Abstract
The ATP-binding cassette (ABC) transporters represent a superfamily of proteins that have important physiological roles in both prokaryotes and eukaryotes. In insects, ABC transporters have previously been implicated in insecticide resistance. The 91-R strain of Drosophila melanogaster has been intensely selected with DDT over six decades. A recent selective sweeps analysis of 91-R implicated the potential role of MDR49, an ABC transporter, in DDT resistance, however, to date the details of how MDR49 may play a role in resistance have not been elucidated. In this study, we investigated the impact of structural changes and an alternative splicing event in MDR49 on DDT-resistance in 91-R, as compared to the DDT susceptible strain 91-C. We observed three amino acid differences in MDR49 when 91-R was compared with 91-C, and only one isoform (MDR49B) was implicated in DDT resistance. A transgenic Drosophila strain containing the 91-R-MDR49B isoform had a significantly higher LD50 value as compared to the 91-C-MDR49B isoform at the early time points (6 h to 12 h) during DDT exposure. Our data support the hypothesis that the MDR49B isoform, with three amino acid mutations, plays a role in the early aspects of DDT resistance in 91-R.
Similar content being viewed by others
Introduction
Post World War II, many agriculturally or medically important pests have been managed through the application of second-generation insecticides. This form of selection pressure has led to the evolution of pesticide resistance in many of the target insect populations1. Insecticide resistance is a valuable model for studying molecular evolution, as it is a well-defined system of man-made selection using known insecticides2,3. One such insecticide is the neurotoxic organochlorine pesticide 4,4′-dichlorodiphenyltrichloroethane (DDT), which affects the arthropod nervous system by interfering with normal nerve impulses. The insecticidal properties of DDT were discovered by Paul Müller in 1939 and due to its widespread use and effectiveness, DDT went from being a panacea for insect control to being banned for use in most countries in the 1970s. Nevertheless, the use of DDT for vector control continues and may increase as insect-borne diseases expand4,5.
Resistance to DDT has been documented across many pest species along with the non-target species, such as Drosophila melanogaster (Drosophila)3,5. DDT resistance in Drosophila has been used for the study of the evolution of insecticide resistance6. Historically, two major mechanisms of DDT resistance in Drosophila have been reported. First, resistance has been associated with amino acid changes in the voltage-gated sodium channel resulting in channel insensitivity to DDT7. Second, metabolic resistance to DDT has been observed in field populations of Drosophila8. Enhanced xenobiotic metabolism is an important form of resistance and is associated with alterations (or some cases structural changes) in the activities or levels of detoxification enzymes, such as cytochrome P450s, glutathione-S-transferases (GSTs), esterases or a combination of these activities8,9,10,11. Furthermore, proteomics-based profiling identified abundant proteins associated with DDT resistance in field- and laboratory-selected resistant Drosophila12.
The DDT resistance phenotype in Drosophila is not uniform, resulting in varying levels of resistance observed across different Drosophila strains, and resistance can be generally categorized into low, medium and high levels as measured by lethal concentration 50 (LC50)8,10,11. One pair of Drosophila strains, which are notably important for the study of the high level DDT resistance phenotype, are 91-R and its DDT susceptible counterpart 91-C. The two strains originated from a common population13,14,15. The laboratory selected DDT resistance strain 91-R has been exposed to prolonged and periodic artificial selection with DDT for 60 years and has thus become highly resistant to DDT whereas 91-C has not been exposed to DDT selection.
In the 91-R strain, constitutive over-expression of Cyp12d1, Cyp6a2 and Cyp6g1 have been observed8,16,17, however, a recent selective sweeps analysis between 91-R and 91-C demonstrated that thirteen major and three minor effect chromosome intervals, with reduced nucleotide diversity, were identified only in the 91-R strain18. Interestingly, of these thirteen major and three minor loci, the only cytochrome P450 observed was Cyp4g1, which is thought to be associated with the reduced curricular penetration phenotype19. Another gene, multidrug resistance 49 (MDR49) was located in one of the other major effect chromosome intervals in the selective sweeps analysis.
