Abstract
Fungal inositol polyphosphate (IP) kinases catalyse phosphorylation of IP3 to inositol pyrophosphate, PP-IP5/IP7, which is essential for virulence of Cryptococcus neoformans. Cryptococcal Kcs1 converts IP6 to PP-IP5/IP7, but the kinase converting IP5 to IP6 is unknown. Deletion of a putative IP5 kinase-encoding gene (IPK1) alone (ipk1Δ) and in combination with KCS1 (ipk1Δkcs1Δ), profoundly reduced virulence in mice. However, deletion of KCS1 and IPK1 had a greater impact on virulence attenuation than that of IPK1 alone. ipk1Δkcs1Δ and kcs1Δ lung burdens were also lower than those of ipk1Δ. Unlike ipk1Δ, ipk1Δkcs1Δ and kcs1Δ failed to disseminate to the brain. IP profiling confirmed Ipk1 as the major IP5 kinase in C. neoformans: ipk1Δ produced no IP6 or PP-IP5/IP7 and, in contrast to ipk1Δkcs1Δ, accumulated IP5 and its pyrophosphorylated PP-IP4 derivative. Kcs1 is therefore a dual specificity (IP5 and IP6) kinase producing PP-IP4 and PP-IP5/IP7. All mutants were similarly attenuated in virulence phenotypes including laccase, urease and growth under oxidative/nitrosative stress. Alternative carbon source utilisation was also reduced significantly in all mutants except ipk1Δ, suggesting that PP-IP4 partially compensates for absent PP-IP5/IP7 in ipk1Δ grown under this condition. In conclusion, PP-IP5/IP7, not IP6, is essential for fungal virulence.
Similar content being viewed by others
Introduction
The fungal pathogen, Cryptococcus neoformans, predominantly infects immunocompromised individuals via the lung and then disseminates to the brain where it establishes life-threatening meningoencephalitis. C. neoformans is responsible for over half a million deaths each year in AIDS patients alone1. Some of the most well-known virulence factors of C. neoformans include a polysaccharide capsule2, melanin3 and urease4,5. A prerequisite to virulence is its ability to grow at human physiological temperature, which can impact the stability of the cell wall6,7. In the host, C. neoformans also encounters oxidative and nitrosative stress, which originate predominantly from macrophages and altered nutritional availability in the lung and brain, which are low in glucose8,9,10,11,12,13.
Several signalling cascades, including calcineurin, mitogen-activated protein kinase/protein kinase C (Mpk1/Pkc1), cyclic adenosine monophosphate/protein kinase A (cAMP/Pka1), high osmolarity glycerol (HOG) and Rim101 pathways, allow C. neoformans to sense, respond and adapt to host stresses encountered throughout the course of infection14,15,16,17,18,19,20. We previously identified a new virulence-related signalling pathway in C. neoformans comprising phospholipase C1 (Plc1) and a series of sequentially acting inositol polyphosphate kinases (IPKs)21,22,23. The IPKs convert the inositol trisphosphate (IP3) product of Plc1 to IP4-IP6 and IP6 to the inositol pyrophosphates, PP-IP5/IP7 and (PP)2-IP4/IP8. Specifically, IP3 is converted to IP4 and IP5 by Arg122,23. An uncharacterised kinase then phosphorylates IP5 to produce the highly abundant IP6 species. IP6 is then phosphorylated by Kcs1 to PP-IP5/IP7. Asp1 further phosphorylates PP-IP5/IP7 to produce (PP)2-IP4/IP823. ARG1 and KCS1 deletion mutants of C. neoformans, which do not produce PP-IP5/IP7 and (PP)2-IP4/IP8, exhibit attenuated growth, compromised cell wall integrity and reduced production of melanin, urease and mating filaments. The KCS1 deletion strain, kcs1Δ, is also unable to utilise alternative carbon sources for growth23. Consequently kcs1Δ has a reduced ability to infect host lung, does not disseminate to the brain and is avirulent in a mouse model23. However, (PP)2-IP4/IP8 plays an insignificant role in cryptococcal virulence per se, since the IP8-deficient mutant strain, asp1Δ, had a similar virulence profile to that of the wild-type strain23. Our results therefore support a crucial role for PP-IP5/IP7 in the pathogenicity of C. neoformans23. While IP6 is the precursor for the synthesis of virulence-promoting PP-IP5/IP7 in C. neoformans, neither the IP5 kinase responsible for production of IP6, nor the contribution of IP6 to fungal pathogenicity is currently known.
In this study we employ combinatorial gene deletion analysis, HPLC-based inositol polyphosphate profiling, phenotypic analysis and a mouse infection model to establish Ipk1 as the major IP5 kinase in C. neoformans and assess the contribution of the Ipk1 product, IP6, to pathogenicity. Similar to Kcs1 we found that Ipk1 is essential for pathogenicity but that its contribution relates more to its indirect role in PP-IP5/IP7 production, rather than its direct role in producing IP6. Our results therefore confirm that PP-IP5/IP7 is the most crucial IP species for cryptococcal pathogenicity and that the contribution of IP6 is relatively insignificant.
Results
Identification of an IP5 kinase in C. neoformans
Homology search
We recently identified Arg1 as the major IP3 kinase in C. neoformans (Cn) converting Plc1-derived IP3 to IP522,23. We also identified Kcs1 as the major cryptococcal IP6 kinase, phosphorylating IP6 to the inositol pyrophosphate PP-IP5/IP7, which is crucial for cryptococcal virulence23. To identify the intermediary kinase in the pathway, we searched the C. neoformans var grubii (strain H99) genomic database (http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans/MultiHome.html) for an IP5 kinase homolog using Ipk1 from Saccharomyces cerevisiae as a query. CNAG_01294 produced the strongest match and was designated CnIPK1. CnIPK1 is 3245 nucleotides in length and is predicted to encode a protein of 415 amino acids. Using the global alignment sequence analysis tool available from https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalw.html, the CnIpk1 protein was found to be just 13.7% identical and 26.68% similar to ScIpk1 and 19.18% identical and 28.96% similar to human IP5 kinase, inositol-pentakisphosphate 2-kinase. Despite the low homology, a domain search at (http://pfam.xfam.org/) confirmed the presence of the inositol-pentakisphosphate 2-kinase protein domain (PF06090) at the N-terminus of CnIPK1.
