Abstract
Neural progenitors differentiated from induced pluripotent stem cells (iPS) hold potentials for treating neurological diseases. Serotonin has potent effects on neuronal functions through multiple receptors, underlying a variety of neural disorders. Glutamate and GABA receptors have been proven functional in neurons differentiated from iPS, however, little is known about 5-HT receptor-mediated modulation in such neuronal networks. In the present study, human iPS were differentiated into cells possessing featured physiological properties of cortical neurons. Whole-cell patch-clamp recording was used to examine the involvement of 5-HT2 receptors in functional modulation of GABAergic synaptic transmission. We found that serotonin and DOI (a selective agonist of 5-HT2A/C receptor) reversibly reduced GABA-activated currents and this 5-HT2A/C receptor mediated inhibition required G protein, PLC, PKC and Ca2+ signaling. Serotonin increased the frequency of miniature inhibitory postsynaptic currents (mIPSCs), which could be mimicked by α-methylserotonin, a 5-HT2 receptor agonist. In contrast, DOI reduced both frequency and amplitude of mIPSCs. These findings suggested that in iPS-derived human neurons serotonin postsynaptically reduced GABAa receptor function through 5-HT2A/C receptors, but presynaptically other 5-HT2 receptors counteracted the action of 5-HT2A/C receptors. Functional expression of serotonin receptors in human iPS-derived neurons provides a pre-requisite for their normal behaviors after grafting.
Similar content being viewed by others
Introduction
Induced neurons from neural stem cells of embryonic stem cell (ES) and induced pluripotent stem cells (iPS) origins not only exhibited neuronal morphology with extensive axon and dendrites, but also possessed mature electrophysiological properties such as repetitive action potentials in response to current stimulation and integrated synaptic connections with host both in vitro and in vivo1,2,3. The iPS-derived neurons recapitulated symptoms of patients with neurological diseases at cellular and synaptic levels, providing a rather distinct insight into the underlying mechanisms4,5. Autologous neuronal progenitors obtained from iPS would provide enough source cells for transplantation, which hold great potentials to treat neurological disorders such as Parkinson’s disease, schizophrenia and stroke6,7,8,9,10,11,12,13. Input-specific long-term potentiation14, homeostatic plasticity, as well as BDNF induced synaptic changes15 were reported for ES-derived neurons, further physiologically supporting the functional integrity of transplanted progenitors.
The development and function of the cerebral cortex is subjected to massive monoaminegic modulation. Serotonin as one of important neuromodulators participates in many neural processes including synaptic transmission, mood, learning and memory16,17,18,19. Serotonergic fibers from raphe nucleus diffusely projecting to the brain exert its modulatory effect through multiple receptor subtypes (1–7)19. So far amounting evidences revealed the existence of glutamate and GABA receptors in neural stem cell derived neurons1,2 and even the role of neuromodulators in the proliferation and differentiation of neural stem cells20,21; however few studies addressed the neuromodulation mediated by serotonin and its receptors. The transplanted neurons have to communicate with host neurons and to be modulated normally to achieve satisfactory therapeutic efficacy. Activation of 5-HT2 receptors has been reported to broadly regulate receptor function and neuronal excitability7,22,23,24,25,26. Functional expression of serotonin receptors in human iPS-derived neurons would endow them with the ability to receive normal serotonergic modulation from the host after grafting and to serve as a cell model to screen serotonin related neuropsychological drugs. Here we differentiated iPS into forebrain-like cortical neurons and whole-cell patch-clamp recording technique was adopted to investigate the involvement of 5-HT2 receptors in the modulation of GABAa receptor function and inhibitory synaptic transmissions.
Results
Acquisition of forebrain-like neural progenitor cells from human iPS
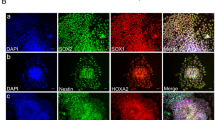
Neural progenitor cells (NPCs) were derived from human iPS reprogrammed from urine cells (UC5) or fibroblasts (GZ2) as described before27,28, as well as ES (H1) as a control. Unless otherwise specified, the majority of the results shown here were obtained from iPS line UC5, which was derived by using a feeder-free, serum-free and virus-free method without oncogene c-MYC, representing a more promising translational application for regenerative medicine. These NPCs expressed Sox1, Pax6 and Nestin of high percentages (Fig. 1a), without mesodermal and endodermal contamination (undetectable markers T and Sox17 by Q-PCR), whereas pluripotency markers including Oct4 and Nanog stopped to express (Fig. 1b,c). The NPCs can stably expand in both suspension and adhesion conditions in neural expanding medium containing EGF and bFGF. More importantly, these cells possessed dorsal forebrain regional identity, which maintained even after long-term passages (Fig. 1d). The NPCs were highly neurogenic and showed typical neuronal morphology and gene expression profile upon differentiation and maturation (Fig. 1e).
Acquisition and identification of forebrain-like neurons from iPS.
