Abstract
Extracellular purinergic products, particularly ATP, have recently been implicated to regulate immune cell functions and contribute to aberrant inflammatory responses of immune diseases. However, regulation of immune responses of colitis by extracellular ATP and its main receptor, P2 × 7, remains to be elucidated. In the study, we induced murine colitis by feeding mice with 4% dextran sulfate sodium (DSS) and noted dramatically heightened extracellular ATP levels in colon tissues during the progression of experimental colitis. Blockade of ATP release by carbenoxolone (CBX) treatment, or promoting ATP degradation by ATP diphosphohydrolase (apyrase), decreased extracellular ATP levels in colon tissues, attenuated DSS-induced colitis, whereas inhibition of extracellular ATP degradation by sodium metatungstate (POM-1) exacerbated tissue damage in the mice with colitis. Moreover, treatment with inhibitor of P2 × 7 receptor, A438079, decreased NFκB activation and active caspase-1 expression in lamina propria immune cells, downregulated proinflammatory cytokine production in colon tissues and attenuated murine colitis. Collectively, these data suggest extracellular ATP participates in regulation of inflammatory responses of experimental colitis, through P2 × 7 receptor and inflammasome and NFκB signaling, which provides potential alternatives to the current clinical approaches to suppress extracellular ATP-mediated immune responsiveness.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD), containing two main clinical forms inclusive of Crohn’s disease (CD) and ulcerative colitis (UC), is a group of chronic intestinal disorders. Whereas etiology of IBD remains largely unknown, recent studies have noted that immune cells in intestine and colon tissues become excessively activated and contribute to progression of IBD1. It has been reported that immune cells including macrophages and T helper cells determine the course of disease progress1,2. After activation triggered by lumen bacterial antigens in intestine, immune cells will produce a large amount of proinflammatory cytokines e.g., tumor necrosis factor (TNF) and interleukin (IL)-1 β and also release substantial bioactive molecules inclusive of ATP and oxygen species2,3. These cytokines and bioactive molecules further induce immune responses, resulting in sustained inflammation and damage in colon tissues4,5. Inhibition of immune cell function has been suggested as one of the crucial targets for the treatment of immune diseases including IBD6,7.
Recently, the roles of extracellular purinergic products in immune regulation are the topics of high interest. Under inflammatory conditions, once becoming activated, immune cells will release abundant ATP via ATP-releasing channels, particularly pannexin 18,9. Injured cells also secrete ATP into extracellular milieu, resulting in high amount levels of ATP in local tissues and organs8. Meanwhile, ATP regulates immune cell functions through its P2 receptors8. Among those P2 receptors, P2 × 7 receptor is preferentially expressed by immune cells, particularly macrophages10,11. It has been reported that via P2 × 7 receptor, ATP induces a variety of bioactivities of immune cells, e.g., enhancing phagocytosis, inducing inflammasome formation and promoting proinflammatory cytokine release8,10.
Extracellular purinergic products have been recently linked with progression of IBD. Upregulation of ecto-nucleotidase expressions has been reported in colon tissue cells of human CD patients and experimental colitis animals12,13,14,15. Meanwhile, genetic deficiency of ecto-nucleotidases in mice, which exhibit dysregulated extracellular purinergic products, has significant impact on experimental colitis16. Among those purinergic products, ATP is the most likely immune-regulatory molecular with capacity to regulate immune responses and its function in IBD has been recently postulated. Administration of extracellular purines, e.g. ATP, exacerbates experimental colitis, through regulation of IL-17-producing T helper cell (Th17) responses17. Meanwhile, extracellular ATP mediates death of enteric neurons during colitis18 and participates in mast cell-dependent intestinal inflammation19.
Immune cells inclusive of macrophages and Th cells are the major effector and mostly pathogenic cells participating in the pathogenesis of IBD and functionalities of those immune cells are associated with disease activity, course and relapse20. However, regulation of immune cell functionalities by extracellular ATP-P2 × 7 receptor signaling and the related mechanisms remain to be elucidated. In the study, we explored the pivotal role of extracellular ATP levels and its P2 × 7 receptor signaling in the development of colitis.
