ hybridization for ntl showed expression in the notochord of MZezh2hu5670/hu5670 embryos, whereas this is not visible in MZezh2hu5670/+ (arrow head). In situ hybridization for krox20 at 1 dpf showed normal expression in MZezh2hu5670/+, but reduced expression in rhombomeres 3 and 5 in MZezh2hu5670/hu5670 embryos (arrow heads). Scale bar is 500 μm for lateral views and 250 μm for dorsal view of krox20 expression. The numbers indicate the number of embryos with the displayed phenotype compared to the total number of embryos analyzed.
Since Ezh1 could potentially take over part of the function of Ezh2, we addressed the expression of ezh1. Until 1 dpf we could not detect ezh1 by qPCR in MZezh2 mutants and wildtype control embryos (Fig. 3c), indicating that during the first 24 hours of development, MZezh2 mutants most likely lack all H3K27 trimethylation activity.
To gain information about developmental processes in the MZezh2 mutants, we performed spatiotemporal expression analyses for eng1 (muscle pioneer marker), myoD (myogenic differentiation marker), ntl (mesodermal marker) and krox20 (neural marker). eng1, myoD and ntl all show expression patterns comparable to expression in heterozygous sibling and wildtype embryos at 1 dpf (Fig. 3d,e, Supplementary Fig. S2)33, indicating that muscle tissue is formed and can differentiate in MZezh2 mutants. However, like for shh we observed sustained expression of ntl in the notochord of MZezh2 mutant embryos at 2 dpf (Fig. 3e), which was not observed in heterozygous siblings and wildtype embryos (Fig. 3e, Supplementary Fig. S2). In addition, expression of krox20 appeared to be less prominent in both rhombomere 3 and 5 in MZezh2 mutant embryos compared to heterozygous siblings and wildtype embryos (Fig. 3e, Supplementary Fig. S2).
These data surprisingly demonstrate that various cellular lineages are properly specified in absence of Ezh2 activity. Interestingly, soon after the body plan has been established, Ezh2 is required for further differentiation of cells in different tissues.
Ezh2 affects the load of maternal mRNA in zygotes
The above results clearly demonstrate that ezh2 is maternally provided and indicate that it has profound effects on zebrafish development, even though MZezh2 mutant embryos survive gastrulation and are able to develop a grossly normal body plan. Given that ZGA occurs around MBT, maternally provided ezh2 may affect gene expression during the first hours of development or in the oocyte, while resulting in detectable phenotypes much later.
To address this we analyzed gene expression in wildtype and MZezh2 mutant embryos at 0 hpf (zygote) and 3.3 hpf (MBT) using an Agilent 4 × 44K array (Fig. 4a). We identified pronounced differences in gene expression between wildtype and MZezh2 mutant zygotes already at 0 and 3.3 hpf (Fig. 4b). Overall, 654 genes are >2-fold higher expressed in MZezh2 mutants versus wildtype and 627 genes are >2-fold lower expressed (Fig. 4b, p < 0.01) at 0 hpf. In addition, 625 genes are upregulated and 206 downregulated in MZezh2 mutants versus wildtype at 3.3 hpf (>2-fold, p < 0.01, Fig. 4b). The differentially expressed genes were divided into 6 clusters using pam with the Euclidean distance metric (Fig. 4b). Clusters 1A and 4A–6A contain genes that are upregulated in MZezh2 mutant embryos compared to wildtype embryos at 0 hpf and 3.3 hpf. (Fig. 4b, Supplementary Fig. S3). To identify enriched biological themes (particularly GO terms) among these genes, we performed DAVID analysis. We identified significant enriched gene functions in cluster 1A, 3A, 5A and 6A. This analysis indicated that genes upregulated in MZezh2 mutants compared to wildtype (cluster 1A, 5A and 6A) are overrepresented for developmental gene functions (Fig. 4c), including previously described Ezh2 targets like hox, pax and tbx (Supplementary Table S1). Genes that are downregulated in MZezh2 mutants compared to wildtype embryos (cluster 3A) are enriched for biological themes including organelle lumen, nucleolus and isomerase.
Gene expression analysis of maternal zygotic ezh2 mutants.
(a) Schematic overview of samples that were used for microarrays and the subsequent workflow. (b) Boxplots of gene expression levels (log2) for genes in cluster 1A–6A, comparing genes that are significantly differently expressed between wildtype versus MZezh2hu5670/hu5670 embryos at 0 hpf and 3.3 hpf. In comparison, expression level (log2) of housekeeping genes actb, eef1a1 and tuba is between 7.3 and 9.3. The mean expression (log2) of the array is between 9.5 and 10.1. (c) DAVID analysis on genes differently expressed between MZezh2hu5670/hu5670 and wildtype embryos at 0 hpf and 3.3 hpf. The fold enrichment of different terms is shown for the different clusters shown in Fig. 4b (Bonferroni corrected p-value < 0.1). (d) Bandplots of H3K27me3 ChIP-sequencing showing presence of H3K27me3 at genes that are significantly (>2-fold, p < 0.01) up- or downregulated in MZezh2hu5670/hu5670 versus wildtype embryos at 0 hpf. The graphs show transcription start site ± 20 kb. The left panel shows the intensity distribution of the H3K27me3 peaks in wildtype embryos at 24 hpf. The mean of the median is depicted as a black line, 50% is red and 90% is pink. (e) Bandplots like in Fig. 4d for genes that are significantly up- or downregulated in MZezh2 mutant versus wildtype embryos at 3.3 hpf. (f) Boxplots of gene expression levels (log2) for genes in cluster 1B–6B (Supplementary Fig. S4), comparing genes that are significantly differently expressed between 0 hpf versus 3.3 hpf in wildtype and MZezh2hu5670/hu5670 embryos. (g) DAVID analysis on genes differently expressed between 3.3 hpf and 0 hpf in MZezh2hu5670/hu5670 and wildtype embryos. The fold enrichment of different terms is shown for the different clusters shown in Fig. 4f (Bonferroni corrected p-value < 0.1).