(a), Immunostaining showed iPS-derived NPCs expressing Pax6, Sox1 and Nestin of high percentages while no expression of pluripotent marker Oct4 (b), which was further confirmed by Q-PCR, (c), however, the mesoderm marker (T) and endoderm marker (Sox17) were undetectable in these NPCs. (d), Q-PCR also confirmed the dorsal forebrain identity of the NPCs, which remained the same characteristics even after long-term passages. Pax6 and Emx2 (not done in H1 NPC): markers for dorsal CNS (central nervous system), Otx2 (not done in UC5 NPC P9) and Foxg1: forebrain markers, En1 and Lmx1b: midbrain markers, Gbx2 and Hoxb2: hindbrain markers. P2, Passage 2; P9, Passage 9. (e), Immunoflurescent photographs showed the NPCs were highly neurogenic with high expression of typical neuronal markers (early Tuj1 and mature Map2). D1: 1 day post-differentiation; D24: 24 day post-differentiation.
Basic electrophysiological properties of iPS-derived forebrain neurons
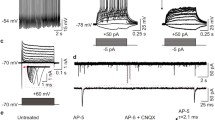
Next, electrophysiological studies were performed on passage 3–8 NPCs-differentiated neurons (Fig. 2a). The majority of them differentiated into glutamate positive neurons (glutamate+, 86.80 ± 0.97%, n = 6; GABA+, 14.19 ± 1.07%, n = 6) (Fig. 3a,b). Voltage-gated sodium and potassium channels of these induced neurons experienced the developmental changes (Fig. 2b,c). Only sparse spikes with lower amplitude and longer duration were elicited at early stages; and progressively maturing neurons displayed repetitive action potentials in response to current injections (Fig. 2d–g), which is consistent with previous reports29,30,31.
Intrinsic membrane properties of iPS-derived neurons.
(a), Phase contrast micrograph showing a typical iPS-derived neuron being approached by a patching pipette. (b), Representative voltage-gated ion currents recorded from an iPS-derived neuron at three developmental stages (upper panel: 1-2 week; middle panel: 2-3 week; lower panel: 4-5 week). The whole cell Na+ (inward) and K+ (outward) currents were elicited by test pulses to potentials between −50 mV and + 60 mV in steps of 10 mV from a holding potential of −70 mV (lower panel). (c), Quantitative analysis of current-voltage relationship respectively of Na+ (negative) and steady sate K+ (positive) ion currents at different development stages (1-2 week, n = 50; 2-3 week, n = 50; 4-5 week, n = 31). (d), Representative traces of action potentials (left panel) in response to injected current steps (20, 30, 40, 50, 60 pA) with 800 ms duration at three developmental stages, Action potentials (right panel) evoked by 50 pA injected current (lowest panel). Quantitative analysis of action potential amplitude (e), number of maximal spikes in response to 800 ms current injection (f) and half width (g) of the action potential at three developmental stages (1-2 week, n = 18; 2-3 week, n = 18; 4-5 week, n = 13). The waveforms of scaled action potentials revealed gradual narrowing of the half width (g1).
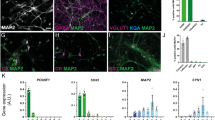
Characterization of neuronal subtypes and functional analyses of receptors currents in iPS-derived neurons.
(a), The majority of spontaneously differentiated iPS-derived neurons were immunopositive to glutamate (left), with a small portion being GABA+ (right). Scale bar: 50 μm. (b), Quantitative analysis of glutamate+ and GABA+ among Map2 positive neurons. (c), A current response to glutamate (1 mM) delivered through puffing electrode (15 psi, 30 ms). (d), The pharmacologically isolated glutamatergic spontaneous excitatory postsynaptic currents could be completely blocked by the AMPA/KA receptor antagonist (DNQX, 20 μM). The current response to GABA (1 mM) (e) delivered through puffing electrode (15 psi, 30 ms) and spontaneous GABAergic inhibitory postsynaptic currents (f) starting from a holding potential of –70 mV to 70 mV in a step of 20 mV. (g), Current-Voltage curve of GABA evoked response revealing its reversal potential at about 0 mV (n = 4). (h), Pharmacologically isolated GABAergic spontaneous inhibitory postsynaptic currents could be completely blocked by a selective GABAa receptor antagonist (Bicuculline, 10 μM).