Results
Extracellular ATP is released in colon tissues during the process of colitis
IBD is characterized by excessive immune cell activation and sustained tissue cell damage21, indicating potential of substantive ATP release into tissue milieu by activated immune cells and injured/dead cells. We thus determined extracellular ATP levels in colon tissues during the progression of DSS-induced colitis. As shown in Fig. 1, extracellular ATP levels in colon tissues of Control mice kept consistent under physical conditions, i.e., from day 1 to day 7. However, in coincidence with histological changes in colon tissues (Supplemental Fig. 1), extracellular ATP levels in colon tissues of colitis mice were dramatically increased since day 5, significantly higher than those Control mice and reach the maximal at day 7. The data indicated significant increase of extracellular ATP levels during the process of colitis in mice.
Blockade of ATP release abrogates DSS induced colitis
Upon a variety of stimulations, ATP can be released by membrane transporters, particularly Pannexin 1 (PANX1)8,22 and thus exhibit its bioactivities. To block ATP release from cells, we employed carbenoxolone (CBX), a specific inhibitor of PANX1 and noted that CBX treatment drastically decreased extracellular ATP levels in colon tissues of colitis mice (Fig. 2a), suggesting that CBX administration significantly inhibits ATP release by colon cells during the process of DSS-induced colitis.
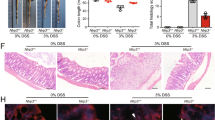
Blockade of ATP release abrogates DSS-induced colitis.
The mice were fed with 4% DSS to induce colitis since day 1. CBX (50 mg/kg body weight) (DSS + CBX) or vehicle (DSS) was injected (i.p) daily into mice from day 3 to day 7. The mice were sacrificed at day 8, ATP levels in colon tissues were determined (n = 5) (a), body weight changes and DAI scores were recorded (n = 10) (b,c), respectively. HE staining was performed and histological scores were graded (n = 10) (d), scale bars: 1000 μm. *p < 0.05; **p < 0.01.
In consistence with decreased extracellular ATP levels, management with CBX attenuated DSS-induced colon inflammation, evidenced with regained body weight (Fig. 2b) and decreased disease activity index (DAI) scores (Fig. 2c). Meanwhile, histological examination showed that DSS colitis mice exhibited severe tissue damage, whereas CBX treatment abrogated DSS-induced histological changes of colitis mice (Fig. 2d).
Blockade of ATP degradation exacerbates tissue damage by colitis
CD39 (also known as Ecto-Nucleoside Triphosphate Diphosphohydrolase 1 or ENTPDase 1), one of ecto-nucleotidases acting to degrade ATP to ADP and AMP, is expressed by a variety of tissue cells, including endothelium, macrophages and a proportion of T cells23. Because CD39 plays pivotal role in regulation of extracellular ATP levels in tissues8, we determined CD39 expression in colon tissues during the process of colitis and noted up-regulated CD39 expression in colon tissues, particularly in lamina propria and submucosal layers, after induction of colitis (Fig. 3a). The data indicate the heightened CD39 expression by immune cells in lamina propria during the progression of intestinal inflammation, in accordance with the previously described observations12,13,15.
Blockade of ATP degradation exacerbates DSS-induced colitis.
After induction of DSS colitis, the mice with colitis were administrated with vehicle, POM1 (10 mg/kg, i.p), or/and ATP (1 mg dissolved in 0.1 mL PBS per mouse, enema) daily since day 3 to day 7. CD39 expression was examined by immunohistochemistry before and 7 days after induction of colitis (a), scale bars: 250 μm. At day 8, ATP levels in colon tissues were determined (n = 5) (b), body weight changes (c) and DAI (d) were recorded and histological scores were graded (e), respectively (n = 10). *p < 0.05; **p < 0.01.
Owing to the potential degradation of extracellular ATP by upregulated CD39 levels in colon tissues, we next studied the effect of inhibition of CD39 bioactivity in intestinal inflammation induced by DSS. Blockade of CD39 bioactivity by sodium metatungstate (POM-1), a pharmacological inhibitor of NTPDase activity, significantly elevated extracellular ATP levels in colon tissues (Fig. 3b). Further measurements showed that, administration of POM-1 resulted in severe disease symptoms including body weight loss (Fig. 3c) and DAI scores (Fig. 3d). Simultaneously, colitis mice with POM1 treatment exhibited worse histological tissue damage in colon, in comparison with the mice of DSS colitis group (Fig. 3e and Supplemental Figure S2). The data indicated that blockade of ATP degradation by POM1 exacerbates tissue damage in mice with colitis.