Since a clear myocardial phenotype in the MZezh2 mutants was observed, the expression of myocardial genes was analyzed in more detail. At both 0 hpf and 3.3 hpf we detected a tendency for myocardial markers to be higher expressed in MZezh2 mutant embryos compared to wildtype embryos (Supplementary Fig. S4).
To determine whether the differentially regulated genes in MZezh2 mutants are indeed enriched for Ezh2 targets, we compared the genes that are up- and downregulated between MZezh2 mutant and wildtype embryos at 0 hpf and 3.3 hpf with previously published ChIP-sequencing data for H3K27me3 at 24 hpf34. We observed that the genes that are upregulated in MZezh2 mutants at 0 hpf and 3.3 hpf are enriched for H3K27me3 peaks under normal conditions (Fig. 4d,e), while such enrichment is not seen for downregulated genes. This suggests that upregulation is due to direct effect of loss of Ezh2 activity and that downregulation most likely stems from indirect effects.
We hypothesize that ezh2 is involved in placing epigenetic signatures during oogenesis that in turn are translated into the establishment of a proper maternal mRNA load of the zygote. This includes mRNAs that do not have a clear role within the oocyte itself, but only function after fertilization and emphasizes the importance of ezh2 in transmitting epigenetic information through the transmission of maternal mRNAs.
Ezh2 affects gene expression during early embryonic development
In order to determine how gene expression is regulated over time, we continued to assess how gene expression changes between 0 hpf and 3.3 hpf and how this is affected in MZezh2 mutant embryos (Fig. 4f, Supplementary Fig. S3). Overall, the changes in gene expression seem to follow the same pattern from 0 hpf to 3.3 hpf in both wildtype and MZezh2 mutant embryos. However, a proportion of transcripts in cluster 1B are downregulated over time in wildtype embryos but not in MZezh2 mutant embryos. In addition, transcripts in cluster 2B show little change in expression in wildtype embryos between 0 hpf and 3.3 hpf, while they become more abundant at 3.3 hpf compared to 0 hpf in MZezh2 mutants (Fig. 4f, Supplementary Fig. S3). This suggests that expression of genes in clusters 1B and 2B is normally controlled in a temporal manner by Ezh2. Furthermore, genes in cluster 3B and 6B are upregulated in both wildtype and MZezh2 mutants from 0 hpf to 3.3 hpf, but the difference in expression is larger in MZezh2 mutants.
We performed DAVID analysis on these genes to identify enriched biological themes on genes that are differently expressed between 0 hpf and 3.3 hpf in wildtype and MZezh2 mutants (Fig. 4g). We only identified significantly enriched gene functions in cluster 3B and 6B (Fig. 4g). As indicated above, both clusters are more upregulated in MZezh2 mutants compared to wildtype embryos at 3.3 hpf and are therefore potential targets of Ezh2. Genes in these clusters are involved in nucleolus, nuclear lumen, non-membrane bounded organelle, transcription factor activity, sequence-specific binding and also include homeobox genes (Supplementary Table S2).
Together, these analyses reveal that Ezh2 not only dictates the maternal load of mRNAs, but also affects the transcription of genes during early embryonic development.
Expression of myocardial markers in MZezh2 mutants
Next, we aimed to better understand the origin of the pleiotropic defects observed in MZezh2 embryos. Since MZezh2 mutants develop a ‘stringy-heart’, which was one of the most pronounced phenotypes, cardiac development was studied in more detail. To gain knowledge about the specification and differentiation of various cardiac lineages, in situ hybridization for different cardiac markers was performed (Fig. 5a–c). Morphologically, the heart fails to undergo cardiac looping resulting in a straight heart tube at 2 dpf in MZezh2 mutant embryos (Fig. 5a, Supplementary Fig. S5). Expression analysis for vmhc revealed a smaller ventricle in MZezh2 mutants compared to heterozygous siblings at 1.5 dpf (Fig. 5b). Next to vmhc, we analyzed expression of hand2 (early marker), myh6 (atrial marker), nppa (late marker) and nfat-c1 (endocardial marker) and showed that these markers are all expressed in the MZezh2 mutant (Fig 5a).
Myocardial development is affected in MZezh2 embryos.
(a) In situ hybridization for different heart markers in MZezh2hu5670/+ and MZezh2hu5670/hu5670 at various time points of development. hand2 is an early myocardial marker. myh6 is a marker for atrial cells. vmhc is a marker for ventricular cells. nppa is a late myocardial marker. nfat-c1 is an endocardial marker. All these markers are expressed in MZezh2hu5670/hu5670, although vmhc, nppa, and nfat-c1 expression show a smaller number of positive cells. (b) In situ hybridization for nkx2.5 at different time points after fertilization in MZezh2hu5670/+ and MZezh2hu5670/hu5670 embryos. Arrow heads point to cells of the pharyngeal arch artery progenitors. This is absent in MZezh2hu5670/hu5670. (c) In situ hybridization for has2 and fgf24 at 2 dpf in MZezh2hu5670/hu5670 and their heterozygous siblings. In MZezh2hu5670/+ expression is restricted to the heart (arrow heads), whereas in the MZezh2hu5670/hu5670 embryos expression is visible in the area surrounding the heart tube (encircled by dashed line). For fgf24 this is also shown from a lateral view (arrow heads). Scale bar is 200 μm. The numbers indicate the number of embryos with the displayed phenotype compared to the total number of embryos analyzed.
