Abstract
Rheumatoid arthritis (RA) is an autoimmune disease and a member of human heat shock protein (HSP) 70 protein family, Binding Immunoglobulin Protein (BiP), has been identified as an important autoantigen for T and B cells. We herein focused on Mycobacterial (Myc) HSPs and immune responses to MycHSPs in RA patients. Serum titers of antibodies against MycHSP70 were significantly elevated in RA patients and correlated with serum anti-BiP antibody titers. A MycHSP70-derived HLA-DR4 major epitope was identified using the proliferative capacity of RA PBMCs as an indicator. The major epitope, MycHSP70287–306, was located at the corresponding position in the major epitope for human BiP336–355 and a strong correlation was found between the proliferation of PBMCs in response to MycHSP70287–306 and BiP336–355. The immunization of HLA-DR4 transgenic mice with MycHSP70 induced the proliferation of T cells and development of anti-BiP antibodies. In contrast, the oral administration of MycHSP70287–306 resulted in the amelioration of collagen-induced arthritis, serum antibody responses and T cell proliferation. In conclusion, immune responses to MycHSP70 were associated with adaptive immunity against BiP in RA and could be an important mechanism underlying the development of autoimmunity.
Similar content being viewed by others
Introduction
Genetic and environmental factors are causative elements in the development of rheumatoid arthritis (RA). The microbiota is an environmental factor that may contribute to uncontrolled immune responses to self-antigens1. Several autoantigens have been reported for RA. Immune responses to citrullinated antigens, such as anti-citrullinated peptide antibodies (ACPAs), have been suggested to play pivotal roles in the pathogenesis of RA. Nevertheless, the precise mechanisms responsible for the breakdown of self-tolerance have not yet been elucidated in detail.
Molecular mimicry is one hypothesis that has been proposed for the development of autoimmunity2. The amino acid sequences of some proteins that are necessary for cell homeostasis have been evolutionarily preserved. Immune responses to such bacterial antigens may cross-react and induce immune responses to the corresponding autoantigens. For example, enolase from Porphyromonas gingivalis is similar to human alpha-enolase and induces autoimmunity to mammalian alpha-enolase3. Vinculin is a membrane-cytoskeletal protein in focal adhesion plaques. van Heemst et al. recently reported that citrullinated vinculin is a novel autoantigen for ACPA antibodies4. Autoreactive T cells that specifically recognize a DERAA-containing vinculin epitope cross-react with DERAA sequences derived from various pathogens.
The heat shock protein (HSP) family is evolutionarily preserved from prokaryotes to mammals. HSPs are molecular chaperones and are required for cell homeostasis. Autoimmune responses to some HSPs, including Mycobacterial (Myc) HSP65 and Binding Immunoglobulin protein (BiP), a member of the HSP70 family, have been reported in RA and the induction of tolerance to these HSPs has been investigated as a new therapeutic approach against this disease5,6. We have shown B cell responses to citrullinated BiP in RA and identified effector and regulatory BiP epitopes for T cells7,8. Previous studies reported the regulatory effects of MycHSP70 via the production of IL-10 and MycHSP70-derived peptide-specific regulatory T cells in mouse models of arthritis9,10. Other studies have established several MycHSP70-specific T cell clones with proliferative capacities and IFN-γ production potentials11. Therefore, the precise features of MycHSP70-specific T cells in RA remain unclear. The results of the present study revealed a close relationship between immune responses to MycHSP70 and human BiP in RA patients, which could support the importance of Myc and human HSPs in RA immunity.
Results
Serum anti-bacterial and human HSP antibodies in RA
Serum antibody titers for human and the corresponding bacterial HSPs were measured in RA patients and healthy donors (HDs) (Fig. 1). Cardiovascular disease patients were excluded because of the presence of serum anti-human HSP70 antibodies in these patients12. Anti-human BiP antibody titers were significantly higher in RA patients than in HDs (Fig. 1A), whereas serum anti-human HSP60 antibody titers were similar (Fig. 1B). Anti-MycHSP70 antibody titers were also increased in RA sera (Fig. 1A), whereas anti-MycHSP65 antibody titers were not (Fig. 1B). The results obtained for anti-human HSP60 and anti-MycHSP65 antibodies were consistent with previous findings13,14. Anti-human HSP40 antibody titers were significantly higher in RA patients than in HDs (Fig. 1C), whereas no significant difference was observed in serum anti-human Cpn10 antibody titers (Fig. 1D). As a model of microbial mucosal exposure, we selected E. coli HSPs as a control. Although sequence similarity between MycHSPs and E.coli HSPs was up to 60%, no significant differences were observed in antibody titers against E. coli HSPs between RA patients and HDs (Fig. 1). We then found a correlation between anti-human HSP antibody titers and anti-MycHSP antibody titers (Fig. 2A,B). Anti-MycHSP70 antibody titers and anti-citrullinated BiP antibody titers, in particular, showed a clear positive correlation (Fig. 2A). Anti-MycHSP70 antibody titers were significantly increased in RA patients and were associated with anti-human BiP and citrullinated BiP antibody titers.