Creation of IPK1 deletion mutant strains
To determine whether CnIpk1 is an IP5 kinase that produces IP6, an IPK1 deletion mutant (ipk1Δ) and an IPK1 reconstituted strain (ipk1Δ + IPK1) were created in the wild-type H99 (WT H99) background using biolistic transformation and homologous recombination as described in the methods. KCS1 was also deleted in ipk1Δ to create an ipk1Δ kcs1Δ double mutant. Targeted gene deletion and genetic reconstitution were confirmed by PCR and antibiotic resistance testing (see supplementary methods and Supplementary Fig. S2).
Comparison of IP profiles of deletion mutants using inositol radiolabelling and HPLC
WT, ipk1Δ, ipk1Δ + IPK1 and the kcs1Δ strains were radiolabelled with [3H] myo-inositol. The IP profile of lysates prepared from each strain was then compared by anion-exchange HPLC (Fig. 1). Similar to many eukaryotic cells including fungi, plants and humans, IP6 was found to be the most abundant IP species in the WT cryptococcal strain (Fig. 1A). PP-IP5/IP7 and (PP)2-IP4/IP8 were detected in the WT strain, but not in kcs1Δ control strain as reported previously23. In contrast, IP6, PP-IP5/IP7 and (PP)2-IP4/IP8 were not detected in ipk1Δ, while IP5 and IP4 were increased (Fig. 1B). These results are consistent with Ipk1 being the major IP5 kinase in C. neoformans. The reintroduction of an intact IPK1 gene into ipk1Δ restored IP5 kinase activity back to the level of the WT strain (Fig. 1C). In addition to IP4 and IP5, an IP species with an elution time corresponding to PP-IP424 accumulated in the ipk1Δ mutant (Fig. 1B) but was absent in the profiles of WT and kcs1Δ (Fig. 1A,D). To determine whether the cryptococcal IP6 kinase, Kcs1, recognises IP5 as an alternative substrate to IP6 in the ipk1Δ mutant and converts it to PP-IP4, the IP profile of the double ipk1Δ kcs1Δ gene deletion mutant was assessed. We hypothesised that PP-IP4 would not be detected in the double mutant if Kcs1 is the progenitor of PP-IP4. The profile of ipk1Δ kcs1Δ (Fig. 1E) confirms this prediction and indicates that Kcs1 synthesises the pyrophosphate-containing PP-IP4 using IP5 as substrate. However, the absence of PP-IP4 in the WT and kcs1Δ strains, which both have active Ipk1, indicates that Kcs1 acts predominantly as an IP6 kinase when Ipk1 is present.
IP profiles of (A) WT, (B) ipk1Δ, (C) ipk1Δ + IPK1, (D) kcs1Δ and (E) ipk1Δ kcs1Δ.
Lysates prepared from [3H] myo-inositol-labelled cells were subjected to anion-exchange HPLC analysis. IP species were eluted using a gradient of increasing phosphate concentration. The elution profile of the IP standards used (IP5 isomer [3H]I(1, 3, 4, 5, 6)P5, [3H]IP6, [3H]PP-IP4 and [3H]PP-IP5/IP7) are indicated by the black arrows; grey arrows indicate the expected elution profile of I(1, 3, 4, 5)P4 and (PP)2-IP4/IP8 species.
Impact of Ipk1 on stress tolerance and production of virulence traits
We recently demonstrated that the PP-IP5/IP7 produced by Kcs1 is crucial for growth of C. neoformans under stress: the kcs1Δ mutant exhibited reduced growth in the presence of cell wall perturbing agents and reduced production of the virulence factors, melanin and urease23. While PP-IP5/IP7 is absent in the kcs1Δ, ipk1Δ and ipk1Δ kcs1Δ mutants, the ipk1Δ and ipk1Δ kcs1Δ mutants also fail to produce IP6 (see Table 1 for a summary). We therefore investigated whether ipk1Δ and ipk1Δ kcs1Δ exhibit these growth and virulence phenotypes and whether they are attenuated to a greater extent than kcs1Δ due to the absence of both IP6 and PP-IP5/IP7. As C. neoformans can replicate inside macrophages, tolerance of oxidative and nitrosative stress was also investigated. The results show that the kcs1Δ mutant, but not the ipk1Δ mutant, displayed a mild growth defect on rich medium (YPD) at 30 °C and 37 °C (Fig. 2A). However, the ipk1Δ mutant exhibited a significant growth defect in the presence of two cell wall perturbing agents, Congo red and, (even more so), caffeine (Fig. 2B). All mutant strains were mildly sensitive to oxidative stress at 37 °C and strongly sensitive to nitrosative stress (Fig. 3). In contrast to our expectation, the ipk1Δ growth defect under all of the conditions tested (Figs 2 and 3) was similar to, or mildly better, than that observed for the kcs1Δ and ipk1Δ kcs1Δ mutant strains, indicating that loss of IP6 in combination with PP-IP5/IP7 does not exacerbate the phenotype. Growth of ipk1Δ + IPK1 was similar to WT under all stress conditions tested.
Effect of temperature (A) and cell wall perturbing agents (B) on growth of ipk1Δ, kcs1Δ and ipk1Δ kcs1Δ.
All strains were serially diluted 10-fold, from 106 cells to 101 cells per 3μL (left to right) and dropped onto media containing the reagents indicated. All plates were incubated at 30 °C/37 °C for 72 hours. The YPD 30 °C plate was used as a control.
Effect of oxidative and nitrosative stress on growth of ipk1Δ, kcs1Δ and ipk1Δkcs1Δ.