Moreover, the puffed glutamate (1 mM) evoked an inward current (Fig. 3c) and spontaneous excitatory postsynaptic currents was blocked by DNQX (20 μM), a selective glutamate receptor antagonist, which indicated that glutamate receptors mediated excitatory synaptic transmission (Fig. 3d). The iPS-derived neuron also responded to puffed 1 mM GABA with an inward current (Fig. 3e). The GABAergic spontaneous IPSCs (Fig. 3f) and GABA-activated currents reversed at 0.92 ± 2.33 mV (n = 4, Fig. 3g), which approximated the theoretical equilibrium potentials of Cl− with CsCl-based internal solutions32. Bicuculline (10 μM), a selective GABAa receptor antagonist completely blocked GABA-activated current and spontaneous IPSCs (Fig. 3h). These indicated that GABAa receptors on the postsynaptic membrane mediated inhibitory synaptic transmission. These electrophysiological evidences suggested that iPS-derived neurons behaved as normal neurons communicating each other through fundamental synaptic connections. To minimize developmental difference of serotonin receptors, at least 4-week-old mature iPS-derived neurons were used to examine the serotonergic modulation.
5-HT2A/C receptor mediated reduction of GABA-activated current
Serotonergic projections diffusely innervate the central nervous system via multiple types of receptors and most 5-HT receptors are metabotropic receptors that are involved in the regulation of neuronal function. Serotonin at 40 μM significantly and reversibly reduced GABA activated currents (reduction%: 45.68 ± 6.14%, n = 5, p = 0.002, Student’s t-test) (Fig. 4a,d) in the iPS-derived neurons. Since 5-HT2 receptors were reported to responsibly modulate GABAa receptors in cortical neurons, we applied DOI (20 μM), a 5-HT2A/C receptor agonist to examine whether 5-HT2A/C receptors accounted for the reduction. We found that DOI similarly reduced GABA-activated currents as serotonin (reduction%: 29.64 ± 2.65%, n = 10, p < 0.001, Student’s t-test) (Fig. 4b,d). However α-methylserotonin, the agonist of 5-HT2 receptor, did not cause any change of GABA-activated currents (reduction%: 0.80 ± 1.61%, n = 6, p = 0.612, Student’s t-test) (Fig. 4c,d), suggesting a counteractive interaction between subtypes of 5-HT2 receptors. The depressive effect of 5-HT and DOI was also replicated in the induced neurons reprogrammed from another cell line (GZ2) (reduction%: 5-HT: 52.87 ± 9.91%, n = 8, p = 0.001; DOI: 28.14 ± 5.37%, n = 6, p = 0.001, Student’s t-test). The reducing effect of 5-HT and DOI on GABA current could be largely attenuated by ketanserin (40 μM), a selective antagonist of 5-HT2A/C receptor (reduction%: 5-HT: 14.59 ± 2.91%, n = 4; DOI: 5.04 ± 1.99%, n = 4) (5-HT + ketanserin vs. 5-HT, p < 0.005; DOI + ketanserin vs. DOI, p < 0.001, Student’s t-test) (Fig. 4d), demonstrating that the reduction of GABA-activated current by serotonin could be majorly ascribed to the activation of 5-HT2A/C receptors at the postsynaptic membrane in human iPS-derived neurons. Notably, the larger reduction caused by 5-HT than DOI, as well as the incomplete blocking by ketanserin, implied the contribution of other 5-HT subtype receptors. And we did observe that 8-hydroxy-2-dipropylaminotetralin (DPAT, 20 μM), a selective 5-HT1A receptors agonist, caused a reversible reduction of GABA-activated current (control: 492.72 ± 118.36 pA; DPAT: 435.56 ± 98.13 pA; n = 5, p = 0.035, Student’s t-test).
5-HT reduced GABA current mainly through activation of 5-HT2A/C receptors.
The representative current traces showing the depressive effect of 5-HT (40 μM) (a1) and DOI (20 μM) (b1) on GABA currents, which was recovered after washout. (a2) and (b2) showed the time course of GABA currents when 5-HT and DOI were applied. (c), Representative traces showing no effect of α-methylserotonin (30 μM) on GABA currents. (d), Quantification data showing the reduction of GABA currents by 5-HT (n = 5), DOI (n = 10) and α-methylserotonin (n = 6). The deceasing effect was largely attenuated by ketanserin (40 μM, a selective 5-HT2A/C receptor antagonist) (n = 4). **p <0.01. NS, no significant.
G-protein is required for 5-HT2A/C receptor mediated reduction of GABA-activated currents
Activation of G-protein is the prerequisite of the action of G-protein-coupled receptors (GPCR) and all 5-HT receptors except ionic 5-HT3 were coupled to different G proteins33. We then examined whether activation of G-protein was the prerequisite of the action of 5-HT and DOI on GABA current. When dialysis of GDP-β-s (0.5 mM) constitutively prevented G-protein complex from dissociating into active subunits of Gα and Gβγ, the depressive effect of 5-HT (Fig. 5a) and DOI (Fig. 5b) on GABA activated currents was completely blocked (5-HT: 98.68 ± 0.49% of control, n = 6; DOI: 100.10 ± 3.21% control, n = 6) (5-HT + GDP-β-s vs. 5-HT, p < 0.005; DOI + GDP-β-s vs. DOI, p < 0.001, Student’s t-test) (Fig. 5c). These data indicated that negative regulation of GABAa receptors by 5-HT2 receptors was dependent on the activation of G-protein. In this and following recordings of applying intracellular blockers, it must be mentioned that at least 5–10 minutes’ waiting period was needed to achieve a successful inhibition34.