Multidrug resistance genes are known to code for ATP-binding Cassette (ABC) transporter proteins. ABC transporters are ATP-dependent efflux pumps belonging to extensive family of transmembrane proteins. The ABC protein family is present in the cellular membrane in all organisms and mediate the efflux of a wide variety of substrates, including sugars, amino acids, lipids, and xenobiotics to the outside of the cell and these transporters prevent the accumulation of harmful toxicants inside the cells20,21. ABC transporters are structurally characterized by four functional units: two highly conserved nucleotide-binding domains (NBDs), which are responsible for ATP-binding and hydrolysis, providing the energy for active transporting substrates across cellular membrane; and, two highly hydrophobic transmembrane domains (TMDs), which are involved in physical pathway for substrate translocation. Unlike the NBDs, TMDs vary in sequence, length and helix number22.
Based on their sequence similarity, domain structures, and organization, ABC transporters can be subdivided into eight subfamilies, designated ABC-A to ABC-H. Insect ABC transporters have diverse functions that affect molting, metabolism, cuticle differentiation, and egg development23,24,25. They are also thought to be associated with defense or resistance to plant defensive compounds and numerous insecticides by reducing toxic concentrations in tissues26. Unlike the other subfamilies, the ABC-B, ABC-C, and ABC-G have been associated with drug resistance and detoxification in insect pests26,27. For example, involvement of ABC transporters in pyrethroid resistance has been reported in Helicoverpa armigera28, Apis mellifera29 and Culex pipiens30.
ABC transporters have also been implicated in DDT resistance in Drosophila. The ABC-B subfamily multiple drug resistance (MDR) genes, MDR50, MDR65, as well as the ABC-C subfamily multidrug resistance-associated protein gene, MRP1, are constitutively overexpressed in the DDT-resistant 91-R strain when compared with the DDT-susceptible Canton-S strain. The ABC-B subfamily multiple drug resistance gene MDR49, however, was not overexpressed in DDT-resistant 91-R strain19,31,32. Additionally, RNAi knockdown of MDR50, MDR65, and MRP1, using transgenic GAL4/UAS-RNAi flies, in conjunction with DDT bioassays, confirmed the potential role of these genes in DDT resistance/susceptibility. Interestingly, MDR49, which was implicated as a putative resistance locus in the selective sweeps analysis, was not over transcribed in 91-R versus the Canton-S strain31,32. These combined observations lead us to test the hypothesis that MDR49 may play a role in DDT resistance through structural changes in the gene (and the resultant proteins) as opposed to increased levels of expression.
In the present study, we focused on the ABC-B subfamily genes, MDR49, MDR50, and MDR65. Using RNA-seq data, we compared open reading frames of all three genes and alternative splicing in MDR49. As MDR49 was the only MDR to be in a major effect chromosome interval in the selective sweeps analysis18, we also created transgenic Drosophila containing both alternative splice forms of MDR49, with the respective amino acid differences between 91-R and 91-C, to determine if structural differences in MDR49 proteins, between 91-R and 91-C, may play a role in DDT resistance.
Results
Sequence differences in MDR49, MDR50, and MDR65 between the 91-R and 91-C strains
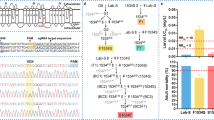
We compared the sequences of the MDR49, MDR50, and MDR65 genes between 91-R and 91-C to determine non-silent mutations within the ORFs that may lead to potential amino acid replacement. Using the GenBank database, two different transcript isoforms were observed from the MDR49 gene, designated as MDR49A and MDR49B. Although the sequences of both transcript isoforms were completely identical, the first four exons were alternatively spliced out of MDR49A, giving rise to MDR49B. Several SNPs were detected when 91-R was compared with 91-C. There were 39 SNPs observed when the MDR49A isoform from 91-R was compared with the MDR49A isoform from 91-C. Similarly, there were 37 SNPs observed when the MDR49B isoform from 91-R was compared with the MDR49B isoform from 91-C. Most SNPs resulted in silent mutations, however, three SNPs resulted in non-silent mutations in both the MDR49A and B genes from 91-R when compared with 91-C.
Thr374, Met388, and Glu666 in MDR49A protein from 91-C were replaced with Ile374, Leu388, and Asp666 from 91-R; Thr173, Met187, and Glu465 in MDR49B protein from 91-C was substitute with Ile173, Leu187, and Asp465 from 91-R (Fig. 1). MDR50 and MDR65 were also determined to have SNPs (4 and 17, respectively) when 91-R was compared with 91-C. These SNPs resulted in three non-silent mutations: Thr393, Ser881, and Val1012 in MDR50 protein from 91-C was replaced with Ala393, Phe881, and Leu1012 in 91-R (Supplementary Fig. S1); Lys277, Ile646, and Leu990 in MDR65 protein from 91-C was substituted with Arg277, Met646, and Pro990 in 91-R (Supplementary Fig. S2). Interestingly, a total of three non-silent mutations were detected in each of the three MDR genes from the 91-R strain and each mutation was novel, suggesting that these residue changes likely cause structural alternation in these three MDR proteins from the DDT-resistant strain.