Serum IgG antibody titers to bacterial and human HSPs in RA.
(A–D) Serum antibody titers to Mycobacterium (Myc) and human HSPs in RA patients and healthy donors (HDs). (A) Antibodies to bacterial HSP70 (DNAK) and human BiP. (B) Antibodies to bacterial HSP65 (GroEL) and human HSP60. (C) Antibodies to bacterial HSP40 (DNAJ) and human HSP40. (D) Antibodies to bacterial HSP10 (GroES) and human HSP10 (Cpn10). Horizontal line: median. *p < 0.05, **p < 0.01, ***p < 0.001.
MycHSP70-derived HLA-DR4 epitopes in RA patients
In order to detect HLA-DR4-restricted, RA-associated MycHSP70 epitopes, we screened 43 MycHSP70-derived peptides using PBMCs from shared epitope (SE)-positive RA patients (Supplementary Figure S1). Similar to BiP-derived epitopes8, at least five peptides induced the proliferation of PBMCs (mean stimulation index >2.5) and, thus, were considered to be candidate peptides for HLA-DR4 epitopes. Of these, MycHSP70287–306 (DRTRKPFQSVIADTGISVSE) provoked the highest proliferative responses, which were dependent on the dose of SE in RA patients (Fig. 3A). MycHSP70287–306–induced proliferation was not observed in HLA-DR4-positive HD PBMCs (Fig. 3A). MycHSP70 and BiP shared 50% amino acid sequence similarity and MycHSP70287–306 located at the corresponding position of the major epitope for human BiP, BiP336–355 (RSTMKPVQKVLEDSDLKKSD), which we previously identified as a RA-related HLA-DR4 effector epitope (Fig. 3B)8. PBMC proliferation to MycHSP70287–306 was significantly dependent on the SE dose and this dependency was similar to our previous findings for BiP336–3558. MycHSP70287–306–induced proliferation correlated with BiP336–355-induced proliferation in RA (Fig. 3C). These proliferation responses to MycHSP70287–306 peptides were clearly inhibited by the blockade of HLA-DR (Fig. 3D). Regarding the production of cytokines, the secretion of interferon (IFN)-γ and interleukin (IL)-17 was induced by MycHSP70287–306 peptides and was also blocked by the anti-HLA-DR antibody (Fig. 3E,F).
MycHSP70287–306 was a HLA-DR4-restricted epitope in RA.
(A) The proliferation of PBMCs in RA patients (Shared epitope (SE) +/+ n = 12, SE +/− n = 22, SE−/− n = 9) and HDs (SE +/− n = 10) in response to MycHSP70287–306. Proliferation was determined by the incorporation of 3-H thymidine. The stimulation index (SI) was calculated using the following equation: (median thymidine uptake to an antigen)/(median thymidine uptake in the absence of an antigen). Horizontal line: the median. (B) Amino acid sequences of MycHSP70 (lower lines) and human BiP (upper lines) near the epitopes. Epitope sequences are indicated in bold letters. (C) Relationship between MycHSP70287–306-induced proliferation and BiP336–355-induced proliferation in RA PBMCs (9 patients with SE +/+ and 14 patients with SE +/−). (D) Blockade of the MycHSP287–306-induced proliferation of PBMCs from HLA-DR4-positive RA patients (n = 6) using an anti-HLA-DR antibody. (E) The secretion of MycHSP287–306-induced IFN-γ from the PBMCs of HLA-DR4-positive HDs (n = 6) and RA patients (n = 8). (F) Blockade of MycHSP287–306-induced cytokine secretion from the PBMCs of HLA-DR4-positive RA patients (n = 6) using an anti-HLA-DR antibody. *p < 0.05, **p < 0.01, ***p < 0.001.
