Abstract
The ability of human metaphase-II arrested eggs to activate following fertilisation declines with advancing maternal age. Egg activation is triggered by repetitive increases in intracellular Ca2+ concentration ([Ca2+]i) in the ooplasm as a result of sperm-egg fusion. We therefore hypothesised that eggs from older females feature a reduced ability to mount appropriate Ca2+ responses at fertilisation. To test this hypothesis we performed the first examination of Ca2+ dynamics in eggs from young and naturally-aged mice. Strikingly, we find that Ca2+ stores and resting [Ca2+]i are unchanged with age. Although eggs from aged mice feature a reduced ability to replenish intracellular Ca2+ stores following depletion, this difference had no effect on the duration, number, or amplitude of Ca2+ oscillations following intracytoplasmic sperm injection or expression of phospholipase C zeta. In contrast, we describe a substantial reduction in the frequency and duration of oscillations in aged eggs upon parthenogenetic activation with SrCl2. We conclude that the ability to mount and respond to an appropriate Ca2+ signal at fertilisation is largely unchanged by advancing maternal age, but subtle changes in Ca2+ handling occur that may have more substantial impacts upon commonly used means of parthenogenetic activation.
Similar content being viewed by others
Introduction
Oocyte aging is a complex multifactorial process resulting in deterioration of oocyte viability with advancing maternal age. Perhaps the best known aspect of oocyte aging is oocyte aneuploidy, in which an age–related increase in chromosome segregation errors during meiosis is associated with a decline in female fertility1,2,3. However, given their extraordinary protracted meiotic arrest, up to 45 years in humans, it is not surprising that oocytes are susceptible to other cellular dysfunctions with age. For example, clinical reports reveal that the ability of the oocyte to resume meiosis and begin embryo development following insemination4 or routine intracytoplasmic sperm injection (ICSI)5,6 also declines with advancing maternal age. Why oocytes from older women are less likely to respond appropriately to sperm is unknown.
At the time of ovulation mammalian oocytes become arrested at metaphase-II (MII) of meiosis, at which point the oocyte can be referred to as an egg. In mammals, liberation from MII arrest and initiation of the embryonic developmental program, commonly termed egg activation, occurs at fertilisation as a result of a spatiotemporal series of increases in intracellular Ca2+ concentration ([Ca2+]i) within the egg cytoplasm known as Ca2+ oscillations. Ca2+ oscillations are initiated by a sperm-borne soluble protein, most probably phospholipase C zeta (PLCζ)7 and persist for several hours until the time of pronucleus formation8. Each Ca2+ oscillation is generated by inositol 1,4,5-trisphosphate (InsP3)-mediated Ca2+ release from the endoplasmic reticulum (ER), the main intracellular Ca2+ store in the egg, followed by a influx of extracellular Ca2+ to replenish stores in time for the next oscillation9. Ca2+ oscillations are not only necessary and sufficient for egg activation10,11, but their temporal dynamics also influence the developmental potential of the resulting embryo12. Importantly, in clinical settings when egg activation fails following ICSI, eggs can sometimes be artificially activated by procedures that promote Ca2+ entry into the egg13. Yet, whether failed egg activation with advancing maternal age is caused by dysregulation of egg Ca2+ dynamics is not known.
A growing body of evidence, largely from naturally-aged mice, reveal changes in Ca2+ handling in various somatic cell types with age. This so-called calcium-hypothesis of aging is considered a major mechanism of age-related somatic cell dysfunction and has particularly been studied with respect to the pathological pathways underlying Alzheimer’s disease14,15,16. Here we hypothesised that mammalian eggs might be similarly vulnerable to Ca2+ dysregulation with advancing maternal age and that this would provide a mechanistic explanation for the reduced ability of eggs from older women to resume development after insemination or ICSI. Although some studies show that the latency period between ovulation and fertilisation either in vivo or in vitro, sometimes referred to as “post-ovulatory aging”, perturbs Ca2+ oscillations at fertilisation17,18,19, whether maternal age affects the egg Ca2+ response is unknown. Therefore, using live fluorescence imaging, we performed the first examination of Ca2+ dynamics in eggs from young and naturally-aged mice. Perhaps surprisingly we find that, in contrast to somatic cells, [Ca2+]i homeostasis remains relatively stable with advancing age, with naturally-aged eggs capable of mounting and responding to an appropriate Ca2+ signal at fertilisation. Instead, our experiments suggest mammalian eggs are adapted to avoid age-related Ca2+ signalling defects that might jeopardise reproductive capacity.
