Abstract
A longer duration of untreated psychosis (DUP) has been associated with poor clinical outcomes in patients with schizophrenia (SZ); however, it remains unclear whether this is due to neurotoxic effects of psychosis. The purpose of this study was to use near-infrared spectroscopy (NIRS) to investigate the influence of DUP on brain function using two verbal fluency tests (VFTs) in patients with first-episode SZ (FES). A total of 28 FES patients and 29 healthy controls (HC) underwent NIRS during VFTs. Group comparisons of cortical activity were made using two-tailed t-tests and the false discovery rate method. We then examined the associations between DUP and hemodynamic changes in each channel to identify any effects of DUP on brain cortical activity. During the letter VFT, the HC group exhibited significantly greater cortical activations over bilateral frontotemporal regions compared to FES patients. However, this distinction was not observed while performing a category version of the VFT. In addition, no associations between DUP and brain cortical activity were observed in the FES group during either VFT. In conclusion, we did not find an association between DUP and frontotemporal cortical activities. This might be because neurodevelopmental disturbances result in neurocognitive deficits long before psychotic symptoms onset.
Similar content being viewed by others
Introduction
Duration of untreated psychosis (DUP) is defined as the time from the first manifestations of psychotic symptoms to the beginning of antipsychotic treatment1. Longer DUP is associated with poor clinical and functional outcomes1,2, but the mechanism underlying this relationship is unknown. The neurotoxicity hypothesis by Wyatt in 1991 proposed that periods of untreated psychosis are ‘biologically toxic’ to the brain3. This idea was a major impetus for programs designed to shorten DUP to prevent further brain tissue loss or cognitive decline in patients with schizophrenia (SZ) and has also been an important part of rationale for developing early intervention services.
In the past two decades, many studies have investigated the effects of DUP on the brain, but they have yielded conflicting results. Some studies showed a deteriorating effect of DUP on various domains of cognitive functions in patients with first-episode SZ (FES)4,5,6, but most others did not observe negative effects7,8,9,10,11,12. Magnetic resonance imaging (MRI) studies focusing on the issue also reported inconsistent results. Some studies demonstrated that longer DUP had negative effects on brain morphological changes13,14,15,16,17, but others did not18,19,20,21,22,23.
More recently, the ‘neurotoxicity’ concept was questioned by several groups. A critical review by Rund et al. concluded that there was limited evidence for a relationship between DUP and changes in neurocognitive functions or brain structures24. Similarly, Anderson et al.’s review listed minimal evidence of an association between untreated psychosis and brain structure25. These neuroimaging and neuropsychological findings may raise the question ‘Is active psychosis neurotoxic?’ In 2006, McGlashan argued that if active psychosis was indeed neurotoxic, certain manifestations would be expected in the course of the illness, but there is little evidence for such a process26.
On the other hand, relatively few functional neuroimaging analyses have addressed this issue. Two studies performed functional MRI (fMRI) to examine the effects of DUP on resting state brain connectivity in drug-naïve FES patients, but they did not find any effects of DUP27,28. One study used near infrared spectroscopy (NIRS) and found negative effects of DUP on cortical activity during a letter version of the verbal fluency test (VFT) in SZ patients treated with antipsychotics for more than 6 months29. However, the study participants were heterogeneous and were in early and chronic stages of SZ; therefore, some confounding factors cannot be totally excluded (i.e. antipsychotic treatment duration or relapsed psychotic episodes).
Uncertainty remains as to whether active psychotic symptoms exacerbate brain cortical activities in patients with SZ. Limiting enrolment to FES patients avoids confounding by illness duration, longstanding substance abuse and treatment effects30. This type of design may provide more generalizable results regarding the nature of the disorder than studies of chronic patients19. The aim of this study was to use NIRS to investigate the relationship between DUP and cortical activity over bilateral frontotemporal regions during VFTs to elucidate the effects of untreated psychosis on brain function. There are two versions of VFT based on the type of cue; the category fluency task (CFT) requires the subject to generate words belonging to a specific semantic category, while the letter fluency task (LFT) requires the generation of words based on phonemic cues. A previous NIRS study investigating DUP effects adopted the LFT as a cognitive activation task29, but another recent report indicated that CFT performance was one of the candidates to identify SZ endophenotype31. Therefore, we employed both VFTs to investigate cortical activity in FES patients. We hypothesised that if psychosis is biologically toxic, longer DUP would be associated with poorer cortical activity during VFTs.