A binding assay showed that MycHSP70287–306 bound to the HLA-DRB1*0401 and 0405 molecule (Fig. 4A). Alanine scanning of this peptide showed that the substitution of F293 (P1), V296 (P4) and D299 (P7) significantly decreased its binding ability (Fig. 4B). These results demonstrated that MycHSP70287–306 is an immunogenic HLA-DR4 epitope in RA patients.
HLA-DR4 binding assay.
(A) Binding activities between a HLA-DRB1*0401 and 0405 molecule and MycHSP70287–306. Binding activities were estimated by the inhibition of peroxidase-labeled HA307–318 peptide binding to a HLA-DRB1 molecule. (B) Binding of MycHSP70287–306 with alanine-substituted peptides to HLA-DRB1*0405 molecules. A MycHSP70287–306-derived peptide in which an amino residue was substituted by an alanine residue. Inhibitory concentration 50% (IC50) was used as a measure of binding activity. *p < 0.05, **p < 0.01, ***p < 0.001.
Immunization of MycHSP70 broke self-tolerance in mice
We next addressed adaptive immunity induced by MycHSP70287–306 in vivo. The immunization of HLA-DR4 transgenic mice with the MycHSP70 protein led to an elevation in serum antibody titers to MycHSP70 and BiP (Fig. 5A). Furthermore, significant CD4+ T cell proliferation was induced in response to the MycHSP70287–306 peptide, providing additional evidence that the MycHSP70287–306 peptide was an HLA-DR4 epitope (Fig. 5B). The significant proliferation of CD4+ T cells in response to the BiP336–355 peptide was also observed (Fig. 5B). These results indicate that immune responses to MycHSP70 induce the loss of self-tolerance to BiP.
Immunization of HLA-DR4 transgenic mice with MycHSP70.
(A) HLA-DR4 transgenic mice were immunized with recombinant MycHSP70. Serum anti-MycHSP70 and anti-mouse BiP antibody titers were measured in the 21 days following the first immunization. (B) Splenic CD4+ T cell proliferation from MycHSP70-immunized HLA-DR4 transgenic mice in response to MycHSP70287–306 and BiP336–355 epitopes. Proliferation was determined by the incorporation of 3-H thymidine. Data were representative of at least three independent trials. ***p < 0.001.
Oral administration of the MycHSP70 epitope ameliorated collagen-induced arthritis
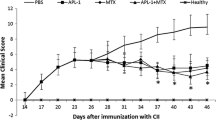
We also examined the therapeutic effects of the oral administration of the MycHSP70287–306 peptide on collagen-induced arthritis (CIA) in DBA/1 J mice. The oral administration of the MycHSP70287–306 peptide significantly improved the development of arthritis and histological scores in CIA (Fig. 6A,B). The proliferation of CD4+ T cells in response to the MycHSP70287–306 and BiP336–355 peptides was reduced by the orally administered MycHSP70287–306 peptide (Fig. 6C). The oral administration of the MycHSP70287–306 peptide also suppressed bovine type II collagen-specific T cell proliferation (Fig. 6C) and pro-inflammatory cytokine secretion from CD4+ T cells (Supplementary Figure S2). Serum anti-BiP and anti-BC II antibody titers were significantly decreased in MycHSP70287–306-treated mice (Fig. 6D). Furthermore, the secretion of IL-10 and TGF-β1 from CD4+ T cells was induced by MycHSP70287–306 and BiP336–355 recall stimulations in MycHSP70287–306-treated mice (Fig. 6E,F). The amount of cytokines released in response to HSP70 epitopes was previously reported to be lower than that from orally tolerized TCR-transgenic CD4+ T cells15,16. This finding suggests that the frequencies of MycHSP70287–306 and BiP336–355-specific T cells were limited in this model. The frequency of CD4+ CD25+ Foxp3+ regulatory T cells was increased by the oral administration of the MycHSP70287–306 peptide to CIA mice (Supplementary Figure S2). These results indicate that the induction of tolerance to MycHSP70 ameliorated inflammatory arthritis and tolerance to autoantigens, including BiP.
Oral tolerance of MycHSP70287–306 peptides in a collagen-induced arthritis model.