Enhancement of ATP degradation ameliorates colitis
Since extracellular ATP levels are heightened in colon tissues during the process of colitis, as shown above, we next studied intestinal inflammation by induction of extracellular ATP degradation. As shown in Fig. 4a, treatment of apyrase, an ATP diphosphohydrolase, significantly decreased extracellular ATP levels in colon of mice with colitis. Intriguingly, apyrase exhibited antagonistic effect on POM1 and reduced extracellular ATP levels after its combination treatment with POM1.
Enhancement of ATP degradation ameliorates colitis.
After induction of DSS colitis, the mice with colitis were administrated i.p with vehicle, POM1 (10 mg/kg), or/and apyrase (2000 U/kg) daily since day 3 to day 7. At sacrifice of the mice at day 8, ATP levels in colon tissues were determined (n = 5) (a), body weight changes (b) and DAI (c) were recorded and histological scores were graded (d), respectively (n = 9). *p < 0.05; **p < 0.01.
Bioactivities of apyrase were further examined in vivo. Apyrase treatment significantly facilitated regain of body weight (Fig. 4b), decreased DAI scores (Fig. 4c) and attenuated tissue damage (Fig. 4d and Supplemental Figure S3). In concordance with extracellular ATP levels in colon tissues as shown in Fig. 4a, apyrase treatment, at least partly, counteracted POM1 bioactivity and ameliorated POM1-induced tissue damages. The data indicate that promotion of ATP degradation by apyrase ameliorates DSS induced colitis.
Blockade of P2 × 7 receptor alleviates colitis
DSS-induced colitis is characterized by excessive activation of immune cells, which are responsible for aberrant immune responses in colon tissues24. We thus hypothesized that, through its receptors, extracellular ATP regulated functionalities of immune cells and mediated inflammatory responses in colon as seen in the mice with DSS-induced colitis. Because P2 × 7 receptor is dominantly expressed by lamina propria cells and myenteric plexus18,25,26 and upregulation of P2 × 7 receptor in intestinal mucosa exhibits potential in the pathogenesis of Crohn’s disease25, we explored the mechanisms of ATP-P2 × 7 receptor signaling in regulation of inflammatory responses of experimental colitis.
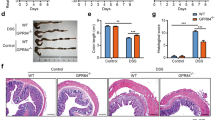
We employed a specific P2 × 7 inhibitor, A43807927, to study the bio-functions of P2 × 7 in the progression of experimental colitis. Administration of A438079 attenuated DSS-induced colitis, as shown by improved body weight regain (Fig. 5a), reduced DAI (Fig. 5b) in DSS+ A438079 group mice, in comparison with those DSS colitis mice. In concomitant with disease symptoms, A438079 treatment alleviated DSS-induced histological tissue damage (Fig. 5c). The data indicate amelioration of experimental colitis by blockade of P2 × 7 receptor.
Blockade of P2 × 7 receptor attenuates DSS induced colitis.
Colitis model was induced since day 1 and A438079 (100 mg/Kg) or vehicle was injected (i.p) into the mice from day 3 to day 7. After the mice were sacrificed at day 8, body weight changes and DAI were recorded (n = 10) (a,b), respectively. HE staining was performed and histological scores were graded (n = 10) (c), scale bars: 1000 μm. *p < 0.05; **p < 0.01.
Because NF-κB has been reported as one of the pivotal factors in regulation of immune responses28, next we have studied whether A438079 treatment could impact on NF-κB activation. As shown in Fig. 6a,c, DSS colitis was characterized by drastic increase of phosphor-NF-κB p65 expression by lamina propria immune cells. However, administration of A438079 significantly decreased phosphor-NF-κB p65 positive cell numbers in lamina propria, suggesting inhibition of NF-κB activation by blockade of P2 × 7 receptor signaling.
A438079 treatment down-regulates NFκB and caspase-1 activation in colon of colitis mice.