G-protein is required for 5-HT2A/C receptor mediated reduction of GABA-activated currents.
In the presence of GDP-b-s (500 μM), which was included in the pipette solution, 5-HT (a) and DOI (b) failed to attenuate the GABA currents. (c), Quantification data showing no effect of 5-HT (n = 6) and DOI (n = 6) on GABA current after blocking the activation of G-protein.
Calcium signaling is involved in the modulation of GABA current by 5-HT2A/C receptor
Ca2+ as an important second messenger participates in many physiological processes and its rise triggered by IP3 would have a potential impact on GABAa receptors as reported before. BAPTA as a potential and rapid chelator of intracellular Ca2+ was used to test the involvement of calcium signaling in the process of 5-HT2A/C receptor mediated reduction of GABA current. When BAPTA (10 mM) was included in the pipette solutions, interestingly both 5-HT (Fig. 6a) and DOI (Fig. 6b) lost their ability to change the amplitude of GABA currents (5-HT: 98.39 ± 1.66%, n = 7; DOI: 99.94 ± 1.32%, n = 5) (5-HT + BAPTA vs. 5-HT, p < 0.005; DOI + BAPTA vs. DOI, p < 0.001, Student’s t-test) (Fig. 6c).
Calcium signaling is involved in the modulation by 5-HT2A/C receptor.
Inclusion of BAPTA (10 mM) in the pipette solution prevented the modulatory effect of 5-HT (a) and DOI (b) on GABA currents. (c), Quantification data showing that intracellular calcium signaling was required to cause the reduction of GABA currents by 5-HT (n = 7) and DOI (n = 5).
Phospholipase C and protein kinase C act as downstream signaling of 5-HT2A/C receptors
Gq protein-coupled 5-HT2A/C receptor theoretically would promote phospholipase C (PLC) to hydrolyze phosphoinositol lipids on the membrane into ionsitol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) and then the rise of Ca2+ and activation of protein kinesis C (PKC) would change the phosphorylation of GABAa receptors. We examined whether the same mechanism occurred in iPS-derived neurons by intracellularly delivering correspondent inhibitors. As expected, when PLC activity was blocked with U-73122 (5 μM) in recording pipette, GABA- activated currents remained unchanged in the presence of DOI (97.03 ± 0.73% control, n = 6) (DOI + U-73122 vs. DOI, p < 0.001, Student’s t-test) (Fig. 7a,d), supporting the involvement of PLC in 5-HT2A/C receptors mediated regulation of GABAa receptors.
Phospholipase C and protein kinase C act as downstream signaling of 5-HT2A/C receptors.
Inclusion of U-73122 (5 μM, n = 6, (a) GF 109203X (2.5 μM, n = 5, (b) in the pipette solution prevented the modulatory effect of DOI on GABA currents, but forskolin (10 μM, n = 5, c) failed to achieve the occluding effect. (c), Quantification data showing that activation of PLC-PKC pathway caused the reduction of GABA currents.
Similar to those reported in rat cortical neurons, the inclusion of GF109203X (2.5 μM), a PKC inhibitor, significantly abolished the reduction of GABA currents by DOI (94.85 ± 2.05% control, n = 5) (DOI + GF109203X vs. DOI, p < 0.001, Student’s t-test) (Fig. 7b,d), implying that the kinase activity of PKC was indispensable for the downstream signaling following activation of 5-HT2 receptor. This conclusion was further confirmed by the failure of adenylyl cyclase activator (forskolin, 10 μM) to occlude the effect of DOI (78.16 ± 2.86% of control, n = 5) (DOI + forskolin vs. DOI, p = 0.063, Student’s t-test) (Fig. 7c,d).
Taken together, these data suggested that PLC and PKC act as downstream signaling cascade to achieve the modulation of GABAa receptors by 5-HT2A/C receptors in human iPS-derived forebrain neurons.