Three mutations that resulted in amino acid replacements, T374I, M388L, and E666D for MDR49A; T173I, M187L, and E465D for MDR49B were observed in 91-R when compared with 91-C. The NBDs were homologous in both strains and each NBD had seven highly conserved motifs (aromatic, Walker A, Q-loop, ABC signature, Walker B, D-loop, and H-loop), which appear in boxes.
Prediction of structure for three MDR proteins
Using the NCBI CDS-Conserved-Domains-prediction-server, the structural features of each of the aforementioned MDR proteins were predicted, including the alternative splice forms for MDR49 (discussed further in the next section). The deduced amino acid sequences for each of the three MDR proteins had characteristic features of ABC transporters, including one cytoplasmic N-terminus nucleotide binding domain (NBD) and several conserved motifs (Walker A motif, Walker B motif, and C-motif) (Fig. 1 and Supplementary Fig. S1, S2). The two alternative splice forms of MDR49 showed atypical variations in their transmembrane domains (TMDs), which were different from each other. For the MDR49A isoform, two TMDs at the C-terminus, both consisting of six transmembrane α-helix segments per TMD were predicted (Fig. 2A). For MDR49B, however, two TMDs at the C-terminus, one consisting of three transmembrane α-helix segments and the other consisting of six transmembrane α-helix segments were predicted (Fig. 2B). Typically, ABC transporters have six predicted membrane-spanning α-helix segments per TMD but the number of segments may vary in each TMD33. The predicted proteins for both MDR50 and MDR65 displayed the more typical features of TMD structure, which consisted of six transmembrane α-helix segments per TMD, respectively (Fig. 3A,B).
(A) Twelve transmembrane segments in two transmembrane domains (TMD 1 and TMD 2) were predicted in the MDR49A isoform. (B) Nine transmembrane segments were predicted in two TMDs in the MDR49B isoform. Amino acid replacements that may result in structural alterations are indicated by red dots. Blue letters represent 91-C; red letters represent 91-R.
The location of the three amino acid replacements in each of the three MDR proteins due to non-silent mutations found in 91-R are given in Figs 2 and 3. T374I, M388L, and E666D for MDR49A; T173I, M187L, and E465D for MDR49B were predicted to be located on the intracellular loop between TMD 1 and TMD 2 for both MDR49A and MDR49B (Fig. 2A,B). For MDR50, T393A was located in the intracellular loop between TMD 1 and TMD 2 and S881F and V1012L were predicted to be in transmembrane segments 7 and 12, respectively (Fig. 3A). For MDR65, K277R was predicted to be in the intracellular loop between transmembrane segment 4 and 5 in TMD 1, I646M in the intracellular loop between TMD 1 and TMD 2, and L992P in the extracellular loop between transmembrane segment 11 and 12 in TMD 2 (Fig. 3B).
Identification of the alternatively spliced transcripts from MDR49, 50 and 65
Transcript discovery analysis was performed using RNA-seq data in order to determine whether alternative splicing occurred in these three MDR genes when 91-R and 91-C were compared. A total average of 157,625,457 raw reads for 91-R and of 150,788,606 raw reads for 91-C were obtained, respectively. The average of 3,186 reads for 91-R and of 4,081 reads for 91-C were mapped to each MDR gene, respectively (Table S2). Two transcript isoforms had previously been reported in GenBank for MDR49 (transcripts A and B, Fig. 4). Interestingly, the current analysis of alternative spliced transcript for MDR49 indicated an additional splice variant in the 91-R strain. Our analysis found average 145 reads, which matched the non-coding RNA region of MDR49 and allowed the prediction of an additional exon (transcript N, Fig. 4B). No reads were found matching to this region in 91-C (Fig. 4A). We confirmed the presence of three alternatively spliced transcripts in 91-R and two alternatively spliced transcripts in 91-C. However, the newly found transcript N in 91-R led to a premature termination of translation. In terms of expression levels between the MDR49A and MDR49B transcripts, both transcripts were expressed at the same ratio between the 91-R and 91-C strains (χ2 = 4.626, df = 2, p = 0.099) (Table S3).