Immunohistochemical staining of phosphor-NFκB p65 (a) and caspase-1 p10 (b) was performed in sections from colonic tissues of three group mice: Control, DSS and DSS+ A438079, scale bars: 250 μm. Phosphor-NFκB p65 (c) or caspase-1 p10 (d) positive cell numbers in per 100 cells in lamina propria of the mice were counted and summarized respectively (n = 7). **P < 0.01; ***p < 0.001.
P2 × 7 receptor signaling has been associated with inflammasome pathway29. We thus determined active caspase-1 expression, i.e., p10, a subset of caspase-130, in lamina propria immune cells. During the progression of colitis, active caspase-1 expression was dramatically induced in lamina propria immune cells (Fig. 6b,d). Intriguingly, A438079 treatment significantly diminished active caspase-1 expression in colitis mice (Fig. 6b,d). The data suggest that blockade of P2 × 7 receptor inhibits inflammasome signaling and active caspase-1 expression.
Expression and production of TNF and IL-1 β as the key cytokines mediating inflammatory responses in colitis31, is regulated by NF-κB and inflammasome pathways32,33. As shown above that NF-κB and inflammasome pathways were regulated by blockade of P2 × 7 receptor, we next determined those cytokine levels in colonic tissues. As shown in Fig. 7a,b, A438079 treatment significantly decreased levels of TNF and IL-1β in colonic tissues of the mice with colitis, in concordance with inhibition of NF-κB and inflammasome pathways in colon tissues.
Discussion
In the present study, we discussed the pivotal role of extracellular purinergic signaling, particularly ATP-P2 × 7 receptor signaling, in regulation of inflammatory responses of experimental colitis. Our data have shown that ATP-P2 × 7 receptor signals regulate immune responses during the progression of DSS colitis, likely through mediating NFκB and inflammasome pathways.
Recently, extracellular purinergic products, e.g. ATP in particular and their impacts on inflammatory responses and diseases become the research topic of high interest. ATP has been shown to regulate phagocytosis of immune cells, promote formation of inflammasome, stimulate cytokine secretion, mediate oxygen release and modulate cell proliferation and apoptosis10,11. ATP plays important roles in pathogenesis of a variety of human diseases, through its P2 receptors which comprise P2X and P2Y receptors10,11.
The pathophysiological role of extracellular ATP in regulation of intestinal inflammation has been postulated. It was reported that rectal administration of ATP boosted Th17 responses and exacerbated experimental colitis17. Meanwhile, extracellular ATP induced death of enteric neurons during colitis18 and participated in mast cell-initiated intestinal inflammation19. In this study, we have noted that substantial extracellular ATP levels are associated with progression of DSS colitis. Our further studies have showed that inhibition of ATP release by pharmacological inhibitor of ATP transporters including PANX1, i.e. CBX, dampens extracellular ATP levels, resulting in amelioration of DSS-induced colitis. The data above show the correlation of extracellular ATP levels with inflammation in colon tissues, indicating the particular role of extracellular ATP levels in pathogenesis of DSS-induced colitis. Meanwhile, we noted that CBX exhibited, likely to less extent, impacts on body weight regain, as compared with other parameters inclusive of extracellular ATP levels in colon and DAI, possibly indicating systematically biological effects of CBX or/and inhibition of ATP transporters.
We and others have previously demonstrated that ectonucleotidases, particularly CD39, can regulate extracellular purinergic product levels inclusive of ATP and thus impact immune cell functionalities and immune responses12,15,34. CD39 sequentially hydrolyzes extracellular ATP and ADP to AMP and the latter is ultimately degraded to adenosine by CD73/ecto-5′-nucleotidase35,36. This process is termed purinergic signaling. We next associated extracellular ATP levels regulated by purinergic signaling with intestinal inflammation. Using POM1, the well used inhibitor of CD39, we noted that blockade of CD39 bioactivity restored substantial extracellular ATP levels in colon tissues and exacerbated intestinal inflammation of DSS-induced colitis. Nevertheless, promotion of ATP hydrolysis by apyrase, a soluble chemical with enzymatic activity identical to CD39, drastically decreased extracellular ATP levels and attenuated experimental colitis. The data above addressed dynamic change of extracellular ATP, inhibition of ATP degradation in particular, in mediating the development of DSS-induced colitis. Meanwhile, we noted that rectal administration of ATP had minimal effect on DSS-induced colitis model. Given that DSS-induced colitis/inflammation drastically enhances the expression of CD39 in colon tissues, it is postulated that increase of CD39 levels boosts degradation of extracellular ATP and counteracts, at least partly, the bioactivities of exogenous ATP.