(A,B) The MycHSP70287–306 peptide or control (PBS alone) was orally administered to bovine type II collagen-immunized DBA/1 J mice after the occurrence of CIA for 5 continuous days (n = 12). Mice were sacrificed on day 35. Arthritis scores were measured by finger and paw swelling and histological scores were measured by cell infiltration (C), pannus formation (P) and bone destruction (B). (C) Splenic CD4+ T cell proliferation from CIA-induced DBA/1 J mice in response to a recall antigen (Ag) stimulation (MycHSP70287–306 and BiP336–355 epitopes and heat-denatured bovine type II collagen (BC II)). Proliferation was determined by3-H thymidine incorporation. (D) Serum anti-BiP and anti-BC II antibody titers were measured by ELISA. (E,F) Mouse IL-10 and TGF-beta1 concentrations in the splenic CD4+ T cell culture were measured by ELISA. Data were representative of at least three independent trials. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Environmental factors account for half of the causes of seropositive RA17. With the exception of a smoking habit, most environmental risks remain unknown because difficulties are associated with following environmental exposure from birth to the age of arthritis onset. Since a T cell clone specific to the Mycobacterial-derived epitope, MycHSP65180–188, has arthritogenic capacity, Mycobacterial exposure was presented as a potential candidate environmental risk factor for RA6. RA has been shown to increase the risk of Mycobacterial infection independent of immunosuppressive therapies18. Nevertheless, evidence for the relationship between Mycobacterial infection with the pathogenesis and exacerbation of RA is limited. We herein demonstrated that T and B cell responses to MycHSP70 were significantly enhanced in RA, which suggests Mycobacterial exposure in RA patients. Moreover, PBMC proliferative responses to MycHSP70287–306 and BiP336–355 were closely associated. T cell repertoire analysis demonstrated the clonal expansion of T cells in RA and the increase observed in proliferation against these epitopes could be partially explained by RA PBMCs containing more antigen-experienced clones19. Moreover, we previously reported that BiP336–355-induced PBMC proliferation correlated with serum anti-BiP antibody titers. The immunological basis for this correlation may be important for understanding the pathogenesis of RA.
MycHSP70 and BiP share 50% of their amino acid sequences and MycHSP70287–306 is located at a position corresponding to that of the BiP-derived HLA-DR4 effector epitope. We previously identified two major BiP-derived HLA-DR4 epitopes in RA. One (BiP336–355) was recognized by effector T cells and the other (BiP456–475) by IL-10-secreting regulatory T cells8. MycHSP70287–306 strongly induces proliferation and is regarded as an effector epitope. MycHSP70287–306 and BiP336–355 share 50% of the amino acids in their core sequences, which bind HLA-DR pockets. They also share the P4V residue, which is regarded as the most important amino acid for HLA-DR4 binding20. This sequence similarity could contribute to the breakdown of T cell tolerance to self BiP in RA and also suggests the role of molecular mimicry; however, we were unable to exclude the contribution of epitope spreading. One possibility is that the same clone recognizes MycHSP70287–306 and BiP336–355. A vinculin-specific TCR has been shown to recognize DERAA-containing epitopes from three bacterial species, Campylobacter coli, Lactobacillus curvatus, and L. sakei4. A second possibility is epitope spreading from the Myc antigen to a self-antigen that shares similarities via recognition by different T cell clones. Epitope spreading is described as the diversification of T cell activation from one epitope to non-cross-reactive autoantigen-derived epitopes and is induced during chronic inflammation. T cell epitope spreading has mainly been examined in mouse models of multiple sclerosis21,22. The findings obtained suggest that intermolecular and intramolecular epitope spreading occurs as a result of inflammation and tissue damage. According to this hypothesis, inflammation induced by MycHSP70-specific T cells could up-regulate the expression of BiP and BiP-specific T cell activation. These possibilities warrant further study.
Mycobacterial species, particularly non-tuberculous mycobacteria, live in the soil and water and a large number of individuals are continuously exposed to several mycobacteria in daily life23. Most of these bacteria are considered to be non-pathogenic for humans. However, difficulties are associated with detecting and identifying these bacteria due to their slow growth rates. Next generation sequencing of 16S rRNA in microbiota has provided new evidence for the “silent” exposure of humans to Mycobacterial species24. The clear correlation between serum anti-MycHSP70 antibody titers and anti-citrullinated BiP antibody titers indicates that a relationship exists between immune responses to mycobacteria with ACPA formation. Mycobacteria in the lungs have been shown to play a role in the pathogenesis of RA because the lungs are a candidate initiator organ in which the triggering of specific immunity occurs in RA24. However, most RA patients have no history of Mycobacterial infection. Since immune responses to mycobacteria were activated in RA patients, our results imply the clinical importance of subclinical exposure to mycobacteria and its association with the pathogenesis of RA. If it is possible to determine the route of exposure, vaccination or tolerance induction to mycobacteria may become a new strategy for RA prevention and therapy. Since the oral administration of the MycHSP70287–306 peptide improved experimental arthritis and suppressed autoreactive T cell reactions, the presence of mycobacteria in the gut, but not in the lungs, may regulate autoimmunity against self-HSPs.