All strains were serially diluted 10-fold, from 106 cells to 101 cells per 5μL (left to right) and dropped onto YNB + 0.5% glucose agar containing the reagents indicated. All plates were incubated at 37 °C for 96 hours.
Production of virulence traits
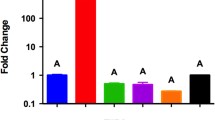
The kcs1Δ mutant exhibits defects in laccase 1 (Lac1)-induced melanisation: in a glucose-deficient environment, expression of the Lac1-encoding gene, LAC1, is repressed in kcs1Δ23. We therefore investigated whether the IPK1-deficient mutants displayed a similar phenotype. Since laccase is cell wall-associated, extracellular laccase activity was quantified by measuring the oxidation of ABTS by whole cells over 120 min following 6 hours of induction in glucose-deficient medium (Fig. 4A). The induction of LAC1 mRNA after 3 hours induction in glucose-deficient medium was also measured by qRT-PCR (Fig. 4B). Relative to WT and ipk1Δ + IPK1, cell-associated laccase activity was reduced in all mutants (Fig. 4A). This reduction in ABTS oxidation correlated with reduced LAC1 mRNA expression (Fig. 4B). Similarly, urease production was reduced in all deletion mutants compared to WT and ipk1Δ + IPK1 as indicated by the diameter of the pink halo surrounding the inoculum, following growth on Christensen’s agar (Fig. 5).
Effect of IPK gene deletion on extracellular laccase activity (A) and LAC1 gene expression (B).
Strains were incubated for 3 and 6 hours at 30 °C in minimal media without glucose for gene induction and enzyme assay, respectively. (A) ABTS was then added and the amount of oxidised ABTS at each time point was measured spectrophotometrically at 436 nm. (B) LAC1 gene expression was determined by qRT-PCR with ACT1 used as the reference gene for normalization. A one-way ANOVA multiple comparisons test of all strains revealed a statistically significant difference between the 3 mutants and both WT and ipk1Δ + IPK1. (****p-value < 0.0001 and error bars represent standard deviation).
Effect of IPK gene deletion on urease production 106 cells of each strain was dropped onto Christensen’s (urea-containing) agar.
All plates were incubated at 30 °C for 72 hours. The extent of urease production, which is observed due to the incorporation of the phenol red pH indicator, is proportional to the diameter of the pink halo surrounding the inoculum.
IPK mutants are hypersusceptible to antifungal drugs
Since the arg1Δ and kcs1Δ mutants are hypersusceptible to antifungal drugs23 we investigated the susceptibility of the ipk1Δ and ipk1Δ kcs1Δ mutants to a range of clinically-available antifungals and included kcs1Δ as a control (Supplementary Table S1). Similar to WT and kcs1Δ, ipk1Δ and ipk1Δ kcs1Δ retained their resistance to the echinocandins: anidulafundin, micafungin and caspofungin. Collectively, the mutants were 2–4 times more sensitive to 5-flucytosine and 4–8 times more sensitive to the azole family (posaconazole, voriconazole, itraconazole and fluconazole). The sensitivity of all mutants to amphotericin B was similar to WT.
Growth on alternative carbon sources
Pathogens of the respiratory system including C. neoformans encounter a low glucose environment during host lung infection9 and cryptococcal metabolic mutants incapable of utilising carbon sources other than glucose are attenuated for virulence in animal models25,26. We previously demonstrated that the PP-IP5/IP7-deficient kcs1Δ strain is impaired in utilising three non-fermentable carbon sources: glycerol, lactate and oleic acid. We therefore compared the growth of the IPK1-deficient mutant strains to that of kcs1Δ when these substrates are used as the sole carbon source (Fig. 6). On the control plate containing glucose, all mutant strains grew at a similar rate to that of WT and ipk1Δ + IPK1. However, on plates containing glycerol, lactate or oleic acid, growth of all of the mutants was reduced. Unlike growth phenotypes in Figs 2 and 3, growth of kcs1Δ and ipk1Δ kcs1Δ on these alternative carbon sources was more severely attenuated than growth of the ipk1Δ mutant. The growth defect seen in kcs1Δ correlated with RNA-seq analysis where expression of genes involved in the utilisation of alternative carbon sources was reduced23. We used the same approach to compare the gene expression profile of ipk1Δ with those of kcs1Δ and arg1Δ. Arg1 encodes the major IP3 kinase in C. neoformans and, like ipk1Δ and kcs1Δ, arg1Δ does not produce PP-IP5/IP7. Figure 7 provides a representative summary of the expression of genes associated with glycolysis (Fig. 7A) and the tricarboxylic acid (TCA) cycle (Fig. 7B). Glycolysis-related genes in Fig. 7A were similarly up-regulated in arg1Δ, ipk1Δ and kcs1Δ, relative to WT. However, although genes encoding TCA cycle enzymes were down-regulated in all mutants, they were down-regulated to a lesser extent in ipk1Δ, compared with other mutants (Fig. 7B). The expression of genes involved in fatty acid β-oxidation and peroxisomal organisation was similarly down-regulated in all mutants (Supplementary Fig. S1).
Effect of IPK1 deletion on alternative carbon source utilisation.
All strains were serially-diluted 10-fold, from 106 cells to 101 cells per 3 μL (left to right) and dropped onto minimal media (MM) containing the carbon sources indicated. MM +1% glucose was used as a control. All plates were incubated at 30 °C for 72 hours.
Expression of glycolysis (A) and TCA cycle (B) associated genes in arg1Δ, ipk1Δ and kcs1Δ mutants, relative to WT H99.
Wild type and mutant cultures grown overnight in YPD broth were used for RNA extraction, followed by RNA-seq gene expression analysis (green, down-regulated expression; red, up-regulated expression). The colour bar to the left of each heat map demonstrates the log2 fold changes from comparison of each mutant to WT.