Inflammasome signaling has been linked with inflammation and implicated in immune diseases37. Upon a variety of stimulations of immune cells, inflammasome becomes activated, leading to cleavage of pro-caspase-1 and release of active caspase-1 from inflammasome complex30. Active caspase-1 comprises two heterodimers inclusive of p20 and p10 which can act to catalyze cytokine maturation process inclusive of IL-1β and IL-1830. In this study, we have noted that DSS-induced colitis is characterized by drastic increment of active caspase-1 expression in lamina propria immune cells, whereas blockade of P2 × 7 receptor by its pharmacological inhibitor dampens active caspase-1 levels in lamina propria of colitis. The data indicate that P2 × 7 receptor signaling regulates immune responses via mediating inflammasome pathway.
NFκB is one of important transcription factors controlling immune cell functions and inflammatory responses of immune diseases28. Upon stimulations of lipopolysaccharides (LPS) and other extracellular bacterial antigens, the dimer of NFκB composed of the P65 and P50 subunits translocates to nucleus of immune cells and regulates various target gene expression including a large amount of inflammatory cytokines and chemokines, resulting in sustained inflammatory responses and tissue damage28,38. Inhibition of NFκB activation and signaling can attenuate immune responses and ameliorate immune diseases in animal models6,39,40, which supports the beneficial effects of inhibition of NFκB signaling. In the present study, we have found that blockade of P2 × 7 receptor signal by A438079 inhibits NFκB activation in lamina propria immune cells, which subsequently results in inhibition of immune responses and TNF levels, a key proinflammatory cytokine for the pathogenesis of IBD41.
Finally, we demonstrate that regulation of extracellular ATP levels in tissues abrogates immune responses of DSS-induced colitis. Under physical conditions, extracellular ATP levels are responsible for functionalities of a wide variety of cells, participating in regulation of cell activation, proliferation and death42,43. However, when excessively released, the heightened extracellular ATP levels induce numerous pathological responses and impact on human diseases such as central nervous system diseases and cardiovascular disease44,45. Here we show that treatment with pharmacologically active reagents, particularly CD39 analogs and P2 × 7 receptor antagonists to decrease extracellular ATP levels or/and block ATP/P2 × 7 receptor signaling, diminishes immune responses in DSS-induced colitis, perhaps indicating that regulation of extracellular ATP levels and ATP/P2 × 7 receptor signaling could be explored as a potential therapeutic target.
Collectively, these data taken together suggest associations of extracellular ATP levels and signaling with progression of experimental colitis. We also demonstrate extracellular ATP participates in regulation of inflammatory responses of colitis, through P2 × 7 receptor and inflammasome and NFκB signaling. Our findings help improve the understanding of the molecular control of ATP-P2 × 7 receptor signaling and immune responses, provide possible new alternatives to the current clinical approaches to suppress extracellular ATP-mediated immune responsiveness.
Experimental Procedures
Animals
Female BALB/c mice were provided by the Experimental Animal Center of Nanchang University and fed under specific pathogen-free conditions. 7 to 8 week-old mice were used in the study, weighing approximately 22 g. All protocols for the projects using mice were reviewed and approved by the Institutional Animal Care Committee of Nanchang University. Animal care, use and treatment were in accordance with the guidelines and regulations.