Results
Resting [Ca2+]i and thapsigargin-sensitive stores are unchanged with age
Naturally-aged mice are an established model of in vivo aging and have been extensively used to identify age-related changes in numerous cell types, including eggs. Here we used CD1 mice at 12–15 months old, which have been well characterised as a model for maternal age-related egg defects, as measured by aneuploidy levels20,21,22,23. Control MII eggs from young CD1 mice and MII eggs from naturally-aged CD1 mice were collected contemporaneously 14 hours after hCG administration. We found that egg yields decreased markedly with age from 19.4 ± 1.2 per mouse in young mice to 2.8 ± 0.4 in aged mice (P < 0.0001) (Fig. 1a). These numbers are consistent with reports in the same mouse strain20 and follow a similar rate of decline in the human ovary24. Eggs from young and naturally-aged mice were morphologically indistinguishable (Fig. 1b). Specifically, young and aged eggs were identical in size, with mean diameters of 75.2 ± 0.4 μm and 75.5 ± 0.3 μm (P = 0.54) respectively, featured clear round zonae pellucidae, easily observable first polar bodies contained within perivitelline spacing of comparable size and showed no obvious differences in egg shape or levels of cytoplasmic granularity (Fig. 1b), which is in line with morphological reports on ovulated human eggs of advanced maternal age25. It is important to note that we also observed a small number of eggs from both young and aged mice that lacked cumulus cells at the time of collection and were reduced in size and darker in appearance. These are commonly observed following a standard superovulation procedure and are presumed to be a result of an earlier ovulation and therefore were not used in this study.
We first set out to determine if advanced age affects the ability to maintain appropriate cytosolic [Ca2+]i. MII eggs from young and naturally-aged mice were collected, microinjected with Calcium GreenTM-1 dextran and Rhodamine B dextran as a ratiometric imaging system and imaged contemporaneously in a side-by-side manner using time-lapse epifluorescence microscopy in normal Ca2+ -containing media (Fig. 1b). The baseline mean fluorescence ratios of young and aged eggs were 0.97 ± 0.01 and 0.96 ± 0.02 (P = 0.47), respectively, revealing no significant difference in cytosolic [Ca2+]i (Fig. 1c). Next we wanted to analyse the contents of intracellular Ca2+ stores. In pilot experiments we compared the Ca2+ response following treatment with ionomycin, a widely used Ca2+ ionophore and found no difference in intracellular Ca2+ content between young and aged eggs (see Supplementary Fig. S1 online). Therefore, to determine more specifically whether ER Ca2+ stores are altered with age, we treated eggs with thapsigargin, a specific inhibitor of the ER Ca2+ -ATPase, that has been used extensively in oocytes26,27. Thapsigargin causes a rise in [Ca2+]i (Fig. 2a,b), which provides an estimate of the amount of Ca2+ contained within thapsigargin-sensitive ER stores in the egg ([Ca2+]ER). The calculated area under the curve for young and aged eggs was 252.8 ± 14.4 and 224.8 ± 16.1 (P = 0.11), respectively and peak fold-change fluorescence levels of young and aged eggs were 1.69 ± 0.02 and 1.65 ± 0.03 (P = 0.34), respectively, revealing no significant difference in [Ca2+]ER stores with egg age (Fig. 2c). Together these data show that cytosolic [Ca2+]i and intracellular [Ca2+]ER stores are maintained with age.
Naturally-aged eggs feature impaired Ca2+ influx
Ca2+ influx from the extracellular milieu is essential to sustain continuous Ca2+ oscillations from fertilisation until pronucleus formation28,29. We therefore tested Ca2+ influx capacity in young and naturally-aged eggs. Following thapsigargin treatment in Ca2+ -free media, normal levels of extracellular Ca2+ were added back to the media (1.7 mM), which triggers an influx of Ca2+ into the egg and is an indication of store refilling (Fig. 2a,b). Strikingly, calculated area under the curve values decreased from 37.24 ± 5.02 in young eggs to 16.36 ± 3.46 in aged (P = 0.0009) and similarly peak fold-change fluorescence levels were reduced from 1.2 ± 0.02 to 1.1 ± 0.01 in young and aged eggs, respectively (P = 0.0002) (Fig. 2d). These data show that MII eggs from mice of advanced age possess a markedly reduced ability to replenish Ca2+ from the extracellular environment.
ICSI- and PLCζ -induced Ca2+ oscillatory patterns are maintained with age
Normal sperm-initiated Ca2+ oscillations are dependent upon Ca2+ influx and cease prematurely in the absence of external Ca2+30,31. We therefore wondered whether the reduced Ca2+ influx capacity that we had detected in naturally-aged eggs would affect the dynamics of Ca2+ oscillations. Thus we performed ICSI on young and aged eggs and recorded [Ca2+]i every 10 seconds for 3.5 hours using epifluorescence imaging. ICSI was chosen for this series of experiments to exclude the possibility of polyspermic fertilisation, which can occasionally occur with standard in vitro fertilisation and could confound the results. As in all experiments young and aged eggs were imaged in a side-by-side contemporaneous manner. Ca2+ oscillations in all eggs persisted for the duration of imaging (Fig. 3a,b). Young and aged eggs displayed a similar number of Ca2+ oscillations, with 3.50 ± 0.23 and 3.30 ± 0.26 Ca2+ spikes (P = 0.61) in the first hour (Fig. 3c) and 5.64 ± 0.37 and 5.20 ± 0.47 Ca2+ spikes (P = 0.46) in the first two hours, respectively (Fig. 3d). Furthermore, peak increases in [Ca2+]i between young and aged eggs were 2.19 ± 0.02 and 2.16 ± 0.02 (P = 0.50), respectively (Fig. 3e), suggesting that aged eggs are capable of mounting Ca2+ oscillations of appropriate amplitude. Together, these data show no significant difference in the ICSI-induced Ca2+ oscillation signature with advanced egg age.