As a limitation of this study, the patients who participated took steroids and/or immunosuppressive drugs, which may have affected their T and B cell functions. Even though these potentially immunosuppressive drugs were used, increased immune responses were observed in RA patients.
In conclusion, we herein demonstrated the presence of T and B cell responses to MycHSP70 in RA. We identified a new effector T cell epitope derived from MycHSP70 and PBMC proliferation induced by the MycHSP70-derived epitope correlated with that induced by the BiP-derived epitope in RA. The sequence similarity between MycHSP70 and human BiP at the T cell epitope level supports a link between Mycobacterial exposure and the breakdown of tolerance to BiP in RA patients. We also demonstrated the potential of the therapeutic application of these epitopes. The induction of tolerance to the MycHSP70-derived epitope could regulate immune responses to self BiP and arthritis, and, in the present study, we provided evidence to suggest that the induction of tolerance to microbial epitopes induces tolerance to related self-antigens. This system may be a suitable model for understanding how autoimmune responses develop in the pathogenesis of RA and may lead to new therapeutic strategies for RA.
Materials and Methods
Patients and HLA-DRB1 typing
Blood samples were obtained from HDs (n = 20) and RA patients without cardiovascular diseases (n = 48). Demographic data are shown in Table S2. We included RA patients who fulfilled the classification criteria of the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism25. Although some established RA patients had been diagnosed by the 1987 ACR criteria, all of them also met the 2010 criteria. No donor had a previous history of active tuberculosis infection. HLA-DRB1 genotyping was performed in accordance with a previous study26.Written informed consent was obtained from all subjects before samples were taken. This study was approved by the Ethical Committee of the University of Tokyo Hospital. The methods were performed in accordance with the approved guidelines.
Proteins
Human recombinant BiP, HSP60, HSP40 and Cpn10 and recombinant MycHSP65 and MycHSP70 were purchased from Enzo Life Sciences. E. coli recombinant DNAK, GroES, DNAJ and GroEL were purchased from ProSpec-Tany TechnoGene. The recombinant MycHSP70 protein (NCBI GenBank AF2122/97) was produced for the mouse immunization study using the pET Directional TOPO Expression Kit (Life Technologies) and purified by the Ni-NTA Fast Start Kit (QIAGEN) according to the manufacturer’s procedures. The preparation of citrullinated BiP was described previously7. Endotoxin was removed by passing through a Detoxi-Gel AffinityPak prepared column (Pierce).
Antibody measurement
Serum antibodies were measured by ELISA as previously reported7. Briefly, 96-well plates (Thermo Fisher Scientific) were coated with 5 μg/mL of each antigen and dissolved in 0.1 M NaHCO3. Diluted samples (100x) were applied, followed by goat anti-human IgG-horseradish peroxidase (HRP) (5000:1,Vector) or goat anti-mouse IgG-HRP (5000:1, Life Technologies) detection antibodies and TMB solution (KPL) for development.
Peptide synthesis
MycHSP70-derived peptides were designed to be 20-mers long and overlap one another by at least 5-mers (Supplementary Table S1) (Operon Biotechnology). The purity of each peptide was greater than 70%. Alanine-substituted peptides, which were derived from MycHSP287–306 and had one amino acid substituted to alanine, were prepared for the binding assay.
Human PBMC culture
PBMCs (1 × 106/mL) isolated with Ficoll-Paque (GE Healthcare) were cultured in the presence or absence of each peptide (10 μg/mL) with RPMI 1640 medium supplemented with 10% human serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified incubator at 37 °C, 5% CO2, for 96 hours. Cells were incubated for 18 h following a 3H-thymidine pulse into each well and harvested using a semiautomated sample harvester. Counts per minute were measured with a scintillation counter. In some experiments, the culture supernatants were collected after a 72-hour incubation and cytokine concentrations were measured in the culture supernatants by a human IFN-γ high sensitivity ELISA kit and human IL-17 high sensitivity ELISA kit (eBioscience). In blocking experiments, 10 μg/mL of an anti-HLA DR monoclonal antibody (L243) (Biolegend, San Diego, CA, USA) was added.