To continue the analyses, we studied expression of nkx2.5, a homeodomain transcription factor and an early myocardial marker. This marker is readily expressed starting at the 12-somite stage in wildtype embryos, but the area of nkx2.5 expression seems to be smaller in the MZezh2 mutant at this stage (Fig. 5b). Also later during development we observed a smaller region of nkx2.5 expressing cells in MZezh2 mutants (Fig. 5b). Interestingly, the posterior group of nkx2.5 positive cells, the pharyngeal arch artery progenitors35,36, is absent in MZezh2 mutants at 1 and 1.5 dpf (Fig. 5a). We conclude from these experiments that, while cardiac cell numbers may be affected in MZezh2 mutants, general differentiation markers for different compartments of the heart tube are grossly expressed normally.
Even though we observed that the above-mentioned myocardial markers are expressed grossly normally, this is not valid for all markers. To start with, the developmental and atrioventricular canal marker has2 is normally and specifically expressed in the heterozygous siblings at 2 dpf. In contrast, in MZezh2 mutant embryos we observe ectopic expression of has2 (Fig. 5c). The same observation was made for fgf24 (Fig. 5c). This gene is downstream of tbx5, a transcription factor essential for heart and limb formation37,38 and fgf24 was upregulated in our expression study (Supplementary Table S1). Whereas fgf24 expression is spatially restricted in heterozygous siblings, a broad ring of expression around the heart tube was observed in MZezh2 mutant embryos (Fig. 5c). Similar results were obtained when performing expression analysis for myocardial markers myl7 and mef2cb at 2 dpf (Supplementary Fig. S5). Overall, these results suggest that myocardial cells are specified, but seem to get dispersed over an area around the regular heart tube over time.
MZezh2 mutants display a loss of myocardial tissue integrity
To further investigate the morphogenetic changes that establish the heart tube over time we performed a time course of in situ hybridization analysis for myl7 (Fig. 6a). At the 12-somite stage, myocardial precursors are present in MZezh2 mutants, even though their location and number appears to be slightly affected as shown by nkx2.5 and hand2 expression analysis (Fig. 5a,b). At 1 dpf the heart of heterozygous siblings starts to jog to the left, like in wildtype embryos, while the heart of MZezh2 mutants frequently remains straight (Supplementary Fig. S5). Despite the lack of jogging, a heart tube is still formed (Fig. 6a,b). At 1.5 dpf the heart of heterozygous siblings starts to undergo cardiac looping (Fig. 6a). This bending of the heart tube did not occur in MZezh2 mutant embryos (Fig. 6a). Remarkably, at this stage myl7 expressing cells are visible outside the heart tube and the heart appears to be smaller in size in MZezh2 mutants (Fig. 6a). At 2 dpf only a small tube of myl7 expressing cells remains in MZezh2 mutants.
MZezh2 mutant embryos display impaired myocardial and gastrointestinal tissue maintenance.
(a) In situ hybridization for myl7 at different time points in MZezh2hu5670/+ and MZezh2hu5670/hu5670. At 1 dpf the heart of MZezh2hu5670/hu5670 is straight. In MZezh2hu5670/hu5670 embryos myl7 expressing cells are found outside the heart at 1.5 dpf (arrow heads). At 2 dpf the number of myl7 expressing cells is decreased in MZezh2hu5670/hu5670 compared to MZezh2hu5670/+. (b) Stills of time lapse (Supplementary Movie S1,2) imaging of Tg(myl7::GFP) MZezh2hu5670/+ and MZezh2hu5670/hu5670 embryos from 1 to 2 dpf. In MZezh2hu5670/hu5670 embryos, GFP-positive cells are moving away from the heart (arrows). Arrow heads point at ventricle and atrium. (c) Immunostaining for GFP to visualize Tg(myl7::GFP) (brown precipitation, arrow heads) combined with in situ hybridization for nkx2.5 (purple staining, arrows) at 2 dpf. In MZezh2hu5670/+ no nkx2.5 expressing cells are present. In MZezh2hu5670/hu5670 cells that are detached from the heart express nkx2.5. The right panel shows a zoom-in of the left panel (white square). Scale bar is 50 μm. (d) Immunostaining for GFP Tg(myl7::GFP) combined with in situ hybridization for nppa at 2 dpf in MZezh2hu5670/+ and MZezh2hu5670/hu5670 embryos. Two embryos per genotype are shown. In MZezh2hu5670/hu5670 embryos nppa expression is absent in one and not ubiquitous in the other embryo (arrow heads). In MZezh2hu5670/+ nppa is expressed in atrium and ventricle (arrow heads). Scale bar is 50 μm. (e) In situ hybridization for different gastrointestinal tract markers at 1 and 2 dpf in MZezh2hu5670/hu5670 and MZezh2hu5670/+. Expression of gata6 is present in both MZezh2hu5670/hu5670 and MZezh2hu5670/+ at 1 and 2 dpf. At 2 dpf the intestinal tube appears straight in the MZezh2hu5670/hu5670, whereas structures like the liver and pancreas can be seen in MZezh2hu5670/+ (arrow heads). MZezh2hu5670/hu5670 are able to form a gastrointestinal tract, observed by in situ hybridization for foxa3 at 2 dpf, although the organs are bilaterally formed (arrow heads). In MZezh2hu5670/hu5670 no expression of terminal differentiation markers for liver, fabp10, and exocrine pancreas, try, was observed. Scale bar is 100 μm. Numbers indicate the number of embryos with the displayed phenotype compared to the total number of embryos analyzed.
We next determined whether the extra-cardiac myl7 positive cells we observed around 1.5 dpf represent cells that are derived from the original heart or they are non-heart-related cells that aberrantly start to express myl7. We performed time-lapse imaging on 1 to 2 dpf MZezh2 mutants and heterozygous siblings carrying a Tg(myl7::GFP) transgene (Fig. 6b, Supplementary Movie S1,2). We observed that at around 33–34 hpf, GFP positive cells detach and move away from the heart tube. These detaching cells appear to be derived from both the ventricle and the atrium (Fig. 6b). These results indicate that the extra-cardial cells are lost from the originally formed heart. They also suggest that a loss of cardiac integrity underlies the reduction of the size of the heart tube and that Ezh2 is required to regulate genes that maintain structural integrity of the cardiac tube.