To further examine Ca2+ oscillation patterns in naturally-aged eggs we set out to compare the effect of artificially introducing PLCζ, a physiological sperm-borne trigger of Ca2+ oscillations32,33. PLCζ was introduced by microinjecting cRNA encoding the PLCζ protein tagged with firefly luciferase34. Analogous to ICSI, PLCζ-induced Ca2+ oscillations in all eggs continued for the duration of imaging (Fig. 4a,b). Young and aged eggs elicited 15.42 ± 1.31 and 14.58 ± 1.15 PLCζ-induced Ca2+ spikes (P = 0.97) in the first two hours (Fig. 4c), respectively, revealing that like ICSI, the frequency of PLCζ-induced Ca2+ oscillations are unchanged with egg age. Moreover, there was no significant difference in the amplitude of PLCζ-induced Ca2+ oscillations between young and aged eggs, with fold-change increases in fluorescence ratio calculated as 3.86 ± 0.12 and 4.02 ± 0.23 (P = 0.51), respectively (Fig. 4d). To correlate oscillation number to PLCζ protein expression, we performed a quantitative analysis of total luminescence on an egg-by-egg basis34. Firstly, young and aged eggs featured similar PLCζ protein expression levels with luminescence counts per second in the first hour of oscillations recorded as 2484.0 ± 116.9 and 2396.6 ± 195.2 (P = 0.69), respectively, indicating inherent translational efficiency is not compromised by age (Fig. 4e), consistent with expression of GFP-tagged fusion proteins by mRNA injection in young and aged germinal vesicle stage oocytes in our lab (JH and GF unpublished). Secondly, quadratic regression analysis of luciferase expression showed no relationship between oscillation frequency and protein expression across young and aged eggs, with R2 values calculated at 0.88 and 0.94 (P = 0.56), respectively, indicating that age does not affect the sensitivity of Ca2+ oscillations initiated by PLCζ expression (Fig. 4e). Collectively, our data show the ability to mount an appropriate ICSI- or PLCζ-induced Ca2+ signal is unaffected by advancing maternal age.
Naturally-aged eggs exhibit substantially fewer oscillations in response to parthenogenetic activation by SrCl2
Parthenogenetic agents are used in clinical settings to artificially activate eggs that do not spontaneously activate following ICSI35 and are commonly used in an experimental context in many mammalian systems26,36. We therefore examined oscillation competence in naturally-aged eggs following activation with SrCl2, a parthenogenetic agent that is widely used in mouse and evokes repetitive oscillations similar to fertilisation37,38,39. To do this, we incubated young and aged eggs side-by-side in SrCl2-containing media and recorded oscillations for 3.5 hours (Fig. 5a,b). SrCl2-induced oscillations were similar in amplitude in young and aged eggs (P = 0.7) (Fig. 5d). Notably however, unlike ICSI- and PLCζ-induced oscillations, 38.5% of aged eggs prematurely ceased oscillating prior to the end of imaging (Fig. 5b), compared to only 7.5% of young eggs, showing that SrCl2-induced oscillation longevity is compromised with age. Overall, aged eggs displayed a 57% reduction in oscillation number compared to young, with 2.77 ± 0.41 oscillations in the first two hours compared to 6.45 ± 0.29 oscillations in young eggs (P < 0.0001) (Fig. 5c). Thus aged eggs feature a reduced ability to mount appropriate oscillatory responses following parthenogenetic activation with SrCl2.
It is known that the cellular events underpinning the egg-to-embryo transition are differentially regulated by a specific number of Ca2+ transients40. Therefore to test whether the reduced longevity and frequency of Sr2+ -induced oscillations observed in aged eggs affected the temporal sequence of egg activation, we recorded the timings of second polar body (Pb2) extrusion and pronucleus (PN) formation in young and aged eggs and correlated the timing of these events with total oscillation number on an individual egg-by-egg basis (Fig. 6a). Regardless of oscillation number, the timing of Pb2 extrusion and PN formation was similar across young and aged eggs (Fig. 6b,c). Together these findings show that advanced maternal age affects the ability of eggs to mount a normal oscillatory pattern in response to parthenogenetic activation with SrCl2, but this does not impact the temporal kinetics of egg activation.
Discussion
Egg activation failure is a prominent barrier to the success of ICSI in the clinic41 and is associated with advancing maternal age5,6. Since the Ca2+ signal at fertilisation is both necessary and sufficient for egg activation, we hypothesised that eggs might be vulnerable to Ca2+ dysregulation with advancing maternal age. Although the effect of time after ovulation (often referred to as post-ovulatory aging) on egg Ca2+ regulation has previously been studied17,18,19, the effect of maternal age was unknown. We took advantage of the recent characterisation of naturally-aged mice as a model of maternal egg aging and found that although Ca2+ influx capacity is markedly impaired, the ability to mount a normal sperm- and PLCζ-induced Ca2+ oscillatory signature is largely unchanged. Interestingly, on the other hand, we find that eggs of advanced age elicit an abnormal oscillatory pattern in response to artificial activation with SrCl2. The discussion that follows will therefore first focus on the effect of aging on egg Ca2+ handling and then comment on the effect of aging upon egg activation in a clinical context.