Impact of Ipk1 on cryptococcal virulence in a mouse model
We found previously that the kcs1Δ mutant is avirulent in a murine inhalational model of cryptococcosis but establishes a persistent asymptomatic infection that remains confined to the lungs for up to 50 days post infection23. Thus, we compared the infection profile of the IPK deletion mutants using the same model. Mice were inoculated with 5 × 105 CFUs of WT, ipk1Δ, ipk1Δ kcs1Δ and ipk1Δ + IPK1 and time to illness (survival) and organ burdens were determined (Fig. 8A). The results for kcs1Δ-infected mice were also included in the analysis as a comparison. All WT- and ipk1Δ + IPK1-infected mice succumbed to infection over a similar time period (median survival time was 14 and 19 days, respectively; p = 0.264). However, 80% and 100% of ipk1Δ- and ipk1Δ kcs1Δ-infected mice, respectively, survived the infection and maintained their weight and vigour over the 50 day time course. The differences in survival between the 3 mutant-infected groups and the WT- and ipk1Δ+IPK1-infected groups was statistically significant (p-value < 0.001) as determined by the Kaplan-Meier log rank test.
Effect of IPK deletion on virulence in a murine inhalation model of cryptococcosis.
(A) Survival analysis. Isoflurane-anaesthetised mice were inoculated intranasally with 5 × 105 CFUs of WT, ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ and ipk1Δ+IPK1 and euthanised after showing debilitating symptoms of infection, or at 50 days post-infection for asymptomatic mice (see Methods). A survival analysis (Kaplan-Meier log rank test) revealed statistically significant differences in survival between the mutant groups and both WT and ipk1Δ+IPK1 (p < 0.001). Median survival of mice infected with WT H99 and ipk1Δ+IPK1 was 14 and 19 days, respectively, but the difference was not statistically significant (p > 0.05). Note that similar to kcs1Δ-infected mice23 all ipk1Δ kcs1Δ-infected mice were healthy with no sign of illness or weight loss for up to at least 50 days post-infection. (B) Organ burden analysis. Infection burdens were determined at time of death or upon termination of the experiment (Day 50). For each strain, organs were harvested from 3 mice. Results represent the mean logCFUs ± SEM. The PP-IP4-deficient strains, kcs1Δ and ipk1Δ kcs1Δ, did not disseminate to the brain.
Lung and brain infection burdens for the WT- and ipk1Δ + IPK1- infected groups (at time of death) and the mutant-infected groups (at time of death or 50 days post-infection for still healthy mice) were also measured (Fig. 8B). Lower lung burdens were obtained for all of the mutant strains relative to WT and ipk1Δ + IPK1, with ipk1Δ burdens being marginally higher than those of ipk1Δ kcs1Δ and kcs1Δ. However, the reduction observed for all mutant strains relative to WT was not statistically significant. Only the PP-IP4-accumulating ipk1Δ mutant strain disseminated to the brain. Our results suggest that the accumulated PP-IP4 in ipk1Δ is compensating for the absence of PP-IP5/IP7 to partially “restore” cryptococcal load in the lung and permit dissemination. However, the improvement is not sufficient to restore virulence. Improved growth of the ipk1Δ mutant strain in lung tissue and its ability to disseminate to the brain may be attributable in part to its improved capability to utilise alterative carbon sources relative to the other mutants (Fig. 6).
Discussion
We previously described Arg1, Kcs1 and Asp1 as major IP3, IP6 and PP-IP5/IP7 kinases in C. neoformans, respectively. We now report that Ipk1 is the major IP5 kinase in C. neoformans. Deletion of the IPK1 gene (ipk1Δ) abolished the synthesis of IP6 and the two inositol pyrophosphates, PP-IP5 and (PP)2-IP4, which are produced by Kcs1 and Asp1, respectively (Fig. 1B). Since PP-IP5/IP7 is crucial for virulence23 and IP6 is highly abundant, we expected that the absence of both IP6 and PP-IP5/IP7 in the ipk1Δ mutant strain would exacerbate the severity of the phenotypic defect as compared to kcs1Δ, which is only deficient in PP-IP5/IP7. However, this was not the case, since loss of IP6 and PP-IP5/IP7 did not attenuate virulence phenotypes or virulence in animal models to a greater extent than the loss of PP-IP5/IP7 alone. Thus, IP6 has a negligible role in fungal physiology and virulence. In S. cerevisiae and S. pombe, Ipk1-generated IP6 has an essential role in Gle1p-mediated mRNA export at the nuclear pore complex, as mutant strains lacking IPK1 failed to effectively export mRNA from the nucleus27,28. So far we have not been able to attribute a role to IP6 in C. neoformans except that it serves as an essential precursor for the synthesis of PP-IP5/IP7.
It remains to be determined how PP-IP5 exerts its effect in C. neoformans. The pleiotropic phenotype of kcs1Δ and its transcriptional profile indicate that PP-IP5 markedly affects gene expression29,30,31,32. PP-IP5/IP7 might regulate gene expression by interacting directly with components of the transcription regulatory complexes/chromatin remodelling machinery, or by pyrophosphorylating transcriptional regulators to alter their activity33,34.
PP-IP4 accumulated in the ipk1Δ mutant, but its production was abolished following co-deletion of KCS1 and IPK1. Similar to Kcs1 in S. cerevisiae24,35, our results demonstrate that cryptococcal Kcs1 is the progenitor of PP-IP4 when Ipk1 is absent. However, when Ipk1 is present, as in the case of WT and the kcs1Δ mutant, PP-IP4 is not produced, indicating that Kcs1 acts predominantly as an IP6 kinase. Thus, cryptococcal Kcs1 is a dual specificity kinase acting primarily as an IP6 kinase to produce PP-IP5 and as an IP5 kinase producing PP-IP4 when IP6 is unavailable. However, the physiological significance of PP-IP4 production remains to be determined. The predominant roles of Ipk1 and Kcs1 in vivo are most likely related to their relative affinities for IP5 and IP6 and the greater abundance of IP6. A model of the complete IP biosynthesis pathway in C. neoformans depicting the role of Ipk1 and the new role for Kcs1 is shown in Fig. 9.
The inositol polyphosphate biosynthesis pathway in C. neoformans.