(A) Two alternative splice variants (transcripts A and B) were predicted from 91-C-MDR49. (B) Three alternative splice variants (transcripts A, B and N) were predicted from 91-R-MDR49. Optional exon (transcript N) was indicated by blue box from 91-R-MDR49. Several reads uniquely matched to the non-coding RNA region. Black letters represent exonic region; red letters represent non-coding RNA region.
Unlike MDR49, only one transcript variant was found and no differences in alternative splicing were observed for either MDR50 or 65 when 91-R was compared with 91-C. All reads clearly matched to the nine exons in MDR50 (Supplementary Fig. S3) and to the twelve exons in MDR65 (Supplementary Fig. S4).
Effects of three mutations in MDR49A and B from 91-R on DDT resistance
As MDR49 was the only gene, of all the MDR genes in Drosophila, found to be associated with a major effect chromosome interval in the selective sweeps analysis18, four transgenic fly strains containing ORFs as follows: (1) MDR49A from 91-R (91-R-MDR49A); (2) MDR49B from 91-R (91-R-MDR49B); (3) MDR49A from 91-C (91-C-MDR49A); and (4) MDR49B from 91-C (91-C-MDR49B), were generated to investigate this finding in more detail.
The susceptibility of these transgenic strains following exposure to DDT is presented in Table 1. When mortality was evaluated at eight different time points (3, 6, 9, 12, 15, 18, 21, and 24 h post-treatment), the test evaluating the hypotheses of equality between the 91-R-MDR49A and 91-C-MDR49A determined that the regression lines were equal (Fig. 5). However, the test evaluating the hypotheses of equality between the 91-R-MDR49B and 91-C-MDR49B determined that regression lines were not equal (Fig. 6). Thus, the LD50 values for 91-R-MDR49B were significantly larger than that from the 91-C-MDR49B after 6, 9, and 12 h following DDT treatment and yielded RR of 3.2, 3.0, and 2.1, respectively (Table 1). There were no significant differences in LD50 values between two transgenic strains, however, after 12 h of DDT treatment and the test evaluating the hypotheses of equality between 91-R-MDR49B and 91-C-MDR49B from 15 to 24 h of DDT treatment determined that the regression lines were equal (Fig. 6). For 3 h post-treatment, precise estimation of the LD50 values for the both 91-R-MDR49B and 91-C-MDR49B were not feasible because its mortality, even at the highest dose examined (50 mg/vial), was still >50%.
Log dose versus percent mortality response curves were determined at different time interval from 6 to 24 h following contact exposure to DDT (μg/vial), respectively. Asterisks indicate statistically significant differences in the dose responses when the two transgenic strains were compared at 6, 9, and 12 h after DDT treatment, as determined by the maximum-log likelihood ratio test (p < 0.05).
Our results support the hypotheses that the MDR49A isoform may not be involved in the DDT resistance phenotype, however, the MDR49B isoform may play a temporal role in DDT resistance at the early time points of DDT exposure. Furthermore, this finding implies that amino acid alternations in the protein coded for by the 91-R allele of the MDR49 gene may be associated with potential resistance mechanisms, including regulating substrate binding affinity at the TMDs or with ATPase activity of the NBDs.
Discussion
To the authors’ knowledge, the present study represents the first paper demonstrating the combination of amino acid replacements and splice form variants of a Drosophila ABC transporter in DDT resistance. Our data demonstrate that one of the two alternative splice forms, MDR49B, contributes to DDT resistance, however, the other isoform MDR49A does not, in spite of the fact that the resultant respective proteins both contain the same amino acid replacements. Transgenic over-transcription of either MDR49A or MDR49B obtained from 91-C did not result in changes in resistance levels to DDT, suggesting that over-transcription of MDR49 does not represent a mechanism by which MDR49 plays a role in resistance. These results are in line with previous work by Gellatly and coworkers, which demonstrated a direct relationship between expression levels of MDR50, MDR65 and MRP1 and DDT resistance whereas MDR49 was not over-transcribed in 91-R31.