Whilst it is well established that oocyte aneuploidy is a major consequence of oocyte aging and a leading cause of age-related infertility1,42, the prolonged lifespan of oocytes provides ample opportunity for other cellular defects to develop. Indeed age-related changes in mitochondrial DNA arrangements43, mitochondrial function44,45 and gene expression profiles46,47 have been reported. Here we report that a maternal age-related deterioration in Ca2+ influx occurs in eggs. Reductions in store-operated Ca2+ entry (SOCE) activity and expression of key SOCE-specific proteins, Stim1 and Orai1, are a characteristic feature of naturally-aged mitotic cells48,49, however their behaviour during egg aging is not known. We speculate that an age-related deterioration in SOCE channel expression or activity likely explains our observation of impaired influx in aged eggs following ER store depletion. Intriguingly however, our data show that Ca2+ oscillations remain robust and unchanged in eggs from older females in the face of substantially reduced store-operated Ca2+ influx. Moreover no obvious difference in the pacemaker potential was observed, though more detailed analysis of this aspect of the Ca2+ oscillation would benefit from more rapid sampling. Whilst fertilisation in nominally Ca2+ -free media results in oscillation termination30, revealing that at least some extracellular Ca2+ is essential for ongoing long-term oscillations, our data suggest that Ca2+ oscillations can persist provided a threshold amount of Ca2+ influx is available. Consistent with this, SOCE inhibition at fertilisation has no effect on the Ca2+ oscillatory response28, similar to the post-fertilisation Ca2+ oscillation phenotype of the aged eggs in this study. Nonetheless an unavoidable conclusion of our data is that, in contrast to other cell types in which an age-related dysregulation of Ca2+ homeostasis is common14,15, in eggs the ability to generate Ca2+ transients is apparently safeguarded. This difference in the pathogenesis of aging between somatic cells and oocytes may be unique to Ca2+ , as aged oocytes feature other hallmarks of somatic cell aging, such an accumulation of reactive oxygen species50, consistent with the involvement of free radicals in aging51. Whilst detailed analyses of other aspects of ionic homeostasis with oocyte age, such as pH regulation52, remain to be studied, we speculate that oocytes are specifically protected from Ca2+ aging, as a mechanism of avoiding endangering the germline.
Whereas we found little effect of natural maternal aging upon sperm- or PLCζ-induced Ca2+ oscillations, our data reveal that aged eggs exhibit a substantially different oscillatory response following activation with SrCl2 compared to young. Perhaps surprisingly, given its widespread use in mouse eggs as a parthenogenetic agent, exactly how SrCl2 initiates a sustained train of oscillations remains poorly understood. Having entered the egg, SrCl2 is thought to stimulate oscillations by potentiating the InsP3 receptors (InsP3R)39. Recent elegant studies identified that TRVP3, a specific transient receptor potential ion channel, is the major route of SrCl2 entry into mouse oocytes, with TrpV3−/− eggs failing to mount an oscillatory response, presumably as a result of failed access to the InsP3R53. Thus a simple potential explanation is that an age-related reduction in TRVP3 might be responsible both for the inhibition of SrCl2-induced oscillations and for the subtle defects in Ca2+ influx. It is also plausible that defects in downstream signalling such as possible activation of oocyte-resident PLCs39, or the overall redox state of oocytes as a result of age-related oxidative stress44, may comprise SrCl2-induced oscillation competence.
Our results have important implications for our appreciation of the effect of maternal aging on oocyte health in clinical settings. In cases where egg activation fails following ICSI, many clinics attempt artificial egg activation using Ca2+ ionophores such as A2318713. However, these treatments only elicit a single rise in Ca2+ that does not reflect the series of Ca2+ oscillations seen at fertilisation and may be suboptimal for development12. Moreover, despite widespread use, the efficacy of these treatments remains unclear. PLCζ is a physiological agent demonstrated by several laboratories to produce a prolonged series of Ca2+ oscillations in mouse and human eggs similar to that of sperm54, providing strong indications for its eventual use as a therapeutic to rescue egg activation following failed ICSI in the clinic. By carefully calibrating PLCζ expression on an egg-by-egg basis using a luciferase tag, we found that young and aged eggs elicit an identical oscillatory signature, as was also the case for ICSI. Thus, at least in mouse, the ability to respond to PLCζ is not affected by maternal age. These data allude that, should PLCζ be used in clinical situations, it may not be necessary to titre according to maternal age, rather that it might be possible to arrive at a universal dose of PLCζ.