Plc1 – produced IP3 is sequentially phosphorylated to IP4, IP5 and IP6 by Arg1 and Ipk1. Kcs1 generates PP-IP4 and PP-IP5/IP7 from IP5 and IP6, respectively. (PP)2-IP4 is derived from Asp1. The Inset represents the position of the phosphates on the inositol ring. Figure was adapted from23.
None of the mutants in this study (ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ) generate PP-IP5/IP7. However, a comparison of the ipk1Δ and kcs1Δ phenotypes revealed that the ipk1Δ phenotype is more robust than the kcs1Δ and ipk1Δ kcs1Δ phenotypes, particularly in the case of virulence in mice. Although Ipk1 was crucial for virulence, with only 20% of the ipk1Δ-infected mice succumbing to infection over a 50 day infection period, no deaths were recorded in the kcs1Δ- or ipk1Δ kcs1Δ-infected groups over the same time period. Furthermore, there was a trend towards ipk1Δ producing higher burdens of infection in the lungs and being the only mutant with an ability to disseminate to the brain despite a similar reduction in urease as compared to the other mutants. Urease has been implicated as a factor enabling C. neoformans to cause meningoencephalitis5.
Nutritional availability in the host lung, glucose in particular, is limited and it is imperative for microbial pathogens to adapt their metabolism accordingly. In agreement with its improved proliferation in the host lung, as compared to Kcs1-deficient mutants, ipk1Δ was able to metabolise glycerol, lactate and oleic acid more efficiently and grew significantly better than the other mutants in the presence of these compounds as a sole carbon source. Glycerol, lactate and the unsaturated fatty acid, oleic acid, are incorporated into the tricarboxylic acid (TCA) cycle via different routes. Glycerol is converted to glyceraldehyde 3-phosphate, lactate is oxidised to pyruvate and oleic acid undergoes β-oxidation to produce acetyl-CoA. Our RNA-seq data demonstrate that genes encoding TCA cycle enzymes are down-regulated in arg1Δ, ipk1Δ and kcs1Δ mutants. However, this down-regulation is less pronounced in ipk1Δ than in arg1Δ and kcs1Δ (Fig. 7B).
A possible explanation for the more robust ipk1Δ phenotype (improved metabolism of alternative carbon sources and, to a lesser extent, growth on rich medium) is that PP-IP4 can (partially) compensate for the absence of PP-IP5/IP7 by fulfilling some of its functions. This is supported by the fact that PP-IP4 and PP-IP5/IP7 are structurally similar (see Fig. 9). Both pyrophosphates have a diphosphate at the 5′ position on the inositol ring. The difference between the two species is the absence of a phosphate group at the 2′ position in PP-IP4, which is replaced by a hydroxyl group. A similar compensation phenomenon was demonstrated in S. cerevisiae, where in the absence of other inositol pyrophosphates, PP-IP4 is sufficient to regulate endocytic trafficking and ensure normal vesicular morphology35. Due to the minor structural difference, PP-IP4 may be able to bind to and/or pyrophosphorylate some, but not all, of the PP-IP5/IP7 targets. The relative stability of the terminal phosphate on the diphosphate of PP-IP4 and its ability to pyrophosphorylate pre-phosphorylated proteins remains to be investigated. Cryptococcal phenotypes uniquely dependent on PP-IP5/IP7 include cell wall integrity, oxidative/nitrosative stress tolerance and laccase and urease production.
C. neoformans encounters oxidative and nitrosative stress initiated by macrophages during infection. IPs have been reported to act as antioxidants, with IP6 being the most potent36,37. Hawkins et al.37 determined that the iron (Fe3+)-chelating properties of IP6 could prevent the formation of toxic reactive oxygen species (ROS). Since iron is also involved in catalysing the production of reactive nitrogen species (RNS)38, we assessed the ability of the IPK mutant strains to tolerate oxidative and nitrosative stress by adding hydrogen peroxide (H2O2) and sodium nitrite (NaNO2), respectively, to the YNB growth medium. However, the growth rates of IP6-deficient ipk1Δ and ipk1Δ kcs1Δ strains and the IP6-producing kcs1Δ strain were similar in the presence of either reagent (Fig. 3) suggesting that the absence of IP6 is not the crucial factor contributing to the oxidative/nitrosative stress sensitivity of ipk1Δ.
Unlike in mammalian cells, IPK enzymes in C. neoformans are not redundant and share a low sequence similarity with their mammalian equivalents. Cryptococcal IPK enzymes therefore represent targets for antifungal drug design. CnIpk1 is only 19.18% identical to the mammalian homolog inositol-pentakisphosphate 2-kinase. CnKcs1 is 12.65%, 11.69% and 12.09% similar to its three equivalent mammalian kinases, inositol hexakisphosphate kinase 1 isoform 1 (IP6K1), IP6K2 and IP6K3, respectively. Mutant strains lacking KCS1 and IPK1 showed increased susceptibility to the azole family of drugs (Supplementary Table S1). This suggests that inhibitors directed against these enzymes, especially Kcs1 due to its crucial role in PP-IP5/IP7 production, could potentially act synergistically with azoles and improve treatment outcome. Furthermore, IP kinases with low homology to mammalian enzymes are also found in other medically important opportunistic fungal pathogens, including Candida albicans, potentially extending the applicability of IPK inhibitors to other fungal pathogens.
Conclusion
Using gene deletion analysis we have identified Ipk1 as the major IP5 kinase in C. neoformans and as a major contributor to cryptococcal pathogenicity. However, the means by which Ipk1 contributes to pathogenicity is due to its indirect role in the production of PP-IP5/IP7, rather than its direct role in producing IP6. Our results using double deletion mutants show that the contribution of IP6 to pathogenicity is, in fact, relatively insignificant and confirm our previous observation that PP-IP5/IP7 is the most crucial IP species. We have also demonstrated an additional kinase activity for Kcs1, which is only evident when Ipk1 is absent. This activity involves production of an additional inositol pyrophosphate species, PP-IP4. In ipk1Δ, PP-IP4 function may overlap with PP-IP5/IP7 to partially restore utilisation of alternative carbon sources, lung infection burdens and dissemination to the CNS, even though ipk1Δ infection remains predominantly asymptomatic.