A loss of cell adhesion may cause the loss of cells from the heart, as it is known that in fn morphants cardiac progenitors fail to form the cardiac disc, which results in two heart fields39. In addition, in mice it was shown that Ezh2 represses regulators of extracellular matrix remodeling in endothelial cells40. To address this, expression of dm-GRASP, a cell-surface adhesion molecule of the immunoglobulin superfamily41, was assessed. Immunostaining for dm-GRASP showed expression of this marker in the hearts of MZezh2 mutants, indicating that cell adhesion is not affected in MZezh2 mutants (Supplementary Fig. S5). In addition, we did not observe a difference in apoptosis between MZezh2 mutant embryos compared to heterozygous siblings (Supplementary Fig. S5).
Finally, to gain insight into the identity and differentiation status of the cells that detach from the heart, we combined in situ hybridization for nkx2.5 with immunostaining for GFP in a Tg(myl7::GFP) background on 1, 1.5 and 2 day old embryos. At 1 dpf there are no major differences between heterozygous siblings and MZezh2 mutant embryos (Supplementary Fig. S5). Remarkably, at 1.5 dpf the expression of nkx2.5 is partially lacking in MZezh2 mutants, whereas it is expressed in heterozygous siblings (Supplementary Fig. S5). The cells of MZezh2 mutants that lack expression of nkx2.5 do express GFP. At 2 dpf, the cells of the heart tubes of both MZezh2 mutants as well as heterozygous siblings are GFP (Myl7) positive but nkx2.5 negative. However, the cells that are detached from the heart tube in MZezh2 mutants are both GFP (Myl7) and nkx2.5 positive (Fig. 6c). Even though GFP has a half-life of 26 hours, meaning that GFP-positive cells do not necessarily express GFP at the RNA level, this result strongly suggests that in total absence of Ezh2, myocardial cells fail to silence nkx2.5. In addition, we also combined immunostaining for GFP with in situ hybridization for nppa, a late myocardial differentiation marker. Next to observing a smaller heart tube at 2 dpf in MZezh2 mutants (Fig 5a), we observed a partial loss of nppa expression in MZezh2 mutants, whereas it was expressed throughout the heart in heterozygous siblings (Fig. 6d). This suggests a defect in terminal differentiation of MZezh2 mutant myocardial cells, possibly related to the observed problems in properly repressing nkx2.5. We conclude that myocardial cells in MZezh2 mutants likely have problems to maintain cardiac differentiation and that this may lead to the structural instability of the heart.
Loss of ezh2 affects terminal differentiation of the liver and pancreas
To address whether this loss of tissue integrity and defects in terminal differentiation is specific for the heart we also addressed cell differentiation in other tissues. For this we chose the gastrointestinal tract and the associated organs. Expression analysis for gata6, an early endoderm marker, showed normal expression at 1 dpf in MZezh2 mutants. The gut of MZezh2 mutants is straight at 2 dpf based on gata6 expression, whereas it has looped in heterozygous siblings (Fig. 6e). Interestingly, expression of foxa3, a definite marker of endoderm, showed incorrect looping or a bilateral gastrointestinal tract in MZezh2 mutants (Fig. 6e). Finally, in situ hybridization for the terminal differentiation markers for liver and the exocrine pancreas, fabp10 and try respectively, revealed a loss of expression suggesting that formation of these organs is delayed or abrogated in MZezh2 mutant embryos (Fig. 6e). These results indicate that the gastrointestinal tract, including the liver and pancreas, is formed initially in MZezh2 mutant embryos, but fails to terminally differentiate. Thus, problems in terminal differentiation in MZezh2 mutants are not heart-specific, but different organs derived from different germ layers are affected.
Discussion
The function of Ezh2 during development has been intensely studied using different model systems, including mouse and Drosophila. Despite these studies, many open questions remain regarding the developmental roles of Ezh2. Our study sheds new light on the requirement of Ezh2 during early vertebrate development. Most importantly, our results indicate that the basic vertebrate body plan can be established without Ezh2, but that Ezh2 is essential for the maintenance of a wide range of tissues, possibly by playing a role in terminal differentiation. In the following sections we will discuss possible scenarios regarding the roles of Ezh2 during vertebrate development.
Function of Ezh2 in germ cells
Our results demonstrate a clear function for ezh2 during embryonic development. Strikingly, even though the maternal-to-zygotic transition occurs around 3–4 hours after fertilization, the first phenotypic differences between wildtype embryos and embryos lacking both maternal and zygotic ezh2 are not evident until hours after gastrulation. This may hint to a mechanism in which maternally expressed ezh2 acts by pre-labeling genes with specific chromatin marks such that they can be properly regulated later during development. It is possible that without this pre-labeling, genes cannot be properly shut down after being activated, like we show for a number of myocardial markers and shh and ntl. Even though we have not timed when this activity would be required, our data suggest that this pre-labeling may in fact already occur during oogenesis. This is supported by observations in Ring1/Rnf2 mutant mice that show that Polycomb group proteins act in the female germline to establish developmental competence20. Also in C. elegans transgenerational inheritance of H3K27me3 has been demonstrated42. In addition, work in Drosophila showed that PRC2 plays a role in determining germ cell fate43,44. We note that this maternal activity is not absolutely essential for viability, since embryos lacking only maternal ezh2, while expressing zygotic ezh2, can develop into fertile adults. Apparently, the embryo is able to handle a wide range of gene expression levels during early development.