5-HT2 receptors mediated modulation of spontaneous inhibitory postsynaptic currents
GABA delivered through pressure puffing directly activated GABA receptors on the cell surface, thus 5-HT receptors on postsynaptic membrane contributed to the above mentioned modulation. Notably, serotonin also exerts a powerful influence on the presynaptic neurotransmitter release32. In order to understand the contribution from presynaptic 5-HT receptors, we performed miniature IPSCs (mIPSCs) recording in iPS-derived neuronal networks in the presence of 1 μM TTX to block the spontaneous action potentials. In contrast to the depressive modulation at postsynaptic site, serotonin robustly enhanced the frequency, but not the amplitude of mIPSCs (frequency: 154 ± 15% of control, p = 0.022; amplitude:108 ± 10% of control, p = 0.453, Student’s t-test, n = 5, Fig. 8a1,d,e) and this could be mimicked by agonist of 5-HT2 receptor, α-methylserotonin (frequency: 149 ± 17% of control, p = 0.034; amplitude:112 ± 8% of control , p = 0.200, Student’s t-test, n = 6, Fig. 8c1,d,e). But DOI still caused a reduction of both frequency and amplitude of mIPSCs (frequency: 63 ± 8% control, p < 0.001; amplitude: 70 ± 1% of control, p = 0.014, Student’s t-test, n = 5, Fig. 8b1,d,e). Cumulative distribution plotting of the amplitude and inter-event interval revealed the same conclusion. Specifically, the inter-event interval cumulative distribution of mIPSCs was shifted significantly leftward by 5-HT (p = 0.004, K–S test, Fig. 8a2) and α-methylserotonin (p = 0.024, K–S test, Fig. 8c2), but rightward by DOI (p < 0.001, K–S test, Fig. 8b2); while the amplitude cumulative distribution was substantially shifted rightward only by DOI (DOI, p < 0.001; 5-HT, p = 0.511; α-methylserotonin, p = 0.127; K–S test, Fig. 8a2,b2,c2). Taken together, our data suggested that several subtypes of presynaptic 5-HT2 receptor differentially modulated inhibitory synaptic transmission in a manner different from those governing the postsynaptic effect.
5-HT2 receptors mediated modulation of spontaneous inhibitory postsynaptic currents.
The representative traces showing the mIPSCs (a1, b1, c1) and the cumulative plots of the mIPSCs (frequency: left panel; amplitude: right panel) in the absence and presence of the 5-HT (a2), DOI (b2) and α–methylserotonin (c2). (d), Quantification data showing the effect of the agonists of 5-HT2 receptors on the frequency of mIPSCs. (e), Quantification data showing the effect of the agonists of 5-HT2 receptors on the amplitude of mIPSCs (5-HT, n = 5; DOI, n = 5; α-methylserotonin, n = 6).
Discussion
Our present study demonstrated presynaptic and postsynaptic 5-HT2 receptors functionally regulated inhibitory synaptic transmissions in human iPS-derived neuronal networks, confirming that these induced neurons highly resemble those of naïve forebrain neurons in terms of physiological properties. Our major findings are: (1) Using our present neural induction protocol, NPCs can be stably and efficiently induced from human iPS with no manual selection needed; (2) These iPS-derived NPCs could be differentiated into morphologically and functionally mature cortical neurons; (3) Serotonin modulated GABAa receptors mainly through 5-HT2A/C receptors; (4) Postsynaptically, 5-HT2A/C receptors exerted its modulating effect through GPCR-PLC-PKC and Ca2+ signaling pathway; (5) Presynaptically, the inhibiting effect mediated by 5-HT2A/C receptors was counteracted by other 5-HT2 receptors.
Neuronal progenitors generated in vitro as donor cells for transplantation hold great promise to treat neurological diseases6,7,8,11,13,35,36. Neural stem cells from iPS have advantages over those from ES in term of less ethic concerns and immunity rejections8,12. Currently virus-free generation of iPS warranted the safety of iPS-derived neurons in translational application1,35. Increasing evidences indicated that transplanted progenitors could survive, migrate, differentiate, integrate into host neural circuitry and even correct the behavioral deficits2,3,6,7,13,36. It has already been reported that ES-derived neurons exhibited homeostatic plasticity and BDNF- induced synaptic plasticity15, as well as input-specific long-term potentiation14. The brain circuitry is known to receive massive monoaminergic projecting fibers engaging in circadian rhythm, motor coordination , learning and memory37. The brain function can’t be properly fulfilled without serotonin modulation16. GABAergic inhibition balances brain state by opposing glutamatergic excitation38,39. The crosstalk between GABA and serotonin systems has been justified in the forebrain23, where 5-HT2 receptors mediate the regulation of GABAergic system both presynaptically and postsynaptically. Therefore the iPS-derived neurons for engrafting should be modulated as its host counterparts, otherwise new disorders might be caused by the transplantation.
Serotonin mainly activated postsynaptic 5-HT2A/C receptors to reduce GABA -activated currents through G protein, PLC, PKC, as well as Ca2+ signaling. DOI reduced inhibitory neurotransmitters activated currents in the cortical neurons either through receptor phosphorylation or trafficking23,40. We postulated that our human iPS-derived neurons likely shared the same mechanism. Presynaptic 5-HT2 receptors were also reported to regulate transmitter release22,25,41. Serotonin significantly influenced mIPSCs through 5-HT2 receptors in our iPS-derived neural network. The enhancement of mIPSCs by serotonin could be mimicked by 5-HT2 receptor agonists. Conversely, a reduction of frequency and amplitude of mIPSCs was caused by DOI. The frequency of mIPSCs is closely related to the probability of transmitter release. The decreased frequency of mIPSCs by DOI means less transmitter release from presynaptic terminals, but this action can be counteracted by other 5-HT2 receptors42. Overall our findings suggested that 5-HT2A/C receptors not only downregulated postsynaptic GABAa receptors, but also attenuated presynaptic GABA release of the iPS-derived neurons.