Induction of dextran sulphate sodium (DSS) colitis and treatment of the mice
Acute DSS colitis was induced in BALB/c mice according to the previously published method with minor modification46,47. The mice were fed 4% (w/v) DSS (molecular mass, 36–50 kDa; MP Biomedicals) dissolved in the drinking water on day one. Fresh DSS solution was provided every other day. Control mice drank only distilled water. Disease symptoms of colitis were assessed daily by measurement of body weight, evaluation of stool consistency and detection of bloody stools. Disease severity was scored using a clinical disease activity index (DAI) ranging from 0 to 4, calculated as previously described46 using the following parameters: stool consistency, presence or absence of fecal blood and weight loss. The mice were killed on day eight and the middle section of colon was fixed in 10% formaldehyde–saline. Hematoxylin and eosin stain (HE)-stained sections were graded based on a scoring system modified from a previous study48,49. Histology scoring was performed and a combined score of inflammatory cell infiltration and tissue damage was determined as follows: score 0, normal colonic mucosa and occasional inflammatory cells in the lamina propria; 1, loss of one-third of the crypts; 2, loss of two-thirds of the crypts; 3, the lamina propria is covered with a single layer of epithelium and mild inflammatory cell infiltration is present; and 4, erosions and transmural extension of infiltrate.
The mice were administrated with various reagents from day 3 to day 7. The doses of the reagents were used according to the related literatures16,17,50,51. DMSO was used to dissolve A438079 (Santa Cruz Biotechnology) and other reagents (from Sigma-Aldrich) were dissolved in saline.
Measurement of extracellular ATP levels in colon tissues
Colon tissues were weighted right after sacrifice of the mice and then kept in PBS containing penicillin (200 U/ml), streptomycin (200 μg/ml) and gentamycin (10 μg/ml) for 5 minutes. After three times of washing, the tissues were incubated in 10 times volume (w/v) of PBS at 37 oC for 1 hour. The supernatants were collected for determination of ATP concentrations herein.
ATP levels in the supernatants were determined according to the instruction of Luminescence ATP Detection Assay System (PerkinElmer, Waltham, MA, USA) with minor modification. In brief, 50 μl of supernatants were added into each well, followed by introduction of 50 μl of the substrate solution. After shaking in dark for 10 min, luminescence intensity of each well was determined.
Enzyme-linked immunosorbent assays (ELISA)
Tumor necrosis factor (TNF) and interleukin (IL) 1β levels in homogenates of colon tissues were determined by ELISA, following manufacturer’s instructions (R&D Systems, Inc). Briefly, polyclonal anti-mouse cytokine antibodies were used as capturing antibodies and biotinylated polyclonal anti-mouse cytokine antibodies for detection and the standard curve of cytokines was set up meanwhile. Color changes were determined at 450 and 540 nm, respectively. The final readings were made after subtraction of readings at 540 nm from the readings at 450 nm.
Immunohistochemistry
Colon tissues of mice were taken and fixed immediately in 10% buffered formalin, embedded in paraffin and cut into 4 μm sections. After blockade of inner peroxidase, sections were incubated sequentially with the first antibody solution including rabbit anti-caspase-1 p10 antibody (M-20, from Santa Cruz Biotechnology), anti-NF-κB p65 (phospho S536, from Abcam) antibody, or anti-CD39/ENTPD1 MAb (Clone 495826, from R&D). After three washes in PBS (pH 7·4), the sections were then incubated in secondary goat anti-rabbit immunoglobulin (Ig)G conjugated with peroxidase labeled polymer, prior to colorization using diaminobenzidine reaction and counterstained with haematoxylin. Negative controls were established using rabbit IgG instead of the first antibodies. The sections were evaluated using light microscopy and 100 cells in lamina propria per high power field were calculated for statistical analysis.
Statistical analysis
All data in the text and figures are expressed as mean ± standard deviation. Comparisons of more than two groups were made with a one-way analysis of variance using Tukey’s post hoc test. When appropriate, comparison with two groups was made using Student’s t-test for unpaired data. Differences were considered statistically significant if P 0.05.
Additional Information
How to cite this article: Wan, P. et al. Extracellular ATP mediates inflammatory responses in colitis via P2×7 receptor signaling. Sci. Rep. 6, 19108; doi: 10.1038/srep19108 (2016).
References
Arseneau, K. O., Tamagawa, H., Pizarro, T. T. & Cominelli, F. Innate and adaptive immune responses related to IBD pathogenesis. Curr Gastroenterol Rep 9, 508–512 (2007).
Brown, S. J. & Mayer, L. The Immune Response in Inflammatory Bowel Disease. Am J Gastroenterol 102, 2058–2069 (2007).