The ezh2 germline mutants are fertile and able to form MZezh2 mutant embryos. The germ cells of ezh2 germline mutants are originally derived from an incross between heterozygous parents. This implies that these germ cells, lacking zygotic expression of ezh2, obtained correct epigenetic labeling from the parents and this may be the reason they can function normally. Whether the germ cells of MZezh2 mutant zebrafish embryos are functional needs to be tested by serial transplantation assays. Previous studies in mouse and human have shown that during germline development H3K27me3 is almost exclusively present at genes important for somatic development45,46 and hence ectopic expression of these genes in MZezh2 mutant germ cells may lead to sterility. In concordance, C. elegans mutants for PRC2 homologs display a maternal effect sterile phenotype47,48,49.
Ezh2 does not affect early zebrafish development
MZezh2 mutant embryos lack Ezh2 and H3K27me3 and show major differences in gene expression even before the zygotic genome is activated. Still, these embryos are able to form a normal body plan and only die at a time point when tissue specification has taken place, indicating zygotic genome activation is not strongly affected. This is in contrast with Polycomb group mutants in other vertebrates, where loss of Polycomb group gene expression results in early lethality, mostly before gastrulation5,6,19,20,50,51,52,53. The reason for this ‘delayed’ lethality in zebrafish is not completely clear. One could argue that Ezh1 is able to compensate for the loss of Ezh2, since it was reported that Ezh1 can also trimethylate H3K2716. However, we think this is highly unlikely, since we show that ezh1 is not maternally loaded into the zebrafish embryo and based on our array and qPCR data, is not expressed in MZezh2 mutant embryos until at least 1 dpf.
A potential explanation for the lack of an early developmental phenotype of MZezh2 mutants in zebrafish is that unlike mice, zebrafish embryos do not form extra-embryonic tissue, which is essential for normal murine development. Another explanation may be found in differences in developmental timing between mice and zebrafish. In mice, maternal contribution lasts only until the 2-cell stage, while in zebrafish embryos this lasts until at least 1,000-cell stage23. Nevertheless, the fact that zebrafish embryos can gastrulate properly in the absence of Ezh2 indicates that this crucial developmental event does not critically depend on Polycomb gene activity. This makes the zebrafish a very interesting and unique model system to study Ezh2 and Polycomb function in general, during tissue specification and maintenance.
Ezh2 function in tissue maintenance
Most of the defects we observed in MZezh2 mutants relate to tissue maintenance. For example, the heart and the gastrointestinal tract can be specified but fail to be properly maintained. The observed loss of tissue maintenance does not seem to be caused by apoptosis. Alternatively, the failure of tissues to terminally differentiate might be caused by an arrest in proliferation, potentially by deregulation of genes involved in cell cycle control. Terminal differentiation defects were also observed in rnf2 mutant zebrafish during pectoral fin and cranial facial development28,54. Although rnf2 was only zygotically absent in these mutants and Rnf2 is part of PRC1 instead of PRC2, this indicates a common mechanism of involvement of Polycomb group genes in terminal differentiation. By more detailed studies of the developing heart tube we show that myocardial integrity cannot be maintained in the absence of Ezh2, while cell adhesion is not affected. In addition to the well-known function of Ezh2 as a suppressor of gene expression, it can also directly methylate non-histone targets. One example of this is the cardiac transcription factor GATA4, where methylation of GATA4 by PRC2 results in inhibition of GATA4 transcriptional activity in mice55. This function of PRC2 potentially plays a role in the observed myocardial phenotype of MZezh2 zebrafish mutants.
Studies in mice, where conditional knockouts for Ezh2 were generated using different heart specific promoters, showed that loss of Ezh2 at an early time point results in cardiac defects, whereas loss of Ezh2 after the heart is fully formed does not show a severe phenotype9,10. Possibly, there is a sensitive period during which Ezh2 represses its targets in progenitor cells to safeguard normal myocardial development, followed by terminal differentiation of myocardial cells, after which Ezh2 becomes dispensable for maintenance of silencing, because other chromatin features may stably lock gene expression status4,6,56,57.
Another mechanism through which Ezh2 may affect tissue maintenance is that Ezh2 may have a critical role within tissue-specific stem cells, such that upon loss of Ezh2 the tissue cannot be properly supported by the addition of newly differentiating cells8,9,10,11,12. Discrimination between these mutually non-exclusive scenarios will require the identification and study of relevant stem cell pools of the affected tissues and tracing experiments in order to follow gene expression within single cells.
Taken together, our work implies that Ezh2 is essential for tissue maintenance and to set up proper maternal mRNA contribution and presents a novel and powerful tool to study how Polycomb group proteins act during early vertebrate development and tissue maintenance.
Methods
Zebrafish genetics and strains
Zebrafish (Danio rerio), were housed according to standard conditions58 and staged according to Kimmel et al59. The ezh2 nonsense mutant (hu5670, R592STOP) was derived from ENU mutagenized libraries using target-selected mutagenesis as described29. Zebrafish with the ezh2 mutant allele were outcrossed against wildtype zebrafish (TL or AB) and subsequently incrossed to obtain homozygous mutants. Tg(myl7::GFP) and Tg(vas::eGFP) zebrafish have been described before60,61. All experiments were carried out in accordance with animal welfare laws, guidelines and policies and were approved by the Utrecht University and the Radboud University Animal Experiments Committee.
Genotyping
DNA was purified from caudal fin tissue taken from anesthetized zebrafish, or from embryos. An ezh2 fragment was amplified by nested PCR with primers indicated in Supplementary Table S3. The ezh2 mutation (hu5670, CCTGGCTGTA(C > T)GAGAGTGTGA) results in the loss of an RsaI restriction site. PCR was followed by RsaI restriction to finalize genotyping (Supplementary Fig. S2).