Methods
Fungal strains and media
Strains used in this study were wild-type C. neoformans var. grubii strain H99 (serotype A, MATα) and ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ and reconstituted ipk1Δ (ipk1Δ + IPK1) which were all created from WT H99. Strains were routinely grown on YPD (1% yeast extract, 2% peptone and 2% dextrose) or Sabouraud (SAB) agar (1% peptone, 4% glucose, 1.5–2% agar). Urease production was assessed on Christensen’s urea agar (2% urea, 1.5% agar, 0.08% NaH2PO4.H2O, 0.12% Na2HPO4, 0.1% peptone, 0.1% glucose, 0.5% NaCl, 0.0012% phenol red, pH 6.8). Plates were incubated at 30 °C for 72 hours.
Creating transgenic strains
The IPK1 gene deletion construct was made using overlap PCR to join the 5′ flanking region, the neomycin resistance cassette (NEOR) and the 3′ flanking region. The flanking regions were amplified from WT H99 genomic DNA and NEOR from plasmid pJAF1 (a gift from Dr John R Perfect, Duke University, Durham, NC, USA)39. The deletion construct, which effectively had NEOR in place of the IPK1 coding region, was then transformed into the WT H99 strain using biolistic transformation40. Successful transformants (ipk1Δ::NEO) whereby the NEOR had replaced the IPK1 gene by homologous recombination, were selected on YPD agar plates containing 0.5M sorbitol and 100μL/mL geneticin (G418).
To construct the reconstituted strain, ipk1Δ + IPK1, the IPK1 gene (with 973 bp of 5′ flank and 622 bp of 3′ flank) was amplified from WT H99 genomic DNA. The hygromycin BR cassette was also amplified by PCR. The two fragments were fused together using overlap PCR. The final fragment was then transformed into the ipk1Δ strain by biolistic transformation. Transformants were selected on YPD agar containing 0.5M sorbitol and 350 μL/mL hygromycin B. To create the double gene deletion mutant, ipk1Δ kcs1Δ, we created a KCS1 deletion cassette, kcs1Δ::NAT, using PCR, which was introduced into the ipk1Δ strain using biolistic transformation to disrupt KCS1. Successful transformants were selected on YPD agar containing 0.5M sorbitol and 100μg/mL nourseothricin. kcs1Δ::NEO was previously created as described in ref. 23.
[3H]-inositol labelling of inositol poly- (IP) and pyrophosphates (PP-IP)
The protocol used for [3H]-inositol labelling of the cryptococcal strains was adapted from41. Overnight YPD cultures of WT, ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ and ipk1Δ + IPK1 were diluted to OD600 = 0.05 in 5 mL YPD containing 10μCi/mL [3H] myo-inositol (PerkinElmer) and incubated with shaking at 200 rpm at 30 °C until the culture OD600 reached at least 12.8 (18–35 hrs). The cells were pelleted by centrifugation (maximum speed for 10 minutes at 4 °C), washed twice with 1 mL ice-cold YPD and snap-frozen in liquid nitrogen. To extract IP/PP-IPs, the cell pellets were resuspended in extraction buffer (1M HClO4, 3mM EDTA, 0.1 mg/mL IP6) and homogenised at 4 °C with a MiniBeadbeater-8 cell disrupter (Daintree Scientific, TAS, Australia): 4 × 30 second cycles with 1 minute rest on ice in between cycles. The debris was pelleted by centrifugation (maximum speed for 5 minutes at 4 °C). The pH of IP extracts was neutralised by titration with neutralisation buffer (1M K2CO3, 3mM EDTA). Samples were then incubated on ice for 2 hours, centrifuged at maximum speed for 10 minutes at 4 °C and supernatants were collected for anion-exchange HPLC as described in41. IP/PP-IP species were identified using the relevant standards. Specifically: 3H-IP6 was acquired from (PerkinElmer NEN (New England Nuclear)); 3H-I(1, 3, 4, 5, 6)P5 was prepared using IPMK as previously described42; PP-IP5/IP7 was prepared using IP6K1 as previously described43. All the radiolabeled inositol phosphates in vitro synthesized were HPLC purified and desalted as described44. PP-IP4 was purified from 3H-inositol radiolabelled Saccharomyces cerevisiae ipk1Δ strain35.
Quantification of laccase activity and LAC1 gene expression
Laccase activity. The assay for quantifying extracellular laccase activity was adapted from23 and is based on the oxidation of the laccase substrate, 2, 2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS). All strains were adjusted to OD600 = 1 and induced in minimal media without glucose (10μM CuSO4, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine and 3μM thiamine) for 6 hours at 30 °C. Following induction, an assay mixture consisting of cells, distilled H2O and 3mM ABTS was incubated at 30 °C over a 120 minute time course, with absorbance readings taken at 15, 30, 60, 90 and 120 mins. At each time point, cells were pelleted by centrifugation and the absorbance of the supernatant at 436 nm was measured using a spectrophotometer.
LAC1 gene expression was measured using SYBR Green qRT-PCR (Corbett Rotor-Gene 6000) with mRNA extracted from cells after a 3 hour induction in minimal media without glucose. ACT1 was used as a reference gene. Relative quantification was determined using the ΔΔCt method45.
Spot dilution assays
WT, ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ and ipk1Δ + IPK1 strains were cultured overnight in YPD broth, serially diluted 10-fold and spotted onto the following medium: YPD agar containing 0.5 mg/mL caffeine or 0.5% Congo red to examine the effect of these cell-wall perturbing agents on growth; minimal media agar supplemented with 1% glucose, 1% glycerol, 1% sodium lactate or 1% oleic acid as the sole carbon source to assess carbon source utilisation; and YNB (pH 4) + 0.5% glucose agar with or without 1mM H2O2/NaNO2 to determine the effect of oxidative/nitrosative stress. Plates were incubated at 30 °C/37 °C for 72–96 hours.