An additional observation from our RNA-seq data was the occurrence of a third alternative splice variant of MDR49 from 91-R. Comparing the additional transcript sequence of MDR49 with the Drosophila genome revealed that the non-coding RNA region generated an optional exon region as a result of alternative splicing with premature stop codon. Thus, this third alternative splice form is unlikely to play any role in the resistance phenotype. Nevertheless, it would be interesting to determine the potential impact of such intense selection pressure by an insecticide upon the prevalence of such “extra” alternative splice forms across the insect genome. One could speculate that the 91-R strain, under intense DDT selection pressure, acquired an additional transcript, including this optional exon from a non-coding RNA region. Additional research is necessary, however, to confirm this hypothesis. However, the phenomenon of an extra alternative splice form originating from intronic sequence is not without precedent, as previous studies have demonstrated that alternative exons with high homology probably originated from exons that were previously constitutively spliced in order to maintain the ancestral transcript as a major form, whereas alternative exons with low homology probably originated from exonization of intronic sequences34.
Nonetheless, previous studies characterized how alternative splicing of a voltage-gated sodium channel in mosquito contributed to insensitivity to pyrethroids, and feasibly indicated a role of splice variants in the pyrethroid resistance phenotype35. The current results from the present study imply that MDR49 may be associated with DDT resistance through a mechanism involving both alternative splicing and amino acid replacements in the protein.
As MDR49 was the only MDR gene previously shown be associated with a major effect chromosome interval in a selective sweep analysis18, we tested the hypothesis that this gene was playing a direct role in DDT resistance through the use of DDT bioassays of transgenic Drosophila expressing the MDR49 isoforms from 91-R and 91-C. Our results suggest that only the MDR49B transcript from 91-R is involved in DDT resistance and importantly, significant mortality differences were observed only after DDT treatment for 6 h to 12 h intervals while no significant difference was observed after 15–24 h DDT exposure. Thus, the data from this study revealed that structural alteration of the MDR49 gene provides partial protection from DDT toxicity and suggests that MDR49B could play a role during the initial phase of the detoxification process after DDT exposure. Interestingly, MDR proteins are known to translocate exogenous substrates to the outside of the cell using energy obtained from ATP hydrolysis by the ATPase activity associated with the NBDs36. In human, two mutations in NBDs disrupt the catalytic activity, causing reduced ATP binding and hydrolysis37. Thus, amino acid replacements near NBDs of MDR49B in 91-R could be directly involved in interrupting the transportation of exogenous substrates like DDT. Furthermore, the role of TMD is to recognize and mediate the passage of substrates across cell membrane. The TMD conformational alteration within MDR49B in 91-R could enhance the large diversity of substrate specificity and mediate substrate transport.
Previously, Pedra et al. (2004) reported that the relative transcript expression of ABC transporter-like gene (CG9892) in the DDT-resistant 91-R strain was greater than in the DDT-susceptible Canton-S strain using whole genome transcript profiles, suggesting a possible association between DDT resistance and ABC transporters8. Recently, Gellatly et al. (2015) also showed that the ABC-B subgroup (MDR50, MDR65) and the ABC-C subgroup (MRP1) were over-transcribed in 91-R when compared to Canton-S and using a UAS/RNAi approach showed that knockdown of these genes increased DDT susceptibility in the transgenic flies31. Furthermore, the association of over transcription of ABC transporters with insecticide resistance has been confirmed in Lygus Hesperus38, Cimex lectularius39, and Myzus persicae40. Nevertheless, our current findings would suggest that 91-R strains may use additional mechanisms allowing for DDT resistance besides over expression of ABC transporters. Indeed, the over expression of ABC transporters associated with resistance to insecticides at different time points has been previously reported in Anopheles stephensi41. The relative expression of an ABC-B subfamily gene was highly upregulated at early time points following permethrin treatment and ABC-G4 was over transcribed at later time points. Also, Atsumi et al. (2012) demonstrated that a single amino acid replacement in the second extracellular loop in an ABC transporter gene causes resistance to the Bt toxin Cry1Ab in the Bombyx mori42. To date, however, ABC transporter mutations associated with resistance to insecticides have not been reported in Drosophila.
In order to elucidate the actual effects of individual amino acid replacements on ABC transporter activity, a logical next step will be to focus on the heterologous expression of MDR49 using cRNAs, containing these three mutation alone and in all combinations, injected into Xenopus laevis oocytes to improve our mechanistic understanding of this novel phase III xenobiotic metabolism reaction involved in DDT resistance in Drosophila.