Graham, D. B. et al. Functional genomics identifies negative regulatory nodes controlling phagocyte oxidative burst. Nature communications 6, 7838, 10.1038/ncomms8838 (2015).
Yamamoto-Furusho, J. K. & Podolsky, D. K. Innate immunity in inflammatory bowel disease. World J Gastroenterol 13, 5577–5580 (2007).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Bai, A. et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol 80, 1708–1717 (2010).
Bai, A. & Peng, Z. Biological therapies of inflammatory bowel disease. Immunotherapy 2, 727–742, 10.2217/imt.10.51 (2010).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. The New England journal of medicine 367, 2322–2333, 10.1056/NEJMra1205750 (2012).
Deaglio, S. & Robson, S. C. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation and immunity. Advances in pharmacology 61, 301–332, 10.1016/B978-0-12-385526-8.00010-2 (2011).
Wiley, J. S., Sluyter, R., Gu, B. J., Stokes, L. & Fuller, S. J. The human P2X7 receptor and its role in innate immunity. Tissue antigens 78, 321–332, 10.1111/j.1399-0039.2011.01780.x (2011).
Costa-Junior, H. M., Marques-da-Silva, C., Vieira, F. S., Moncao-Ribeiro, L. C. & Coutinho-Silva, R. Lipid metabolism modulation by the P2X7 receptor in the immune system and during the course of infection: new insights into the old view. Purinergic signalling 7, 381–392, 10.1007/s11302-011-9255-6 (2011).
Bai, A. et al. CD39 and CD161 Modulate Th17 Responses in Crohn’s Disease. Journal of immunology 193, 3366–3377, 10.4049/jimmunol.1400346 (2014).
Neshat, S. et al. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol 296, G399–405 (2009).
Doherty, G. A. et al. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 42, 3062–3072 (2012).
Bai, A. et al. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nature communications 6, 8819, 10.1038/ncomms9819 (2015).
Friedman, D. J. et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proceedings of the National Academy of Sciences of the United States of America 106, 16788–16793 (2009).
Atarashi, K. et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008).
Gulbransen, B. D. et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18, 600–604 (2012).
Kurashima, Y. et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nature communications 3, 1034, 10.1038/ncomms2023 (2012).
Dave, M., Papadakis, K. A. & Faubion, W. A., Jr. Immunology of inflammatory bowel disease and molecular targets for biologics. Gastroenterology clinics of North America 43, 405–424, 10.1016/j.gtc.2014.05.003 (2014).
Corridoni, D., Arseneau, K. O. & Cominelli, F. Inflammatory bowel disease. Immunology letters 161, 231–235, 10.1016/j.imlet.2014.04.004 (2014).
Lohman, A. W. et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nature communications 6, 7965, 10.1038/ncomms8965 (2015).
Burnstock, G. & Boeynaems, J. M. Purinergic signalling and immune cells. Purinergic signalling 10, 529–564, 10.1007/s11302-014-9427-2 (2014).
Abdelbaqi, M. et al. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Laboratory investigation; a journal of technical methods and pathology 86, 380–390, 10.1038/labinvest.3700398 (2006).
Neves, A. R. et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflammatory bowel diseases 20, 444–457, 10.1097/01.MIB.0000441201.10454.06 (2014).
Antonioli, L. et al. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PloS one 9, e116253, 10.1371/journal.pone.0116253 (2014).
Yan, Y. et al. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. American journal of physiology. Cell physiology 308, C463–472, 10.1152/ajpcell.00245.2014 (2015).
Gasparini, C. & Feldmann, M. NF-kappaB as a target for modulating inflammatory responses. Current pharmaceutical design 18, 5735–5745 (2012).
Gombault, A., Baron, L. & Couillin, I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Frontiers in immunology 3, 414, 10.3389/fimmu.2012.00414 (2012).
Gattorno, M. & Martini, A. Beyond the NLRP3 inflammasome: autoinflammatory diseases reach adolescence. Arthritis and rheumatism 65, 1137–1147, 10.1002/art.37882 (2013).
Perrier, C. & Rutgeerts, P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy 3, 1341–1352 (2011).