Taken together, our findings demonstrate 5-HT2 receptors functionally modulated GABAergic synaptic transmission in the neural networks composed of human iPS-derived neurons, suggesting that iPS-derived neurons would receive diffused serotonin neuromodulation after transplantation just like host cells, but also could sever as an ideal in vitro model for studying neurological diseases and screening serotonin related neuropsychological drugs.
Methods
All experiments were carried out in accordance with the guidelines of the Human Subject Research Ethics Committee at Guangzhou Institutes of Biomedicine and Health (GIBH), Chinese Academy of Sciences (CAS) and the Committee approved the experiments. Formal informed consent was obtained from all subjects.
Cell culture and neural differentiation
Two human iPS lines from healthy human, UC5 (Passage 15–25)28 and GZ2 (Passage 10–20)27, established and maintained in our laboratory and one human ESCs line, H1 (Passage 40–50, Wicell, Madison, WI, USA), were adopted in the present study. UC5 cell line was derived from urine cells using a feeder-free, serum-free and virus-free method without oncogene c-MYC. GZ2 cell line was reprogrammed from skin fibroblasts by using Yamanaka factors43. All these pluripotent cells were cultured as described elsewhere44,45 on plates coated with Matrigel (BD Biosciences, San Jose, CA, USA) in mTesR1 medium (Stemcell Technologies, Vancouver, BC, Canada) and routinely passaged by EDTA (Ethylene Diamine Tetraacetic Acid, 0.5 mM) dissociation every 4–6 day.
Neural induction was performed as previously reported via a monolayer strategy46 by dual inhibition of SMAD signaling with empirical modifications to get highly homogenous neural progenitor cells (NPCs) of dorsal forebrain identity. Briefly, once the pluripotent cells got 100% confluence (Day 0 of neural induction), the medium was changed to neural induction medium (NIM). NIM basically contained N2B27 medium (DMEM/F12: Neurobasal (1:1), 0.5% N2, 1% B27, 1% Glutamax (GIBCO), 1% non-essential amino acid (GIBCO)), plus 2 inhibitors (5 μM S431542 (Sigma), 5 μM Dorsomophin (Sigma)). By the 8-9th day post induction, when a uniform packed neuroepithelial layer appeared routinely, cell aggregates dissociated from the neuroepithelium by manually scratching and gentle pipetting with no selection, were replated on new Matrigel plate and continually fed with NIM without inhibitors for another 8 days. Then NPCs were harvested by floating the neural rosettes with Dispase digestion without picking and further purified via 1-2 passage(s) with Accutase digestion into single cells. The dorsal forebrain identity of NPCs was confirmed by immunostaining and Q-PCR.
For neuron differentiation, NPCs were digested into single cells with Accutase, plated on Matrigel-coated glass coverslips in 24-well plate at a density of 40,000 cells/well and fed with neural differentiation medium (NDM). NDM consisted of Neurobasal (Life Technologies), 2% B27, 1% Glutamax (GIBCO) and 1% non-essential amino acid (GIBCO), supplemented with BDNF, GDNF, IGF1 (all at 10 ng/ml, Peprotech), ascorbic acid (200 ng/ml) and cAMP (100 nM). Expression of neuron markers Tuj1 and Map2, astrocyte marker GFAP, as well as neuronal subtype markers glutamate and GABA was examined by immunostaining.
Immunofluoresent microscopy
Immunostaining of cells was done as previously described1. Briefly, neural stem cells or differentiated neurons plated on glass coverslips were fixed by 4% PFA solution for 20 min at room temperature, followed by blocking with 5% bovine serum albumin in PBS for 1 h at room temperature (RT). Subsequently, samples were incubated with primary antibodies overnight at 4 °C and then with appropriate fluorescent probe-conjugated secondary antibodies for 1 h at RT. Nuclei were counterstained with DAPI. Images were acquired using Carl Zeiss microscope. The primary antibodies used here included: Sox1 (1:1000, Millipore), Sox2 (1:100, R&D Systems), Nestin (1:200, Millipore), Pax6 (1:300, R&D Systems), Tuj1 (1:500, Covance), Map2 (1:500, Millipore), Glutamate (1:1000, Sigma), GABA (1:1000, Sigma).