Methods
Drosophila melanogaster strains
DDT-resistant 91-R and DDT-susceptible 91-C strains were obtained from Dr. Ranjan Ganguly (University of Tennessee-Knoxville). Strains were reared on brown diet (Jazz-Mix Drosophila Food, Fischer Scientific, Cat. No. AS153) at 25 °C with 8:16 L:D in plastic bottles and transferred to new bottles about every three weeks. Populations of the 91-R and 91-C strains have been maintained in the Pittendrigh laboratory for over a dozen years and 91-R strain has been under continuous selection at DDT concentrations of 100 mg/ml. For the transgenic lines w1118 was used.
Structure analysis of MDR proteins
Sequence similarity and analysis of protein-specific motifs were performed using BLAST programs on NCBI. Sequence alignments were performed with Clustal Omega (EMBL-European Bioinformatics Institute, Cambridge, UK). The transmembrane domain and membrane topology was predicted with TOPCONS online software (http://topcons.cbr.su.se/)43.
Identification of alternatively spliced transcripts
All RNA-seq databases from 91-R and 91-C were imported to the CLC genomic workbench 8.5 software according to the manufacture’s manual (Qiagen, Valencia, CA, USA). The “Transcript Discovery” plug-in was used to predict alternative splicing transcripts and genes. Our trimmed reads of each sample with three replications were mapped against MDR49, MDR50, and MDR65 Drosophila genome sequences extracted from Drosophila genome assembly release 6.07 (file dmel-all-chromosome-r6.07.fasta downloaded from Flybase.org) including intergenic regions. The BAM files with mapped reads were deposited to NCBI Short Read Archive (SRA) with the accession number SRP068789. The matched reads for each transcript were visualized in the CLC interface. Each generated transcript was manually examined by comparing RNA-seq reads with the Drosophila genomic sequence to identify alternatively spliced variants.
Cloning of MDR49 from 91-R and 91-C strains for transgenic expression
Two MDR49 splice isoforms were cloned and sequenced using total RNA from each of the two strains (91-R and 91-C). First-strand cDNA was synthesized by using Superscript™ III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) primed with oligo-d(T). Internal cDNA fragments of the MDR49A and MDR49B genes were amplified from the first-strand cDNA with a set of gene-specific primers for each strain. The amplified cDNA-specific products were purified using a PCR clean-up kit (Qiagen, CA) and directly sequenced using the gene-specific primers for both ends to cover the full length. All sequences were assembled and compared by using Vector NTI (Invitrogen, CA).
Transgenic expression of the two MDR49 splice isoforms in Drosophila
The full-length MDR49A and MDR49B splice isoforms were amplified from the cDNA of both 91-R and 91-C strains using Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA). The sequence-specific primer pairs were used (Table S1). The PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, CA) and cloned into the pCR2.1 TOPO vector (Invitrogen, CA). Individual clone from both strains were purified with a QIAprep Miniprep kit (Qiagen, CA) and sequenced to locate the open reading frame and validate the correct amino acid sequences for MDR49A and MDR49B. After sequence analysis, the selected clones for both MDR49 transcripts were sub cloned into the pUAST vector. Transgenic flies were generated by the BestGene Inc (Chino Hills, CA) using the w1118 strain. For the expression of the transgene MDR49A and B, ubiquitous driver strain (P{w[+mC] = GAL4-elav.L}2/CyO) was obtained from Bloomington Drosophila Stock Center (Bloomington, IL). This female ubiquitous driver strain was crossed with our male transgenic strains and selected F1 progeny that showed both GAL4-MDR49A and B by examining wing shape and eye color for mortality bioassay.
Mortality bioassays
We used a previously described bioassay approach with some modifications31. To determine diagnostic doses of DDT, flies were exposed to vials coated a series of concentration of DDT, and mortality was determined after the flies were exposed for 3, 6, 9, 12, 15, 18, 21, and 24 h in the vials. Flies were considered dead when all movement and leg twitching had ceased. The median lethal dose (LD50) values and their 95% confidential limits (CLs) were determined by Probit analysis (POLOPC, LeOra Software, Berkeley, CA). Test for the hypotheses of equality (slopes and intercepts are not significantly different) was performed as described by Robertson et al. (2007)44. The maximum-log likelihood test was used to determine whether the resulting mortality curves from differently treated fly groups were statistically different (p < 0.05).
Additional Information
How to cite this article: Seong, K. M. et al. Splice form variant and amino acid changes in MDR49 confers DDT resistance in transgenic Drosophila. Sci. Rep. 6, 23355; doi: 10.1038/srep23355 (2016).