Peptide binding assay
The procedure to assess peptide binding to HLA-DRB1*0401 and 0405 was described in a previous study8. In brief, HLA-DRA1B1*0401 and 0405 was cloned and transfected into S2 Drosophila cells. Soluble DR4 molecules in the supernatant were collected and purified through an affinity column coupled with the anti-DR antibody LB3.1. In the peptide binding assay, a 40 nM solution of purified DR4 was incubated at 37 °C for 4 h with the HA307–319 peptide (0.5 nM), which had been labeled at the N terminus with biotin. The indicated concentrations of the peptides were added as competitors to HA peptide binding. Bound peptides were separated from free peptides by immobilizing the DR molecules on microtiter plates coated with LB3.1. The antibody was coated to the plate by the overnight incubation of a 5 μg/mL solution at 4 °C. Bound biotinylated peptides were detected by an incubation with streptavidin-alkaline phosphatase. An automatic microplate reader (Bio-rad 550, Bio-rad) was used to measure optical density.
HLA-DR4 transgenic mice
HLA-DR4 transgenic mice were obtained from Taconic, immunized twice (day 0, 14) with 100 μg of MycHSP70 with the same volume of complete Freund’s adjuvant subcutaneously into the tails and sacrificed on day 21. MACS-purified CD4+ T cells (1 × 105/mL) were cultured with irradiated splenocytes (1 × 106/mL) and an antigen (10 μg/mL) in RPMI 1640 medium supplemented with 5% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 5 × 10−5 M 2-mercaptoethanol in a humidified incubator at 37 °C, 5% CO2, for 72 h. 3H-thymidine was pulsed to each well, cells were harvested after an 18-hour incubation and analyzed with a scintillation counter.
CIA
CIA in the background of DBA/1 J female mice (SLC Japan) was performed and arthritis scores were acquired as previously described7. In the therapeutic experiments, mice were administered 200 μg of MycHSP287–306 peptides orally for 5 continuous days following the onset of arthritis. Mice were sacrificed on day 35 and the CD4+ T cell culture was performed as described above. Mouse cytokine concentrations in the supernatants were measured using a mouse IL-10 ELISA kit and mouse/human TGF-beta 1 ELISA kit (eBioscience) according to the manufacturer’s protocol. Tissue samples were embedded in paraffin wax after 10% formaldehyde fixation and decalcification. Sections were stained with hematoxylin and eosin. Synovial tissues were graded by mononuclear cell infiltration, pannus formation and cartilage erosion, as described previously7. All animal experiments were conducted in accordance with Institutional and National Guidelines and the protocols were approved by the Ethical Committee on Animal Experiments of the University of Tokyo.
Statistical analysis
Data were expressed as the median ± SEM. Differences were compared with the Mann-Whitney U test. Comparisons of more than three groups were performed using a one-way analysis of variance followed by a Bonferroni multiple comparison. Correlations were evaluated using Spearman’s rank correlation coefficient. P values less than 0.05 were considered significant.
Additional Information
How to cite this article: Shoda, H. et al. Immune responses to Mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. Sci. Rep. 6, 22486; doi: 10.1038/srep22486 (2016).
References
Tobon, G. J., Youinou, P. & Saraux, A. The environment, geo-epidemiology and autoimmune disease: Rheumatoid arthritis. Autoimmun Rev 9, A288–92 (2010).
Albert, L. J. & Inman, R. D. Molecular mimicry and autoimmunity. N Engl J Med 341, 2068–2074 (1999).
Kinloch, A. J. et al. Immunization with Porphyromonas gingivalis enolase induces autoimmunity to mammalian alpha-enolase and arthritis in DR4-IE-transgenic mice. Arthritis Rheum 63, 3818–23 (2011).
van Heemst, J. et al. Crossreactivity to vinculin and microbes provides a molecular basis for HLA-based protection against rheumatoid arthritis. Nat Commun 6, 6681 (2015).
Corrigall, V. M. et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol 166, 1492–1498 (2001).
van Eden, W., van der Zee, R. & Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5, 318–330 (2015).
Shoda, H. et al. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res Ther 13, R191 (2010).