Electrophysiological Recording
Whole-cell patch-clamp recordings were obtained at room temperature from iPS-derived neurons as described previously26,47. Signals were amplified with a MultiClamp 700B amplifier, digitized with a Digidata 1440 and acquired with pClamp 10 software (Molecular Devices,USA). The bath solution contained (in mM): 127 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES and 12 glucose (pH 7.4 with NaOH, 300 osmol/L). Patch pipettes with resistance between 8–10 MΩ were pulled from borosilicate glass (WPI, USA) with a Sutter P97 puller (Sutter, USA). Pipettes were filled with solutions containing (in mM): 145 K-gluconate, 0.2 EGTA, 10 HEPES, 5 NaCl, 1 MgCl2, 4 Mg-ATP and 0.3 Na-GTP (pH 7.2 with KOH, 285 osmol/L). In recording GABA activated currents and spontaneous inhibitory synaptic currents, CsCl-based solution was used to get inward chloride currents (in mM):130 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 5 EGTA, 2 QX-314, 2 MgATP and 0.2 Na-GTP (pH 7.2 with CsOH, 285 osmol/L).
To directly activate GABAa receptors on the membrane of recorded iPS-derived neuron, GABA (1 mM) was locally applied with a pressure of 15 psi controlled by a custom-made puffing device48. An external solution flowed following the GABA delivery to reduce receptor desensitization and neurons were generally held at −70 mV to record inward ion currents. Gamma-aminobutyric (GABA) receptor-mediated inhibitory postsynaptic currents (IPSCs) were recorded in a voltage-clamp mode. The external solutions including different drugs were exchanged to perfuse the neurons. 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM) and R-2-amino-5- phosphonopentanoate (APV, 50 μM) were used to block glutamatergic transmission. 1 μM TTX was routinely added in the external solution to prevent spontaneous action potentials of the neural networks. Serotonin and α-methylserotonin was purchased from Sigma-Aldrich and other receptor agonist and antagonists were purchased from Tocris Cookson Ltd.
Data analysis
Off-line data analysis was performed by using Clampfit 10.2 (Molecular Devices, USA). The Minianalysis (Synaptosoft, USA) was used to extract events for analyzing the frequency and amplitude of spontaneous IPSCs and waveforms of action potentials. Processed data were further imported into Origin 8.0 (OriginLab Corporation, USA) for plotting graphs. Numerical data were reported as mean ± SE (standard error). Student’s t-test and Kolmogorov-Smirnov test (K-S test) were used to evaluate significance level unless otherwise stated and p < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Wang, H. et al. 5-HT2 receptors mediate functional modulation of GABAa receptors and inhibitory synaptic transmissions in human iPS-derived neurons. Sci. Rep. 6, 20033; doi: 10.1038/srep20033 (2016).
References
Wang, L. et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods 10, 84–89 (2013).
Weick, J. P., Liu, Y. & Zhang, S. C. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc Natl Acad Sci USA 108, 20189–20194 (2011).
Ideguchi, M., Palmer, T. D., Recht, L. D. & Weimann, J. M. Murine embryonic stem cell-derived pyramidal neurons integrate into the cerebral cortex and appropriately project axons to subcortical targets. J Neurosci 30, 894–904 (2010).
Farra, N. et al. Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations. Mol Psychiatry 17, 1261–1271 (2012).
Wen, Z. et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418 (2014).
Lamba, D. A., Gust, J. & Reh, T. A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79 (2009).
Komlosi, G. et al. Fluoxetine (prozac) and serotonin act on excitatory synaptic transmission to suppress single layer 2/3 pyramidal neuron-triggered cell assemblies in the human prefrontal cortex. J Neurosci 32, 16369–16378 (2012).
Yu, D. X., Marchetto, M. C. & Gage, F. H. Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell 12, 678–688 (2013).
Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A. & Baraban, S. C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci 16, 692–697 (2013).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551 (2011).
Nori, S. et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA 108, 16825–16830 (2011).
Morizane, A. et al. Direct Comparison of Autologous and Allogeneic Transplantation of iPSC-Derived Neural Cells in the Brain of a Nonhuman Primate. Stem Cell Reports 1, 283–292 (2013).
Oki, K. et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 30, 1120–1133 (2012).
Sorensen, A. T., Rogelius, N., Lundberg, C. & Kokaia, M. Activity-dependent long-term plasticity of afferent synapses on grafted stem/progenitor cell-derived neurons. Exp Neurol 229, 274–281 (2011).
Copi, A., Jungling, K. & Gottmann, K. Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network. J Neurophysiol 94, 4538–4543 (2005).
Ciranna, L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4, 101–114 (2006).
Doya, K. Metalearning and neuromodulation. Neural Netw 15, 495–506 (2002).
Benekareddy, M., Goodfellow, N. M., Lambe, E. K. & Vaidya, V. A. Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci 30, 12138–12150 (2010).
Andrade, R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci 861, 190–203 (1998).
Belinsky, G. S. et al. Dopamine receptors in human embryonic stem cell neurodifferentiation. Stem Cells Dev 22, 1522–1540 (2013).