References
Gassmann, A. J., Onstad, D. W. & Pittendrigh, B. R. Evolutionary analysis of herbivorous insects in natural and agricultural environments. Pest Manag. Sci. 65, 1174–1181 (2009).
Li, X., Schuler, M. A. & Berenbaum, M. R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253 (2007).
Barnola, F. V., Camejo, G. & Villegas, R. Ionic channels and nerve membrane lipoproteins: DDT-nerve membrane interaction. Int. J. Neurosci. 1, 309–316 (1971).
Edman, J. D. Emerging vectorborne diseases and their control in New discoveries in agrochemicals, ACS symposium series 892, ACS Books (eds Clark, J. M. et al. ) 314–325 (Washington, D.C., 2004).
Hemingway, J. Vector biology diagnostics and public health pesticide development through the product development partnership route in Advances in vector human vector control ACS symposium series 1014, ACS Books (eds Clark, J. M. et al. ) 1–12 (Washington, D.C., 2009).
Busvine, J. R. DDT-resistance in Drosophila melanogaster . Bull. World Health Organ. 16, 206–208 (1957).
Pittendrigh, B. R., Reenan, R., ffrench-Constant, R. H. & Ganetzky, B. Point mutations in the Drosophila sodium channel gene para associated with resistance to DDT and pyrethroid insecticides. Mol. Gen. Genet. 256, 602–610 (1997).
Pedra, J. H., McIntyre, L. M., Scharf, M. E. & Pittendrigh, B. R. Genome-wide transcription profile of field- and laboratory-selected dichlorodiphenyltrichloroethane (DDT)-resistant Drosophila . Proc. Natl. Acad. Sci. USA 101, E7034–7039 (2004).
Amichot, M. et al. Point mutations associated with insecticide resistance in the Drosophila cytochrome P450 Cyp6a2 enable DDT metabolism. Eur. J. Biochem. 271, 1250–1257 (2004).
Le Goff, G. et al. Microarray analysis of cytochrome P450 mediated insecticide resistance in Drosophila . Insect Biochem. Mol. Biol. 33, 701–708 (2003).
Festucci-Buselli, R. A. et al. Expression of Cyp6g1 and Cyp12d1 in DDT resistant and susceptible strains of Drosophila melanogaster . Insect Mol. Biol. 14, 69–77 (2005).
Pedra, J. H. et al. Profiling of abundant proteins associated with dichlorodiphenyltrichloroethane resistance in Drosophila melanogaster . Proteomics 5, 258–269 (2005).
Merrell, D. J. & Underhill, J. C. Selection for DDT resistance in inbred, laboratory, and wild stocks of Drosophila melanogaster . J. Econ. Entomol. 49, 300–306 (1956).
Merrell, D. J. Heterosis in DDT resistant and susceptible populations of Drosophila melanogaster . Genetics 45, 573–581 (1960).
Merrell, D. J. Lethal frequency and allelism in DDT resistant populations and their controls. Am. Nat. 99, 411–417 (1965).
Giraudo, M., Unnithan, G. C., Le Goff, G. & Feyereisen, R. Regulation of cytochrome P450 expression in Drosophila: Genomic insights. Pestic. Biochem. Physiol. 97, 115–122 (2010).
Qiu, X. et al. Genome-wide analysis of genes associated with moderate and high DDT resistance in Drosophila melanogaster . Pest. Manag. Sci. 69, 930–937 (2013).
Steele, L. D. et al. Selective sweep analysis in the genomes of the 91-R and 91-C Drosophila melanogaster strains reveals few of the ‘usual suspects’ in dichlorodiphenyltrichloroethane (DDT) resistance. PLoS One 10, e0123066 (2015).
Strycharz, J. P. et al. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pestic. Biochem. Physiol. 107, 207–217 (2013).
Dassa, E. & Bouige, P. The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152, 211–229 (2001).
Dean, M., Hamon, Y. & Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017 (2001).
Linton, K. J. Structure and function of ABC transporters. Physiology 22, 122–130 (2007).
Dow, J. A. & Davies, S. A. The Malpighian tubule: rapid insights from post-genomic biology. J. Insect Physiol. 52, 365–378 (2006).
Vache, C. et al. A potential genomic biomarker for the detection of polycyclic aromatic hydrocarbon pollutants: multidrug resistance gene 49 in Drosophila melanogaster . Environ. Toxicol. Chem. 26, 1418–1424 (2007).
Broehan, G., Kroeger, T., Lorenzen, M. & Merzendorfer, H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum . BMC Genomics 14, 6 (2013).