Our data show that egg Ca2+ dynamics are largely maintained with advancing maternal age and therefore do not afford a simple explanation for age-related failed activation following assisted reproductive procedures. One possible alternative is that whilst the gross Ca2+ oscillatory signature is unchanged, the downstream molecular messengers decoding the Ca2+ signal could be compromised. For example, the Ca2+ signal is instrumental in inactivation of cytostatic factor (CSF), the cytoplasmic activity that maintains MII arrest. CSF inactivation occurs via Ca2+ -dependent calmodulin-dependent kinase II activity and is entirely dependent upon Ca2+ oscillations55. We consider that, at least in mouse, a defect in Ca2+ -sensing appears unlikely, since egg activation was unaffected by maternal age. This was even the case for SrCl2, where oscillation frequency decreased with age, but careful analysis revealed no difference in the temporal dynamics of polar body and pronucleus formation, though we cannot exclude the possibility of more subtle effects of aging on the subsequent kinetics of preimplantation embryonic development. Thus, whilst in our experiments Ca2+ dysregulation does not explain failed activation, the question of what causes egg activation failure following ICSI in maternally aged eggs remains unanswered. The role of paternal age in ICSI failure is often hard to analyse in clinical studies4,5,6. Investigations into PLCζ protein levels with paternal age would therefore be extremely valuable, as multiple studies show that human sperm with little to no PLCζ expression fail to initiate egg activation56,57. Alternatively, zinc has recently emerged as an intriguing potential determinant of egg fertilisation success, sperm-egg fusion inducing a series of ‘zinc sparks’ that occur rapidly in response to Ca2+ oscillations58. Examination of zinc dynamics and other aspects of ionic homeostasis with advancing maternal age may provide insight into why fertilisation failure increases in older women. Finally, though our study shows conclusively that Ca2+ dysregulation is minor in a well established model of mammalian oocyte aging, we cannot formally exclude that Ca2+ perturbations may be more severe in human eggs from aged patients in suboptimal culture conditions, where fertilisations are performed at various times after egg collection (post ovulatory aging). Future studies of Ca2+ responsiveness in failed ICSI cases under controlled conditions in the clinic will thus be invaluable.
In conclusion, our data show advanced maternal age leads to a deterioration in the oocyte’s ability to replenish Ca2+ from the extracellular environment, however this has no effect on overall physiological output of Ca2+ oscillations. We speculate that, unlike some somatic cells, oocytes may have adapted a defence mechanism to prevent Ca2+ dysregulation in the germline, to avoid jeopardising reproductive capacity.
Methods
Egg collection
MII eggs were collected from the oviducts of 3 month old (referred to as ‘young’ eggs) and 12–15 month old (‘naturally-aged’ eggs) female Swiss CD1 mice (Harlan and Charles River Laboratories) following stimulation with pregnant mare’s serum gonadotrophin (i.p., young 5 IU, aged 10 IU) and superovulation with human chorionic gonadotropin (hCG) (i.p., young 5 IU, aged 10 IU) at 48 hour intervals. Naturally-aged mice were acquired as retired breeders at 7–9 months of age and housed for a further 5–7 months. Mice were sacrificed 14 hours post-hCG and cumulus masses were released into M2 media containing hyaluronidase (0.3 mg/ml) by rupture of the oviduct with a 27-gauge needle. Cumulus-free eggs were washed through three drops of M2 under mineral oil at 37 °C. All experiments were performed with young and naturally-aged eggs collected contemporaneously. All animal experiments were approved by the Comité Institutionnel de Protection des Animaux du CHUM (CIPA) or the UK Home Office. All animal experiments were performed in accordance with relevant guidelines and regulations of CIPA or the UK Home Office.
Microinjection
Eggs were microinjected using Narishige manipulators mounted on a Leica DMI4000 inverted microscope, as described previously59. Briefly, eggs were placed in a drop of M2 under oil and immobilised using a holding pipette. The injection pipette was inserted into the egg cytoplasm and the oolemma breached using a short pulse of negative capacitance from an intracellular electrometer (Warner Instruments). A controlled fixed-pressure injection that displaced a sphere of cytoplasm with a diameter of ~10 μm (<5% total egg volume) was then delivered using a Picopump (WPI, Sarasota, FL). Following injection, eggs were left to recover for ~30 minutes in M2 under oil at 37 °C.
Intracellular Ca2+ measurements
To measure [Ca2+]i, eggs were co-microinjected with Calcium GreenTM-1 dextran (1 mM) and Rhodamine B dextran (1 mM), or incubated in M2 containing Cal-520 AM (5 μM) for 30 minutes at 37 °C before the experiment. Where necessary, the zona was removed with brief exposure to acidified Tyrode’s solution. Imaging was performed in a glass bottom petri dish heated to 37 °C on a Leica DMI4000 inverted epifluorescence microscope. Images were acquired every 10 seconds and captured for up to 3.5 hours. Ratiometric calculations were performed by dividing Calcium GreenTM-1 dextran values by Rhodamine B after background subtraction. Cal-520 AM fluorescence and the luminescent signal from firefly luciferase were imaged concurrently in the same eggs using a Zeiss Axiovert S100TV microscope. Cal-520 AM was excited from 450–490 nm. The fluorescent and luminescent emission light was collected through the same filter set at 515 nm. The luminescence values presented represent the number of measured photon counts per second.
Ca2+ assays and egg activation
To examine [Ca2+]ER content, thapsigargin, an inhibitor of the ER Ca2+ -ATPase, was pipetted directly into the petri dish (final concentration 10 μM). After [Ca2+]i returned to baseline, CaCl2 (1.7 mM) was added to measure Ca2+ influx. ICSI was performed as described60 using Piezo-actuated micromanipulation in M2 media containing cytochalasin B (5 μg/ml). Spermatozoa were collected from caudal epididymides of proven-breeder male CD1 mice and a sperm suspension created with 12% PVP360 (w/v) (Sigma) to immobilise for Piezo-pulsed decapitation. PLCζ cRNA tagged with firefly luciferase34 was introduced by microinjection and strontium activation achieved by incubating eggs in SrCl2-containing (10 mM) Ca2+ -free M2 media.