Scharl, M. et al. Protein tyrosine phosphatase N2 regulates TNFalpha-induced signalling and cytokine secretion in human intestinal epithelial cells. Gut 60, 189–197, 10.1136/gut.2010.216606 (2011).
dos Santos, G. et al. Vimentin regulates activation of the NLRP3 inflammasome. Nature communications 6, 6574, \10.1038/ncomms7574 (2015).
Clayton, A., Al-Taei, S., Webber, J., Mason, M. D. & Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. Journal of immunology 187, 676–683 (2011).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine 204, 1257–1265 (2007).
Borsellino, G. et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110, 1225–1232 (2007).
Elliott, E. I. & Sutterwala, F. S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunological reviews 265, 35–52, 10.1111/imr.12286 (2015).
Rakonczay, Z., Jr., Hegyi, P., Takacs, T., McCarroll, J. & Saluja, A. K. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut 57, 259–267 (2008).
Ohira, H. et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb 20, 425–442 (2013).
Bai, A., Guo, Y. & Lu, N. The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand J Immunol 66, 538–545 (2007).
Mahida, Y. R. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflammatory bowel diseases 6, 21–33 (2000).
Nauseef, W. M. Biological roles for the NOX family NADPH oxidases. The Journal of biological chemistry 283, 16961–16965 (2008).
Lee, I. T. & Yang, C. M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84, 581–590 (2012).
Sperlagh, B. & Illes, P. P2X7 receptor: an emerging target in central nervous system diseases. Trends in pharmacological sciences 35, 537–547, \10.1016/j.tips.2014.08.002 (2014).
Menzies, R. I., Unwin, R. J. & Bailey, M. A. Renal P2 receptors and hypertension. Acta physiologica 213, 232–241, 10.1111/apha.12412 (2015).
Bai, A., Lu, N., Guo, Y., Chen, J. & Liu, Z. Modulation of inflammatory response via alpha2-adrenoceptor blockade in acute murine colitis. Clin Exp Immunol 156, 353–362 (2009).
Hong, K. et al. All-trans retinoic acid attenuates experimental colitis through inhibition of NF-kappaB signaling. Immunology letters 162, 34–40, 10.1016/j.imlet.2014.06.011 (2014).
Murthy, S. N. et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Digestive diseases and sciences 38, 1722–1734 (1993).
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M. F. Chemically induced mouse models of intestinal inflammation. Nature protocols 2, 541–546, 10.1038/nprot.2007.41 (2007).
Grenz, A. et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. Faseb J 21, 2863–2873 (2007).
Ying, Y. L. et al. Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Experimental neurology 261, 836–843, 10.1016/j.expneurol.2014.09.007 (2014).
Acknowledgements
This work was supported by National Natural Science Foundation of China, No. 81470828, 81270472, 81070310 (AB) and 81170564 (XL); Principle Investigator Program of Jiangxi Province (AB); Science and Technology Program of Education Department of Jiangxi Province, No. GJJ13138 (AB); and Natural Science Foundation of Jiangxi Province, No. 20142BAB205048 (AB).
Author information
Authors and Affiliations
Contributions
A.B. and X.L. convinced and designed the study. P.W., X.L., Y.X., Y.R., J.C., N.L., Y.G. and A.B. conducted the experiments and performed analyses. P.W., X.L., Y.G. and A.B. wrote and finalized the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wan, P., Liu, X., Xiong, Y. et al. Extracellular ATP mediates inflammatory responses in colitis via P2 × 7 receptor signaling. Sci Rep 6, 19108 (2016). https://doi.org/10.1038/srep19108
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19108
- Springer Nature Limited
This article is cited by
-
Administration of an AAV vector coding for a P2X7-blocking nanobody-based biologic ameliorates colitis in mice
Journal of Nanobiotechnology (2024)
-
Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease
Nature Medicine (2021)
-
Resolution of ulcerative colitis
Seminars in Immunopathology (2019)
-
Zidovudine ameliorates pathology in the mouse model of Duchenne muscular dystrophy via P2RX7 purinoceptor antagonism
Acta Neuropathologica Communications (2018)
-
Inhibition of neddylation ameliorates DSS-induced colitis
Cellular & Molecular Immunology (2018)