Dermauw, W. & Van Leeuwen, T. The ABC gene family in arthropods: comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 45, 89–110 (2014).
Buss, D. S. & Callaghan, A. Interaction of pesticides with p-glycoprotein and other ABC proteins: A survey of the possible importance to insecticide, herbicide and fungicide resistance. Pestic. Biochem. Physiol. 90, 141–153 (2008).
Srinivas, R., Shamsundar, G. S., Jayalakshmi, S. K. & Sreeramulu, K. Effect of insecticides and inhibitors on P-glycoprotein ATPase (M-type) activity of resistant pest Helicoverpa armigera . Curr. Sci. 88, 1449–1452 (2005).
Hawthorne, D. J. & Dively, G. P. Killing them with kindness? In-hive medications may inhibit xenobiotic efflux transporters and endanger honey bees. PLoS One 6, e26796 (2011).
Buss, D. S., McCaffery, A. R. & Callaghan, A. Evidence for p-glycoprotein modification of insecticide toxicity in mosquitoes of the Culex pipiens complex. Med. Vet. Entomol. 16, 218–222 (2002).
Gellatly, K. J. et al. RNAi validation of resistance genes and their interactions in the highly DDT-resistant 91-R strain of Drosophila melanogaster . Pestic. Biochem. Physiol. 121, 107–115 (2015).
Strycharz, J. P., Lee, S. H., Sun, W., Pittendrigh, B. R. & Clark, J. M. RNAi knockdown of ABC transporters causes decreased tolerance in highly DDT-resistant 91-R strain of Drosophila melanogaster in Picogram: Abstract Book. ACS/AGRO 78, 116 (2010).
Biemans-Oldehinkel, E., Doeven, M. K. & Poolman, B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 580, 1023–1035 (2006).
Kim, E., Goren, A. & Ast, G. Alternative splicing: current perspectives. BioEssays 30, 38–47 (2008).
He, L., Li, T., Zhang, L. & Liu, N. Multiple sodium channel variants in the mosquito Culex quinquefasciatus . Int. J. Biol. Sci. 8, 1291–1309 (2012).
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992).
Frelet, A. & Klein, M. Insight in eukaryotic ABC transporter function by mutation analysis. FEBS lett. 580, 1064–1084 (2006).
Hull, J. J. et al. Transcriptome-based identification of ABC transporters in the western tarnished plant bug Lygus hesperus . PLoS One 9, e113046 (2014).
Zhu, F. et al. Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci. Rep. 3, 1456 (2013).
Silva, A. X., Jander, G., Samaniego, H., Ramsey, J. S. & Figueroa, C. C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS One 7, e36366 (2012).
Epis, S. et al. Temporal dynamics of the ABC transporter response to insecticide treatment: insights from the malaria vector Anopheles stephensi . Sci. Rep. 4, 7435 (2014).
Atsumi, S. et al. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 109, E1591–1598 (2012).
Bernsel, A., Viklund, H., Hennerdal, A. & Elofsson, A. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 37, W465–468 (2009).
Bruck, D. Bioassays with arthropods: 2nd Edition. J. Econ. Entomol. 102, 466–466 (2009).
Author information
Authors and Affiliations
Contributions
K.M.S. designed the experiments, analyzed the data and wrote the manuscript. W.S. performed experiments and data analysis. B.R.P. supervised the research, participated in the design of the experiments, interpretation of the results, and in the writing and editing of the manuscript. J.M.C. revised and discussed the results and commented on the manuscript. All authors contributed to the discussion and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Seong, K., Sun, W., Clark, J. et al. Splice form variant and amino acid changes in MDR49 confers DDT resistance in transgenic Drosophila. Sci Rep 6, 23355 (2016). https://doi.org/10.1038/srep23355
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23355
- Springer Nature Limited
This article is cited by
-
Post-transcriptional modulation of cytochrome P450s, Cyp6g1 and Cyp6g2, by miR-310s cluster is associated with DDT-resistant Drosophila melanogaster strain 91-R
Scientific Reports (2020)
-
Seven-Membered Lactam Derivatives of Podophyllotoxins as New Pesticidal Agents
Scientific Reports (2017)
-
Design, Synthesis and Evaluation of Novel Isoxazolines/Oxime Sulfonates of 2′(2′,6′)-(Di)Chloropodophyllotoxins as Insecticidal Agents
Scientific Reports (2016)