Benninghoff, J. et al. Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology 35, 893–903 (2010).
Zhou, F. M. & Hablitz, J. J. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol 82, 2989–2999 (1999).
Feng, J., Cai, X., Zhao, J. & Yan, Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci 21, 6502–6511 (2001).
Goodfellow, N. M., Benekareddy, M., Vaidya, V. A. & Lambe, E. K. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci 29, 10094–10103 (2009).
Deng, P. Y. & Lei, S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K + channels. Mol Cell Neurosci 39, 273–284 (2008).
Bin Luo, Lingli Hu, Chunhua Liu, Yiping Guo & Wang, H. Activation of 5-HT2A/C receptor reduces glycine receptor-mediated currents in cultured auditory cortical neurons. Amino Acids (2015).
Li, S. et al. Valproic acid-induced hepatotoxicity in Alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model. Hepatology 61, 1730–1739 (2015).
Xue, Y. et al. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS One 8, e70573 (2013).
Belinsky, G. S., Moore, A. R., Short, S. M., Rich, M. T. & Antic, S. D. Physiological properties of neurons derived from human embryonic stem cells using a dibutyryl cyclic AMP-based protocol. Stem Cells Dev 20, 1733–1746 (2011).
Belinsky, G. S. et al. Patch-clamp recordings and calcium imaging followed by single-cell PCR reveal the developmental profile of 13 genes in iPSC-derived human neurons. Stem Cell Res 12, 101–118 (2014).
Moore, A. R. et al. Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb Cortex 19, 1795–1805 (2009).
Wang, H. T., Luo, B., Zhou, K. Q., Xu, T. L. & Chen, L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res 215, 77–83 (2006).
Hannon, J. & Hoyer, D. Molecular biology of 5-HT receptors. Behav Brain Res 195, 198–213 (2008).
Wang, M., Tashiro, M. & Berlin, J. R. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J Physiol 555, 383–396 (2004).
Han, S. S., Williams, L. A. & Eggan, K. C. Constructing and deconstructing stem cell models of neurological disease. Neuron 70, 626–644 (2011).
Tornero, D. et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136, 3561–3577 (2013).
Lesch, K. P. & Waider, J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76, 175–191 (2012).
Sun, B. et al. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 5, 624–633 (2009).
Moreau, A. W., Amar, M., Le Roux, N., Morel, N. & Fossier, P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex 20, 456–467 (2010).
Luo, B., Hu, L., Liu, C., Guo, Y. & Wang, H. Activation of 5-HT receptor reduces glycine receptor-mediated currents in cultured auditory cortical neurons. Amino Acids doi: 10.1007/s00726-015-2086-y (2015).
Wang, H. T., Luo, B., Huang, Y. N., Zhou, K. Q. & Chen, L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res 236, 42–51 (2008).
Yuen, E. Y., Jiang, Q., Chen, P., Feng, J. & Yan, Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 283, 17194–17204 (2008).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Moody, J. Feeder-independent culture systems for human pluripotent stem cells. Methods Mol Biol 946, 507–521 (2013).
Liu, J. et al. A reciprocal antagonism between miR-376c and TGF-beta signaling regulates neural differentiation of human pluripotent stem cells. FASEB J 28, 4642–4656 (2014).
Shi, Y., Kirwan, P. & Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7, 1836–1846 (2012).
Wang, H., Han, Y. F., Chan, Y. S. & He, J. Stimulus-specific adaptation at the synapse level in vitro. PLoS One 9, e114537 (2014).
Guo, Y. et al. An economic method to build a puffing instrument for drug application in vitro. J Neurosci Methods 256, 122–126 (2015).
Acknowledgements
This work was supported by the National Key Basic Research Program of China (2012CB966300, 2014CB964600); the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01020202); the National Science Foundation of China (31300901, 31200852, 31371514, 81301340); the Bureau of Science and Technology of the Guangzhou Municipality (201400000003-2) and the “Youth Innovation Promotion Association of the Chinese Academy of Sciences” (to Dr. Y Guo and Dr. H Wang).
Author information
Authors and Affiliations
Contributions
J.H., Y.G. and H.W. designed and conceived the experiments. H.W. and L.H. collected and interpreted the electrophysiological data. Y.G. and C.L. performed the neural induction and differentiation. L.W. assisted in neural differentiation. C.L. and Z.S. did the immunostaining and Q-PCR. H.W. and Y.G. wrote the manuscript and produced the figures. J.H. and G.P. supervised the experiments and commented on the manuscript. All authors approved the submitted version.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, H., Hu, L., Liu, C. et al. 5-HT2 receptors mediate functional modulation of GABAa receptors and inhibitory synaptic transmissions in human iPS-derived neurons. Sci Rep 6, 20033 (2016). https://doi.org/10.1038/srep20033
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20033
- Springer Nature Limited