Germ cell transplantation
Germ cell transplantation was performed as described previously30. At 4 hpf cells from the margin of the embryo were transplanted into wildtype hosts that were injected with the dead end morpholino, resulting in death of the primordial germ cells of the host62. Transplanted cells were labeled with Tg(vas::eGFP) and were derived from an ezh2(hu5670) heterozygous incross. After transplantation the donors were genotyped. At 1 dpf it was assessed whether the transplantation was successful, after which these embryos were raised to adulthood, obtaining a wildtype zebrafish harboring an ezh2 mutant germline. The adult female germline mutants were checked for being 100% mutant by crossing them to a male germline mutant or a male ezh2 heterozygous mutant zebrafish and determine the phenotype and genotype of the progeny. For all germline mutants used in this study the resulting progeny was 100% or 50% homozygous mutant, depending on the genotype of the zebrafish it was crossed with. The germline mutant zebrafish displayed normal fertility and produced 200–600 embryos per cross. The MZezh2 mutant embryos all displayed the same phenotype. For the experiments below we used siblings from a cross of ezh2 germline mutant females with heterozygous ezh2 mutant males and genotyped them afterwards. For the gene expression analysis we crossed ezh2 germline mutant females with ezh2 germline mutant males to obtain 100% MZezh2 mutant progeny. Since the MZezh2 mutant embryos display a lethal phenotype, the embryos that were used were the first generation after germline transplantation.
Histological analysis
Zebrafish embryos were sacrificed with Tricaine and ice-cold water, fixed overnight in 4% PFA in PBS at 4 °C. After fixation the embryos were gradually transferred to 75% ethanol after which they were embedded in plastic for sectioning. Plastic sections were stained with haemotoxylin and eosin for histological analysis.
Whole mount in situ hybridization
Embryos were fixed overnight at 4 °C in 4% PFA in PBS, after which they were gradually transferred to 100% methanol. Embryos older than 24 hpf were treated with proteinase K. In situ hybridization was performed as described previously63. The embryos were imaged by light microscopy or embedded in plastic for sectioning and imaging.
Immunostainings
Immunostainings were performed as described previously63,64. Embryos were fixed in 4% PFA in PBS at 4 °C overnight. After fixation they were gradually transferred to 100% methanol. Rabbit anti-Ezh2 antibody from Cell Signalling Technologies was used (1:200). The epitope of this antibody is located upstream of the SET domain and the identified nonsense mutation in ezh2. Rabbit anti-H3K27me3 antibody from Millipore was used (1:750). Cy3-anti-rabbit antibody from Jackson ImmunoResearch was used as secondary antibody. Immunostainings were analyzed using a confocal fluorescent microscope (Leica, SP5). Immunostainings after in situ hybridization and for dm-GRASP and active Caspase-3 were performed with a rabbit anti-GFP from Gentaur (1:200), mouse anti-dm-GRASP from DSHB (1:200) and anti-Caspase-3 from BD Biosciences (1:500), respectively, followed by a peroxidase labeled polymer (Immunovision and Dako) for DAB staining. The immunostainings were analyzed using a light microscope. When embedded in paraffin, the sections were stained with neutral red.
qPCR analysis
Total RNA was isolated from 0 hpf, 3.3 hpf and 1 dpf wildtype and MZezh2 mutant embryos using Trizol. cDNA was synthesized using Superscript II (Invitrogen). Standard qPCR using SYBR Green was performed using the primers shown in Supplementary Table S4. Relative expression was corrected for primer efficiency and calculated based on expression of housekeeping genes β-actin and ef1α.
Time lapse imaging
Embryos of 1 dpf were dechorionated and mounted in glass bottom plates using 0.25% agarose in E3 embryo medium containing Tricaine. Confocal imaging was performed overnight using a LEICA AF7000 microscope. Pictures were taken with 7.5-minute intervals.
Gene expression microarrays
Custom 4 × 44k microarrays for zebrafish from Agilent were used according to manufacturer’s protocol. 200 ng of total RNA from 1 cell stage embryos and embryos of 3.3 hpf was converted into cRNA and labeled with Cy3 or Cy5. Samples were subsequently hybridized overnight and washed. A dye swap was included as a technical replicate. The experiment was performed in duplicate using biological replicates. The arrays were processed using R/Bioconductor and limma65. After background correction, within-array normalization (loess) and between-array normalization (Aquantile) was performed. Differential expression was determined using eBayes method. The expression profiles were clustered using pam with the Euclidean distance metric. We used the biomaRt package66,67 to provide the Ensembl annotation with systematic name and genomic location based on the probe identifiers.
H3K27me3 ChIP-seq data for 24 hpf was obtained from NCBI GEO (GSE3505034) and mapped to the zebrafish genome (danRer7/Zv9) with bwa68. The bandplots were created using fluff69 for the transcription start sites of differentially expressed genes (fold change >=2) and genes present on the array with or without H3K27me3 enrichment. DAVID annotation70,71 was obtained from https://david.ncifcrf.gov/.
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE64618 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64618).
Additional Information
How to cite this article: San, B. et al. Normal formation of a vertebrate body plan and loss of tissue maintenance in the absence of ezh2. Sci. Rep. 6, 24658; doi: 10.1038/srep24658 (2016).
References
Marks, P. et al. Histone deacetylases and cancer: causes and therapies. Nature reviews. Cancer 1, 194–202, doi: 10.1038/35106079 (2001).
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews. Genetics 13, 484–492, doi: 10.1038/nrg3230 (2012).
Bracken, A. P. & Helin, K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nature reviews. Cancer 9, 773–784, doi: 10.1038/nrc2736 (2009).
Jones, R. S. & Gelbart, W. M. Genetic analysis of the enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics 126, 185–199 (1990).
O’Carroll, D. et al. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol 21, 4330–4336 (2001).
Pasini, D., Bracken, A. P., Hansen, J. B., Capillo, M. & Helin, K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol 27, 3769–3779 (2007).
van der Stoop, P. et al. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. Plos One 3, e2235, doi: 10.1371/journal.pone.0002235 (2008).