Results
Study participant characteristics
The study participants’ demographic characteristics are presented in Table 1. There were no significant differences between the healthy control (HC) and FES groups regarding age, sex, education, or socioeconomic status (SES). For both the LFT and CFT, the HC group performed significantly better than the FES group (LFT; P = 0.004, CFT; P = 0.004). LFT performance was significantly lower than that of the CFT in HC (P = 0.001) and FES groups (P = 0.001).
Cortical activity during the VFT
As shown in Fig. 1, there were significant increases in [oxy-Hb] changes during the LFT relative to the pre-task baseline over bilateral frontotemporal regions at 51 channels (except ch10; FDR-corrected, P = 0.000 ~ 0.021) in the HC group and 44 channels (ch1-3, ch9-14, ch17-42, ch44-52; FDR- corrected, P = 0.000 ~ 0.037) in the FES group. With regard to CFT, there were significant increases in [oxy-Hb] changes during the task period at 47 channels (ch1-2, ch4-6, ch10-16, ch18-52; FDR-corrected, P = 0.000 ~ 0.030) in the HC group and 31 channels (ch11, ch13, ch20, ch22-26, ch29-37, ch39-52; FDR-corrected, P = 0.000 ~ 0.029) in the FES group (see Supplementary Table S1, S2, S3 and S4 online). The grand averaged waveforms of [oxy-Hb] and [deoxy-Hb] changes during two types of VFTs in both groups are shown in Fig. 2.
Grand average waveforms.
(A) LFT in healthy controls. (B) CFT in healthy controls. (C) LFT in patients with schizophrenia. (D) CFT in patients with schizophrenia. Mean [Oxy-Hb] and [deoxy-Hb] changes during VFTs are presented as grand average waveforms in 52 channels in red and blue lines, respectively.
Group comparison of cortical activity during the VFT
Compared with the HC group, the FES group demonstrated significantly lower cortical activity in 30 channels during the LFT (ch1, ch9, ch11, ch15, ch19-20, ch22, ch24-27, ch30-36, ch39-42, ch44-47, ch49-52; FDR-corrected, P = 0.000 ~ 0.028; Fig. 3). These channels were approximately located at bilateral superior frontal gyrus (SFG), bilateral middle frontal gyrus (MFG), bilateral inferior frontal gyrus (IFG), bilateral precentral gyrus, bilateral postcentral gyrus, bilateral supramarginal gyrus, bilateral superior temporal gyrus and bilateral middle gyrus (Fig. 3). On the other hand, we found no significant differences in cortical activity over bilateral frontotemporal regions between the two groups during the CFT (see Supplementary Table S5 and S6 online).
Topographic maps of different hemodynamic response patterns during LFT over bilateral frontotemporal regions.
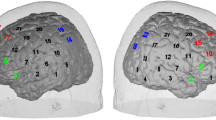
Left: Topographic maps showing clusters of channels with significantly higher cortical activities in the HC group. Right: Dot plots of the mean [oxy-Hb] changes in typical channels during the LFT period. Bars show the averages of the [oxy-Hb] changes at ch32 and asterisks show significant differences between groups (FDR-corrected P < 0.05).
Correlational analyses
In the FES group, we did not find significant associations between DUP and cortical activities over frontotemporal regions during the LFT or CFT. No associations between DUP and LFT or CFT performances were found. In addition, there were no significant relationships between DUP and PANSS positive (rho: 0.28, n.s.), negative (rho: 0.31, n.s.) or general psychopathology scores (rho: 0.23, n.s.). With regard to the associations between antipsychotic dosage and VFT performance, no significant associations were found. On the other hand, longer DUP was significantly associated with poor SES (rho = 0.42, P = 0.02) (see Supplementary Table S7, S8, Fig. S1 and Fig. S2 online). In addition, we did not find any associations between cortical activities and duration of illness (DOI) (see Supplementary Table S9 and S10 online).