Shoda, H. et al. Autoantigen BiP-derived HLA-DR4 epitopes differentially recognized by effector and regulatory T cells in rheumatoid arthritis. Arthritis Rheumatol 67, 1171–1181 (2015).
Wieten, L. et al. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS One 4, e4186 (2009).
van Herwijnen, M. J. et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci USA 109, 14134–14139 (2012).
Adams, E. et al. Human T-cell clones to the 70-kilodalton heat shock protein of Mycobacterium leprae define mycobacterium-specific epitopes rather than shared epitopes. Infect Immun. 65, 1061–1070 (1997).
Kocsis, J. et al. Antibodies against the human heat shock protein hsp70 in patients with severe coronary artery disease. Immunological Investigations. 31, 219–231 (2002).
Worthington, J. et al. Lack of association of increased antibody levels to mycobacterial hsp65 with rheumatoid arthritis: results from a study of disease discordant twin pairs. Ann Rheum Dis 52, 542–544 (1993).
van Halm, V. P. et al. Antibodies against human 60 kDa heat shock protein are not associated with cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis 65, 590–594 (2006).
Zang, X., Izikson, L., Liu, L. & Weiner, H. L. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 167, 4245–4253 (2001).
Maron, R., Slavin, A. J., Hoffmann, E., Komagata, Y. & Weiner, H. Oral tolerance to copolymer 1 in myelin basic protein (MBP) TCR transgenic mice: cross-reactivity with MBP-specific TCR and differential induction of anti-inflammatory cytokines. Int Immunol. 14, 131–138 (2001).
Jiang, X. et al. To what extent is the familial risk of rheumatoid arthritis explained by established rheumatoid arthritis risk factors? Arthritis Rheumatol 67, 352–362 (2015).
Winthrop, K. L. & Iseman, M. Bedfellows: Mycobacteria and rheumatoid arthritis in the era of biologic therapy. Nat Rev Rheumatol 9, 524–531 (2013).
Ishigaki, K. et al. Quantitative and qualitative characterization of expanded CD4+ T cell clones in rheumatoid arthritis patients. Sci Rep 5, 12937 (2015).
Scally, S. W. et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination and rheumatoid arthritis. J Exp Med 210, 2569–2582 (2013).
McRae, B. L., Vanderlugt, C. L., Dal Canto, M. C. & Miller, S. D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 182, 75–85 (1995).
McMahon, E. J., Bailey, S. M., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 11, 335–339 (2005).
Wallace, R. J. et al. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. J Clin Microbiol 51, 1747–1752 (2013).
Catrina, A. I. et al. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol 10, 645–653 (2014).
Aletaha, D. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69, 1580–1588 (2010).
Kawai, S. et al. Routine low and high resolution typing of the HLA-DRB gene using the PCR-MPH (microtitre plate hybridization) method. Eur J Immunogenet 23, 471–486 (1996).
Acknowledgements
The authors are grateful to Ms Jun Takizawa and Ms Kayako Watada for their excellent technical assistance. This study was supported by the Ministry of Health, Labour and Welfare, Ministry of Education, Culture, Sports, Science and Technology KAKENHI Grant-in-Aid for Scientific Research (C) (26461462), as well as by a Grant-in-Aid for Scientific Research (S) (23229007) and the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
H.S. designed, performed and analyzed the experiments in this study. K.F. designed the study and provided advice. N.H., S.S. and T.O. performed and analyzed the experiments in this study. K.Y. provided overall coordination with respect to conception, design and supervision of the study. H.S. and K.Y. wrote the manuscript with comments from co-authors.
Ethics declarations
Competing interests
K.Y. received financial support or fees from AbbVie, Astellas, BMS, Daiichi-Sankyo, MitsubishiTanabe, Pfizer, Sanofi, Santen, Takeda, Teijin., Boehringer Ingelheim, Chugai, Eisai, Ono, Taisho Toyama,UCB., ImmunoFuture, Asahi Kasei and Janssen. K.F. received financial support or fees from Astellas, BMS, Daiichi-Sankyo, MitsubishiTanabe, Pfizer, Santen, Takeda, Chugai, Eisai, Taisho Toyama and UCB. and Janssen. All other authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shoda, H., Hanata, N., Sumitomo, S. et al. Immune responses to Mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. Sci Rep 6, 22486 (2016). https://doi.org/10.1038/srep22486
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22486
- Springer Nature Limited