RNA-seq
The methodology pertaining to RNA-seq is described in23. The following equation was used to generate expression values for heat maps: log2(FPKMmutant/FPKMWT). Clustering was performed using Gene Cluster 3 (University of Tokyo, Human Genome Center http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm#ctv) and the heat maps drawn using Java TreeView (http://jtreeview.sourceforge.net/). Expression data used to generate the heat maps are listed in Supplementary spreadsheet 1. The whole RNA-seq dataset has been deposited in the NCBI GEO database under the accession number GSE78824.
Murine inhalation model of cryptococcosis
All procedures described were approved and governed by the Sydney West Local Health District Animal Ethics Committee, Department of Animal Care, Westmead Hospital and all procedures were carried out in accordance with the guidelines and regulations of this institute. Survival and organ burden were conducted using 7-week-old female BALB/c mice obtained from the Animal Resource Centre, Floreat Park, Western Australia. Mice were anaesthetised using isoflurane (in oxygen) delivered via an isoflurane vapouriser attached to a Stinger Small Animal Anaesthetic Machine (Advanced Anaesthesia Specialists).
Groups of 10 mice were inoculated intranasally with WT, ipk1Δ, kcs1Δ, ipk1Δ kcs1Δ and ipk1Δ + IPK1 strains (5 × 105 cells/20μl PBS). Viable yeast cells inoculated into the nares were quantified following culture on SAB plates. Mice were observed daily for 50 days for signs of ill-health. Mice were deemed to have succumbed to infection if they had lost 20% of their pre-infection weight and/or if they showed debilitating clinical signs such as respiratory distress, hunching, excessive ruffling and reduced mobility. Sick mice were euthanised by CO2 inhalation followed by cervical dislocation. Lungs and brain were removed from 3 mice and assessed for infection burden following homogenisation and quantitative culture as previously described23. All healthy mice were also euthanised as above at the end of the study, 50 days post-inoculation and organ burdens were quantified as above. Differences in survival were analysed with SPSS (Version 20) statistical software, using the Kaplan-Meier method (Mantel-Cox log-rank test), where a p-value < 0.001 was considered statistically significant.
Additional Information
How to cite this article: Li, C. et al. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that PP-IP5/IP7, not IP6, is essential for virulence. Sci. Rep. 6, 23927; doi: 10.1038/srep23927 (2016).
References
Park, B. J. et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23, 525–530, doi: 10.1097/QAD.0b013e328322ffac (2009).
Chang, Y. C. & Kwon-Chung, K. J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 14, 4912–4919 (1994).
Salas, S. D., Bennett, J. E., Kwon-Chung, K. J., Perfect, J. R. & Williamson, P. R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184, 377–386 (1996).
Cox, G. M., Mukherjee, J., Cole, G. T., Casadevall, A. & Perfect, J. R. Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68, 443–448 (2000).
Olszewski, M. A. et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 164, 1761–1771, doi: 10.1016/S0002-9440(10)63734-0 (2004).
Kraus, P. R. et al. Identification of Cryptococcus neoformans temperature-regulated genes with a genomic-DNA microarray. Eukaryot Cell 3, 1249–1260, doi: 10.1128/EC.3.5.1249-1260.2004 (2004).
Kraus, P. R., Fox, D. S., Cox, G. M. & Heitman, J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol Microbiol 48, 1377–1387 (2003).
Missall, T. A. et al. Posttranslational, translational and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryot Cell 5, 518–529, doi: 10.1128/EC.5.3.518-529.2006 (2006).
Hu, G., Cheng, P. Y., Sham, A., Perfect, J. R. & Kronstad, J. W. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Molecular microbiology 69, 1456–1475, doi: 10.1111/j.1365-2958.2008.06374.x (2008).
Upadhya, R., Campbell, L. T., Donlin, M. J., Aurora, R. & Lodge, J. K. Global transcriptome profile of Cryptococcus neoformans during exposure to hydrogen peroxide induced oxidative stress. PLoS One 8, e55110, doi: 10.1371/journal.pone.0055110 (2013).
Feldmesser, M., Tucker, S. & Casadevall, A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 9, 273–278 (2001).
Kalsi, K. K. et al. Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflugers Arch 457, 1061–1070, doi: 10.1007/s00424-008-0576-4 (2009).
Garnett, J. P., Baker, E. H. & Baines, D. L. Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. The European respiratory journal 40, 1269–1276, doi: 10.1183/09031936.00052612 (2012).
Odom, A. et al. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 16, 2576–2589, doi: 10.1093/emboj/16.10.2576 (1997).
D’Souza, C. A. et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol 21, 3179–3191, doi: 10.1128/MCB.21.9.3179-3191.2001 (2001).
Bahn, Y. S. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell 7, 2017–2036, doi: 10.1128/EC.00323-08 (2008).
Kim, S. Y. et al. Hrk1 plays both Hog1-dependent and -independent roles in controlling stress response and antifungal drug resistance in Cryptococcus neoformans. PLoS One 6, e18769, doi: 10.1371/journal.pone.0018769 (2011).
O’Meara, T. R. et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6, e1000776, doi: 10.1371/journal.ppat.1000776 (2010).
Kozubowski, L., Lee, S. C. & Heitman, J. Signalling pathways in the pathogenesis of Cryptococcus. Cell Microbiol 11, 370–380, doi: 10.1111/j.1462-5822.2008.01273.x (2009).
Gerik, K. J., Bhimireddy, S. R., Ryerse, J. S., Specht, C. A. & Lodge, J. K. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell 7, 1685–1698, doi: 10.1128/EC.00146-08 (2008).
Chayakulkeeree, M. et al. Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans. Molecular microbiology 69, 809–826, doi: 10.1111/j.1365-2958.2008.06310.x (2008).
Lev, S. et al. Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase. Infect Immun 81, 1245–1255, doi: 10.1128/IAI.01421-12 (2013).