Kamminga, L. M. et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood 107, 2170–2179 (2006).
He, A. et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 110, 406–415, doi: 10.1161/CIRCRESAHA.111.252205 (2012).
Delgado-Olguin, P. et al. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nature genetics 44, 343–347, doi: 10.1038/ng.1068 (2012).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes & development 25, 485–498, doi: 10.1101/gad.2019811 (2011).
Juan, A. H. et al. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes & development 25, 789–794, doi: 10.1101/gad.2027911 (2011).
Son, J., Shen, S. S., Margueron, R. & Reinberg, D. Nucleosome-binding activities within JARID2 and EZH1 regulate the function of PRC2 on chromatin. Genes Dev 27, 2663–2677, doi: 10.1101/gad.225888.113 (2013).
Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Molecular cell 32, 491–502, doi: 10.1016/j.molcel.2008.10.016 (2008).
Margueron, R. et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell 32, 503–518, doi: 10.1016/j.molcel.2008.11.004 (2008).
Luis, N. M., Morey, L., Di Croce, L. & Benitah, S. A. Polycomb in stem cells: PRC1 branches out. Cell Stem Cell 11, 16–21, doi: 10.1016/j.stem.2012.06.005 (2012).
Kalb, R. et al. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat Struct Mol Biol 21, 569–571, doi: 10.1038/nsmb.2833 (2014).
Donohoe, M. E. et al. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol Cell Biol 19, 7237–7244 (1999).
Faust, C., Lawson, K. A., Schork, N. J., Thiel, B. & Magnuson, T. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development 125, 4495–4506 (1998).
Posfai, E. et al. Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev 26, 920–932, doi: 10.1101/gad.188094.112 (2012).
Yokobayashi, S. et al. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature 495, 236–240, doi: 10.1038/nature11918 (2013).
Harvey, S. A. et al. Identification of the zebrafish maternal and paternal transcriptomes. Development 140, 2703–2710, doi: 10.1242/dev.095091 (2013).
Tadros, W. & Lipshitz, H. D. The maternal-to-zygotic transition: a play in two acts. Development 136, 3033–3042, doi: 10.1242/dev.033183 (2009).
Lee, M. T. et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 503, 360–364, doi: 10.1038/nature12632 (2013).
Andersen, I. S. et al. Epigenetic marking of the zebrafish developmental program. Current topics in developmental biology 104, 85–112, doi: 10.1016/B978-0-12-416027-9.00003-6 (2013).
Lindeman, L. C. et al. Prepatterning of developmental gene expression by modified histones before zygotic genome activation. Developmental cell 21, 993–1004, doi: 10.1016/j.devcel.2011.10.008 (2011).
Vastenhouw, N. L. et al. Chromatin signature of embryonic pluripotency is established during genome activation. Nature 464, 922–926, doi: 10.1038/nature08866 (2010).
van der Velden, Y. U., Wang, L., van Lohuizen, M. & Haramis, A. P. The Polycomb group protein Ring1b is essential for pectoral fin development. Development 139, 2210–2220, doi: 10.1242/dev.077156 (2012).
Wienholds, E. et al. Efficient target-selected mutagenesis in zebrafish. Genome Res 13, 2700–2707 (2003).
Ciruna, B. et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci USA 99, 14919–14924 (2002).
Krauss, S., Concordet, J. P. & Ingham, P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444 (1993).
Krauss, S., Johansen, T., Korzh, V. & Fjose, A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 113, 1193–1206 (1991).
Weinberg, E. S. et al. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271–280 (1996).
Irimia, M. et al. Extensive conservation of ancient microsynteny across metazoans due to cis-regulatory constraints. Genome Res 22, 2356–2367, doi: 10.1101/gr.139725.112 (2012).
Paffett-Lugassy, N. et al. Heart field origin of great vessel precursors relies on nkx2.5-mediated vasculogenesis. Nat Cell Biol 15, 1362–1369, doi: 10.1038/ncb2862 (2013).
Nagelberg, D. et al. Origin, Specification and Plasticity of the Great Vessels of the Heart. Curr Biol 25, 2099–2110, doi: 10.1016/j.cub.2015.06.076 (2015).
Fischer, S., Draper, B. W. & Neumann, C. J. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development 130, 3515–3524 (2003).
Garrity, D. M., Childs, S. & Fishman, M. C. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129, 4635–4645 (2002).
Trinh, L. A. & Stainier, D. Y. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell 6, 371–382 (2004).
Delgado-Olguin, P. et al. Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Development 141, 4610–4617, doi: 10.1242/dev.112607 (2014).
Fashena, D. & Westerfield, M. Secondary motoneuron axons localize DM-GRASP on their fasciculated segments. J Comp Neurol 406, 415–424 (1999).
Gaydos, L. J., Wang, W. & Strome, S. Gene repression. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science 345, 1515–1518, doi: 10.1126/science.1255023 (2014).
Iovino, N., Ciabrelli, F. & Cavalli, G. PRC2 controls Drosophila oocyte cell fate by repressing cell cycle genes. Dev Cell 26, 431–439, doi: 10.1016/j.devcel.2013.06.021 (2013).
Eun, S. H., Shi, Z., Cui, K., Zhao, K. & Chen, X. A non-cell autonomous role of E(z) to prevent germ cells from turning on a somatic cell marker. Science 343, 1513–1516, doi: 10.1126/science.1246514 (2014).
Hammoud, S. S. et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature 460, 473–478, doi: 10.1038/nature08162 (2009).
Brykczynska, U. et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol 17, 679–687, doi: 10.1038/nsmb.1821 (2010).
Capowski, E. E., Martin, P., Garvin, C. & Strome, S. Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics 129, 1061–1072 (1991).
Holdeman, R., Nehrt, S. & Strome, S. MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila Polycomb group protein. Development 125, 2457–2467 (1998).