Data analysis and statistics
Ratiometric images were analysed using Fiji software (Image J) (http://fiji.sc/Fiji) and fluorescence intensity from each egg was plotted against time. All changes in [Ca2+]i were statistically analysed using GraphPad Prism Software version 6. The normality of all data sets was assessed using the Shapiro-Wilk test. Data were then analysed using either a Student’s two-tailed unpaired t test (parametric data) or a Mann-Whitney test (nonparametric data) as appropriate. Statistical significance was defined as P < 0.05. Actual P values are presented except where P < 0.0001. Data is presented as mean ± standard error (s.e.m.).
Additional Information
How to cite this article: Haverfield, J. et al. Ca2+ dynamics in oocytes from naturally-aged mice. Sci. Rep. 6, 19357; doi: 10.1038/srep19357 (2016).
References
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 (2001).
Kuliev, A., Zlatopolsky, Z., Kirillova, I., Spivakova, J. & Cieslak Janzen, J. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod. Biomed. Online 22, 2–8 (2011).
Howe, K. & FitzHarris, G. Recent insights into spindle function in mammalian oocytes and early embryos. Biol. Reprod. 89, 71 (2013).
Schwartz, D. & Mayaux, M. J. Female fecundity as a function of age. N. Engl. J. Med. 306, 404–406 (1982).
Bhattacharya, S., Maheshwari, A. & Mollison, J. Factors associated with failed treatment: an analysis of 121,744 women embarking on their first IVF cycles. PLoS ONE 8, e82249 (2013).
Devroey, P. et al. Female age predicts embryonic implantation after ICSI: a case-controlled study. Hum. Reprod. 11, 1324–1327 (1996).
Swann, K., Larman, M. G., Saunders, C. M. & Lai, F. A. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction 127, 431–439 (2004).
Marangos, P., FitzHarris, G. & Carroll, J. Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development 130, 1461–1472 (2003).
Wakai, T. & Fissore, R. A. Ca2+ homeostasis and regulation of ER Ca2+ in mammalian oocytes/eggs. Cell Calcium 53, 63–67 (2013).
Kline, D. & Kline, J. T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 149, 80–89 (1992).
Fulton, B. P. & Whittingham, D. G. Activation of mammalian oocytes by intracellular injection of calcium. Nature 273, 149–151 (1978).
Ozil, J. P., Banrezes, B., Tóth, S., Pan, H. & Schultz, R. M. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev. Biol. 300, 534–544 (2006).
Ebner, T. et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod. Biomed. Online 30, 359–365 (2015).
Thibault, O., Gant, J. C. & Landfield, P. W. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6, 307–317 (2007).
Khachaturian, Z. S. Calcium hypothesis of Alzheimer’s disease and brain aging. Ann. N. Y. Acad. Sci. 747, 1–11 (1994).
Hartmann, H., Eckert, A., Velbinger, K., Rewsin, M. & Müller, W. E. Down-regulation of free intracellular calcium in dissociated brain cells of aged mice and rats. Life Sci. 59, 435–449 (1996).
Igarashi, H., Takahashi, E., Hiroi, M. & Doi, K. Aging-related changes in calcium oscillations in fertilized mouse oocytes. Mol. Reprod. Dev. 48, 383–390 (1997).
Takahashi, T., Takahashi, E., Igarashi, H., Tezuka, N. & Kurachi, H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol. Reprod. Dev. 66, 143–152 (2003).
Jones, K. T. & Whittingham, D. G. A comparison of sperm- and IP3-induced Ca2+ release in activated and aging mouse oocytes. Dev. Biol. 178, 229–237 (1996).
Merriman, J. A., Jennings, P. C., McLaughlin, E. A. & Jones, K. T. Effect of aging on superovulation efficiency, aneuploidy rates and sister chromatid cohesion in mice aged up to 15 months. Biol. Reprod. 86, 49 (2012).
Yun, Y., Lane, S. I. R. & Jones, K. T. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development 141, 199–208 (2014).
Jiao, Z.-X., Xu, M. & Woodruff, T. K. Age-related increase in aneuploidy and alteration of gene expression in mouse first polar bodies. J. Assist. Reprod. Genet. 31, 731–737 (2014).
Sebestova, J., Danylevska, A., Novakova, L., Kubelka, M. & Anger, M. Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle 11, 3011–3018 (2012).
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil. Steril. 101, 633–4 (2014).
Figueira, R. de C. S. et al. Metaphase II human oocyte morphology: contributing factors and effects on fertilization potential and embryo developmental ability in ICSI cycles. Fertil. Steril. 94, 1115–1117 (2010).
Jones, K. T., Carroll, J. & Whittingham, D. G. Ionomycin, thapsigargin, ryanodine and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J. Biol. Chem. 270, 6671–6677 (1995).
Kline, D. & Kline, J. T. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J. Biol. Chem. 267, 17624–17630 (1992).
Miao, Y. L., Stein, P., Jefferson, W. N., Padilla-Banks, E. & Williams, C. J. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc. Natl. Acad. Sci. USA 109, 4169–4174 (2012).
Cheon, B., Lee, H. C., Wakai, T. & Fissore, R. A. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol. Biol. Cell 24, 1396–1410 (2013).
Igusa, Y. & Miyazaki, S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J. Physiol. 340, 611–632 (1983).
Winston, N. J., McGuinness, O., Johnson, M. H. & Maro, B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J. Cell Sci. 108(Pt 1), 143–151 (1995).