Lev, S. et al. Fungal Inositol Pyrophosphate IP7 is Crucial for Metabolic Adaptation to the Host Environment and Pathogenicity. MBio 6, e00531–00515, doi: 10.1128/mBio.00531-15 (2015).
Saiardi, A., Caffrey, J. J., Snyder, S. H. & Shears, S. B. The inositol hexakisphosphate kinase family. Catalytic flexibility and function in yeast vacuole biogenesis. J Biol Chem 275, 24686–24692, doi: 10.1074/jbc.M002750200 (2000).
Price, M. S. et al. Cryptococcus neoformans requires a functional glycolytic pathway for disease but not persistence in the host. MBio 2, e00103–00111, doi: 10.1128/mBio.00103-11 (2011).
Panepinto, J. et al. The DEAD-box RNA helicase Vad1 regulates multiple virulence-associated genes in Cryptococcus neoformans. J Clin Invest 115, 632–641, doi: 10.1172/JCI23048 (2005).
York, J. D., Odom, A. R., Murphy, R., Ives, E. B. & Wente, S. R. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 285, 96–100 (1999).
Sarmah, B. & Wente, S. R. Dual functions for the Schizosaccharomyces pombe inositol kinase Ipk1 in nuclear mRNA export and polarized cell growth. Eukaryot Cell 8, 134–146, doi: 10.1128/EC.00279-08 (2009).
Alcazar-Roman, A. R. & Wente, S. R. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117, 1–13, doi: 10.1007/s00412-007-0126-4 (2008).
Odom, A. R., Stahlberg, A., Wente, S. R. & York, J. D. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287, 2026–2029 (2000).
El Alami, M., Messenguy, F., Scherens, B. & Dubois, E. Arg82p is a bifunctional protein whose inositol polyphosphate kinase activity is essential for nitrogen and PHO gene expression but not for Mcm1p chaperoning in yeast. Molecular microbiology 49, 457–468 (2003).
Auesukaree, C., Tochio, H., Shirakawa, M., Kaneko, Y. & Harashima, S. Plc1p, Arg82p and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. The Journal of biological chemistry 280, 25127–25133, doi: 10.1074/jbc.M414579200 (2005).
Shears, S. B. Inositol pyrophosphates: why so many phosphates? Adv Biol Regul 57, 203–216, doi: 10.1016/j.jbior.2014.09.015 (2015).
Wilson, M. S., Livermore, T. M. & Saiardi, A. Inositol pyrophosphates: between signalling and metabolism. Biochem J 452, 369–379, doi: 10.1042/BJ20130118 (2013).
Saiardi, A., Sciambi, C., McCaffery, J. M., Wendland, B. & Snyder, S. H. Inositol pyrophosphates regulate endocytic trafficking. Proc Natl Acad Sci USA 99, 14206–14211, doi: 10.1073/pnas.212527899 (2002).
Graf, E. & Eaton, J. W. Antioxidant functions of phytic acid. Free Radic Biol Med 8, 61–69 (1990).
Hawkins, P. T. et al. Inhibition of iron-catalysed hydroxyl radical formation by inositol polyphosphates: a possible physiological function for myo-inositol hexakisphosphate. Biochem J 294 (Pt 3), 929–934 (1993).
Bouton, C., Raveau, M. & Drapier, J. C. Modulation of iron regulatory protein functions. Further insights into the role of nitrogen- and oxygen-derived reactive species. J Biol Chem 271, 2300–2306 (1996).
Fraser, J. A., Subaran, R. L., Nichols, C. B. & Heitman, J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot Cell 2, 1036–1045 (2003).
Toffaletti, D. L., Rude, T. H., Johnston, S. A., Durack, D. T. & Perfect, J. R. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175, 1405–1411 (1993).
Azevedo, C. & Saiardi, A. Extraction and analysis of soluble inositol polyphosphates from yeast. Nat Protoc 1, 2416–2422, doi: 10.1038/nprot.2006.337 (2006).
Maffucci, T. et al. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res 65, 8339–8349, doi: 10.1158/0008-5472.CAN-05-0121 (2005).
Azevedo, C., Burton, A., Bennett, M., Onnebo, S. M. & Saiardi, A. Synthesis of InsP7 by the Inositol Hexakisphosphate Kinase 1 (IP6K1). Methods Mol Biol 645, 73–85, doi: 10.1007/978-1-60327-175-2_5 (2010).
Menniti, F. S., Miller, R. N., Putney, J. W. Jr. & Shears, S. B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem 268, 3850–3856 (1993).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, doi: 10.1006/meth.2001.1262 (2001).
Acknowledgements
We thank Christabel F. Wilson and Dr Keren Kaufman-Francis for their technical assistance with the animal study and the sequencing team at the Ramaciotti Centre for Genomics (UNSW, Sydney, Australia). This work was supported by a National Health and Medical Research Council of Australia project grant (APP1058779_Djordjevic/Saiardi/Sorrell/Lev) and by Bioplatforms Australia through the Commonwealth Government National Collaborative Research Infrastructure Strategy (RNA-seq). C.L. is supported by an Australian Postgraduate Award. A.S. is supported by the Medical Research Council (MRC) core support to the MRC/UCL Laboratory for Molecular Cell Biology University Unit (MC_UU_1201814). T.C.S. is supported by the Sydney Medical School Foundation.
Author information
Authors and Affiliations
Contributions
C.L., S.L., T.S. and J.D. designed the study. C.L., S.L., A.S., D.D. and J.D. performed all experiments and statistical analyses. All authors edited the manuscript. All authors reviewed the manuscript and approved the manuscript for publication.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, C., Lev, S., Saiardi, A. et al. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that PP-IP5/IP7, not IP6, is essential for virulence. Sci Rep 6, 23927 (2016). https://doi.org/10.1038/srep23927
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23927
- Springer Nature Limited
This article is cited by
-
The intersection between stress responses and inositol pyrophosphates in Saccharomyces cerevisiae
Current Genetics (2020)
-
Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans
Nature Communications (2016)