Xu, L. & Strome, S. Depletion of a novel SET-domain protein enhances the sterility of mes-3 and mes-4 mutants of Caenorhabditis elegans. Genetics 159, 1019–1029 (2001).
Pasini, D., Bracken, A. P., Jensen, M. R., Denchi, E. L. & Helin, K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 23, 4061 (2004).
Suzuki, M. et al. Involvement of the Polycomb-group gene Ring1B in the specification of the anterior-posterior axis in mice. Development 129, 4171–4183 (2002).
Voncken, J. W. et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc.Natl.Acad.Sci.USA 100, 2468 (2003).
Wang, J., Mager, J., Schnedier, E. & Magnuson, T. The mouse PcG gene eed is required for Hox gene repression and extraembryonic development. Mamm.Genome 13, 493 (2002).
van der Velden, Y. U., Wang, L., Querol Cano, L. & Haramis, A. P. The polycomb group protein ring1b/rnf2 is specifically required for craniofacial development. Plos One 8, e73997, doi: 10.1371/journal.pone.0073997 (2013).
He, A. et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes & development 26, 37–42, doi: 10.1101/gad.173930.111 (2012).
Montgomery, N. D. et al. The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation. Curr.Biol. 15, 942 (2005).
Chamberlain, S. J., Yee, D. & Magnuson, T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells 26, 1496–1505, doi: 10.1634/stemcells.2008-0102 (2008).
Westerfield, M. In The zebrafish book, A guide for the laboratory use of zebrafish (Danio rerio) 5th edn, (ed Westerfield, M. ) Chs. 1-3 (University of Oregon Press, 2007).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310 (1995).
Huang, C. J., Tu, C. T., Hsiao, C. D., Hsieh, F. J. & Tsai, H. J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 228, 30–40, doi: 10.1002/dvdy.10356 (2003).
Krovel, A. V. & Olsen, L. C. Expression of a vas::EGFP transgene in primordial germ cells of the zebrafish. Mech Dev 116, 141–150 (2002).
Weidinger, G. et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13, 1429–1434 (2003).
Houwing, S. et al. A Role for Piwi and piRNAs in Germ Cell Maintenance and Transposon Silencing in Zebrafish. Cell 129, 69–82 (2007).
Huang, H. Y. et al. Tdrd1 acts as a molecular scaffold for Piwi proteins and piRNA targets in zebrafish. EMBO J 30, 3298–3308, doi: 10.1038/emboj.2011.228 (2011).
Smyth, G. K. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor Statistics for Biology and Health (eds Robert Gentleman et al.) Ch. 23, 397–420 (Springer New York, 2005).
Durinck, S. et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440, doi: 10.1093/bioinformatics/bti525 (2005).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nature protocols 4, 1184–1191, doi: 10.1038/nprot.2009.97 (2009).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760, doi: 10.1093/bioinformatics/btp324 (2009).
Georgiou, G. & van Heeringen, S. J. fluff : 1.62. doi: 10.5281/zenodo.34209 (2015).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4, 44–57, doi: 10.1038/nprot.2008.211 (2009).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37, 1–13, doi: 10.1093/nar/gkn923 (2009).
Acknowledgements
The authors want to thank Dr. Sylvia Boj of the Hubrecht Institute for providing the fabp2 probe, Dr. Anna Pavlina Haramis of the Institute of Biology in Leiden for providing us with the hox probes, the members of the Bakkers lab at the Hubrecht Institute for providing myocardial and developmental markers for in situ hybridization and antibodies for immunohistochemistry, Marjo den Broeder from the Institute for Environmental Studies providing us with the shh, eng1, and fgf24 probes, Henk van Roekel and Prof. dr. Edwin Cuppen of the Hubrecht Institute for identifying the ezh2 (hu5670) mutant in the ENU library, Jeroen Korving of the Hubrecht Institute for excellent histotechnical support, Dr. Klaas Mulder and Prof. dr. Gert Jan Veenstra of the Radboud University for critically reading the manuscript and the animal caretakers for taking care of the zebrafish. We thank Dr. Federico Tessadori and Dr. Emily Noël of the Hubrecht Institute and the members of the Ketting laboratory of the Institute of Molecular Biology for stimulating discussions. The work was funded by the Innovative Research scheme of the Netherlands Organisation for Scientific research (www.nwo.nl, NWO-Veni 916.96.021 and NWO-Vidi 864.12.009, L.M.K.) and the Radboud University Nijmegen Medical Centre tenure track fellowship (www.radboudumc.nl, L.M.K.).
Author information
Authors and Affiliations
Contributions
B.S. and N.D.C. performed experiments and wrote and edited the manuscript. S.v.H. assisted in the analysis of the microarray data and edited the manuscript. N.W. and A.K.L. assisted with experiments and edited the manuscript. M.A. performed experiments. J.B. designed experiments and edited the manuscript. R.F.K. designed experiments and wrote and edited the manuscript. L.M.K. designed and performed experiments and wrote and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
San, B., Chrispijn, N., Wittkopp, N. et al. Normal formation of a vertebrate body plan and loss of tissue maintenance in the absence of ezh2. Sci Rep 6, 24658 (2016). https://doi.org/10.1038/srep24658
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24658
- Springer Nature Limited
This article is cited by
-
Inhibition of methyltransferase activity of enhancer of zeste 2 leads to enhanced lipid accumulation and altered chromatin status in zebrafish
Epigenetics & Chromatin (2020)
-
The round goby genome provides insights into mechanisms that may facilitate biological invasions
BMC Biology (2020)
-
Loss of the Polycomb group protein Rnf2 results in derepression of tbx-transcription factors and defects in embryonic and cardiac development
Scientific Reports (2019)
-
Ezh1 arises from Ezh2 gene duplication but its function is not required for zebrafish development
Scientific Reports (2019)