Saunders, C. M. et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129, 3533–3544 (2002).
Rogers, N. T. et al. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 128, 697–702 (2004).
Swann, K., Campbell, K., Yu, Y., Saunders, C. & Lai, F. A. Use of luciferase chimaera to monitor PLCzeta expression in mouse eggs. Methods Mol. Biol. 518, 17–29 (2009).
Sfontouris, I. A. et al. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Hum. Reprod. 30, 1831–1841 (2015).
Fernandes, C. B. et al. Artificial activation of bovine and equine oocytes with cycloheximide, roscovitine, strontium, or 6-dimethylaminopurine in low or high calcium concentrations. Zygote 22, 387–394 (2014).
Tomashov-Matar, R. et al. Strontium-induced rat egg activation. Reproduction 130, 467–474 (2005).
Méo, S. C., Leal, C. L. V. & Garcia, J. M. Activation and early parthenogenesis of bovine oocytes treated with ethanol and strontium. Anim. Reprod. Sci. 81, 35–46 (2004).
Zhang, D. et al. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors and requires activation of phospholipase and the synergistic action of InsP3. Hum. Reprod. 20, 3053–3061 (2005).
Ducibella, T. et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev. Biol. 250, 280–291 (2002).
Shinar, S. et al. Total fertilization failure in intra-cytoplasmic sperm injection cycles-classification and management. Gynecol Endocrinol. 30, 593–596 (2014).
Jones, K. T. & Lane, S. I. R. Molecular causes of aneuploidy in mammalian eggs. Development 140, 3719–3730 (2013).
Barritt, J. A., Cohen, J. & Brenner, C. A. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod. Biomed. Online 1, 96–100 (2000).
Eichenlaub-Ritter, U., Wieczorek, M., Lüke, S. & Seidel, T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 11, 783–796 (2011).
Udagawa, O. et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr. Biol. 24, 2451–2458 (2014).
Grøndahl, M. L. et al. Gene expression profiles of single human mature oocytes in relation to age. Hum. Reprod. 25, 957–968 (2010).
Pan, H., Ma, P., Zhu, W. & Schultz, R. M. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 316, 397–407 (2008).
Shen, B. et al. Attenuated mesangial cell proliferation related to store-operated Ca2+ entry in aged rat: the role of STIM 1 and Orai 1. Age 35, 2193–2202 (2013).
Yang, Y. et al. Contrasting Patterns of Agonist-induced Store-operated Ca2+ Entry and Vasoconstriction in Mesenteric Arteries and Aorta With Aging. J. Cardiovasc. Pharmacol. 65, 571–578 (2015).
Tarín, J. J. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol. Hum. Reprod. 2, 717–724 (1996).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
FitzHarris, G. & Baltz, J. M. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction 138, 619–627 (2009).
Carvacho, I., Lee, H. C., Fissore, R. A. & Clapham, D. E. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 5, 1375–1386 (2013).
Swann, K. & Lai, F. A. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium 53, 55–62 (2013).
Lorca, T. et al. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366, 270–273 (1993).
Yoon, S. Y. et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J. Clin. Invest. 118, 3671–3681 (2008).
Durban, M. et al. PLCζ disruption with complete fertilization failure in normozoospermia. J. Assist. Reprod. Genet. 32, 879–886 (2015).
Que, E. L. et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 7, 130–139 (2015).
FitzHarris, G. A shift from kinesin 5-dependent metaphase spindle function during preimplantation development in mouse. Development 136, 2111–2119 (2009).
Yoshida, N. & Perry, A. C. Piezo-actuated mouse intracytoplasmic sperm injection (ICSI). Nat. Protoc. 2, 296–304 (2007).
Acknowledgements
This work was supported by Project Grants from Fondation J-Louis Lévesque (Canada), the Canadian Institutes of Health Research and the Medical Research Council (UK) to GF. JH is supported by a Postdoctoral Fellowship from The Lalor Foundation. MN is supported by a European Union FP7 Marie-Curie Intra-European Fellowship (628634). We thank Randa Sanusi for excellent technical assistance.
Author information
Authors and Affiliations
Contributions
J.H. and G.F. designed the study and wrote the manuscript. J.H. performed experiments shown in Figs 1,2,3,5 and 6. J.H. analysed the results for Figs 1, 2, 3, 4, 5, 6. S.N. performed experiments shown in Fig. 3. E.T., D.L., M.N., F.A.L. and K.S. designed and performed the experiments for Fig. 4. All authors reviewed the manuscript.
Cytosolic [Ca2+]i concentrations are unchanged with maternal age.
(a) Comparison of MII egg numbers collected from young and naturally-aged mice. Note the marked decrease in egg yields with advancing maternal age (P < 0.0001). Data collected from 20 independent experiments and presented as average number per mouse (n = 87 aged mice, n = 37 young mice). (b) Top panel: Young aged eggs were imaged side-by-side for all experiments. Bottom panel: Representative ratiometric image of young and aged eggs injected with Calcium GreenTM-1 dextran and Rhodamine B dextran. (c) Quantification of cytosolic [Ca2+]i in young (n = 34) and aged (n = 22) eggs (Ca2+ -containing media) revealed no difference with maternal age (P = 0.47). Data collected from 4 independent experiments. ns indicates not statistically significant. a.u. represents arbitrary units. Error bars are s.e.m.
Naturally-aged eggs feature a reduced Ca2+ influx capacity.
(a) A typical Ca2+ response to thapsigargin followed by Ca2+ add-back is demonstrated with pseudocoloured time-lapse images, with warmer colours indicating higher [Ca2+]i. Numbers in the top left of each image panel represent minutes (mins). (b) Representative Ca2+ response curve in MII eggs from young and naturally-aged mice after treatment with thapsigargin (10 μm) followed by Ca2+ (1.7 mM) add-back. The Ca2+ add-back response curve has been magnified (grey box outline). (c) Quantitative analyses of the thapsigargin response in young (n = 18) and aged (n = 10) eggs. Area under the curve (AUC) and fold-change fluorescence ratio calculations revealed maternal age does not affect thapsigargin-sensitive [Ca2+]ER stores (P = 0.11 and P = 0.34, respectively). (d) Quantitative analyses of the Ca2+ -add back response in young (n = 17) and aged (n = 10) eggs. AUC and fold-change fluorescence calculations showed Ca2+ influx capacity is reduced with maternal age (P = 0.0009 and P = 0.0002, respectively). Experiments performed in Ca2+ -free media. ns indicates not statistically significant. a.u. represents arbitrary units. Error bars are s.e.m.
ICSI-induced [Ca2+]i oscillations are unchanged with maternal age.
(a,b) Typical Ca2+ oscillatory patterns in young and naturally-aged eggs following ICSI. (c,d) Quantification of the number of Ca2+ spikes in the first hour (c) and first two hours (d) in young (n = 14) and aged (n = 10) eggs revealed no difference in oscillation frequency (P =0.61 first hour, P = 0.46 first two hours), indicating that aged eggs are capable of mounting and responding to an appropriate Ca2+ signal at fertilisation. (e) Quantification of fold-change fluorescence in young (n = 14) and aged (n = 10) eggs revealed the amplitude of Ca2+ oscillations is unaffected by age (P = 0.50). Experiments performed in Ca2+ -containing media. ns indicates not statistically significant. a.u. represents arbitrary units. Error bars are s.e.m.
Naturally-aged eggs show a marked reduction in oscillation number following parthenogenetic activation with SrCl2.
(a,b) Typical SrCl2-induced oscillatory patterns in young and naturally-aged eggs. (b) Note that two different oscillatory responses were observed for aged eggs. i) 61.5% of aged eggs oscillated for the duration of imaging (left panel). ii) 38.5% of aged eggs ceased oscillating prematurely (right panel). (c) Quantification of the number of oscillations in the first two hours in young (n = 40) and aged (n = 13) eggs revealed a marked reduction in oscillation frequency with maternal age (P < 0.0001). (d) Quantification of the fold-change fluorescence ratio in young (n = 40) and aged (n = 13) eggs revealed oscillation amplitude is unaffected by age (P = 0.7). Experiments performed in Ca2+ -free media. a.u. represents arbitrary units. ns indicates not statistically significant. Error bars are s.e.m.
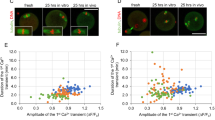
Egg activation kinetics are not affected by maternal age.
(a) Timing of second polar body extrusion (Pb2) and pronucleus formation (PN) in young and aged eggs activated with SrCl2. Note that the timing of both egg activation events are similar, regardless of egg age. (b) Correlation between second polar body timing and oscillation number (total) between young and aged eggs. (c) Correlation between pronuclear formation timing and oscillation number (total) between young and aged eggs.
PLCζ-induced [Ca2+]i oscillations are unchanged with maternal age.
(a,b) Typical Ca2+ oscillatory pattern in young and naturally-aged eggs following microinjection with PLCζ cRNA. (c) Quantification of the number of Ca2+ spikes in two hours following the first oscillation in young (n = 31) and aged (n = 12) eggs showed that maternal age does not affect the eggs ability to respond to a PLCζ signal at fertilization (P = 0.97). (d) Quantification of the average oscillation amplitude in young (n = 31) and aged (n = 12) eggs showed no difference with age (P = 0.51). (e) Quadratic regression analysis of oscillation number and PLCζ protein expression level (measured as luminescence levels) in young (black) and aged (red) eggs revealed age does not affect the sensitivity of PLCζ-induced Ca2+ oscillations. R2 values are 0.88 and 0.94 (P = 0.56) for young and aged eggs, respectively. Experiments performed in Ca2+ -containing media. F/F0 represents fluorescent intensity relative to baseline. cps represents counts per second. ns indicates not statistically significant. Error bars are s.e.m.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Haverfield, J., Nakagawa, S., Love, D. et al. Ca2+ dynamics in oocytes from naturally-aged mice. Sci Rep 6, 19357 (2016). https://doi.org/10.1038/srep19357
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19357
- Springer Nature Limited
This article is cited by
-
Assessment of intracellular calcium and plasmalemmal membrane potential in cryopreserved metaphase II mouse oocytes
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Calcium Oscillatory Patterns and Oocyte Activation During Fertilization: a Possible Mechanism for Total Fertilization Failure (TFF) in Human In Vitro Fertilization?
Reproductive Sciences (2021)