In FES patients, during the LFT, there was no significant correlation between mean [oxy-Hb] changes in any channel and PANSS positive scores (rho: −0.50 to 0.00, n.s.), PANSS general psychopathology scores (rho: −0.61 to 0.13, n.s.), or daily dosage level of antipsychotic drugs (rho: −0.33 to 0.55, n.s.), but a significantly negative correlation was observed between cortical activities and PANSS negative scores at 14 channels (ch20, ch30–33, ch40–45, ch50–52; rho = −0.71 to −0.48; FDR-corrected, P = 0.000 ~ 0.013). On the other hand, during the CFT, mean [oxy-Hb] changes were not significantly correlated with PANSS positive scores (rho: −0.33 to 0.23, n.s.), PANSS negative scores (rho: −0.52 to 0.34, n.s.) or daily dosage level of antipsychotic drugs (rho: −0.37 to 0.42, n.s.) in any channel, but a significantly negative correlation was observed with PANSS general psychopathology scores at 2 channels (ch20, ch30; rho: −0.67 to −0.63; FDR-corrected, P = 0.000 ~ 0.001).
Discussion
Our results did not reveal an association between DUP and cortical activity over bilateral frontotemporal regions during either VFT in FES patients. To the best of our knowledge, this is the first study to use multichannel NIRS to investigate DUP effects on brain cortical activity during two versions of the VFT in FES patients. There main findings can be summarised as follows. (1) There were significant increases in both groups’ cortical activities over bilateral frontotemporal regions during both VFT. (2) There was a difference in the cortical activity patterns between the 2 groups during the LFT but not the CFT, indicating that deficits in cortical activity during phonemic processing may occur early in the course of SZ. (3) There was a lack of association between DUP and cortical activities during both VFTs.
Increased cortical activity of frontotemporal regions during VFT
We found significantly increased cortical activities in the HC group during both types of VFT over bilateral frontotemporal regions, which is consistent with several previous NIRS studies32,33,34,35,36. However, during the LFT, we found significantly increased cortical activities over bilateral frontotemporal regions in the FES group, which is similar to the results reported by Takizawa et al. and Marumo et al. using 52 multi-channel NIRS36,37. However, Ehlis et al. did not show a significant increase in cortical activities over prefrontal regions32. With regard to CFT, the FES group showed significantly increased cortical activities over bilateral frontotemporal regions, which is similar to findings reported by Marumo et al.37, but again, inconsistent with those reported by Ehlis et al.32. These discrepancies may be attributable to at least two reasons. Firstly, Ehlis et al. used 22 channel NIRS whereas we used a 52-channel NIRS instrument that enabled us to examine cortical activities over a wider area. Secondly, these differences might be due to different participant characteristics. Variable patterns of cortical activity have been reported according to the progression of SZ clinical stages34. In the present study, we recruited SZ patients during the early stage of their illness, which is different from previous studies (mean duration of illness > 10 years)32,33,35,37.
Group comparisons of NIRS signals and performance during the VFT
Our findings showed reduced cortical activities over bilateral frontotemporal regions during the LFT in the FES patients, which was consistent with previous NIRS studies focusing on FES34 as well as chronic SZ patients32,33,35,36,38. On the other hand, with regard to CFT, we did not find significant differences in cortical activities between FES and HC groups. Our results were consistent with those reported by Ikezawa et al.33, but inconsistent with those reported by others32,35,37. However, because those NIRS studies focusing on CFT recruited patients at a relatively chronic stage (mean illness duration > 10 years), it is difficult to directly compare the results. On the other hand, similar to previous studies32,33,37, group differences in cortical activations were more significant for the LFT than the CFT, indicating more pronounced phonological (letter fluency) deficits in FES patients32. Thus, it may suggest that cortical activities during the LFT may be a more sensitive indicator of frontal dysfunction in SZ than the CFT in SZ patients Taiwan.
With regard to the performance of VFTs, FES group scores lower in both types of VFT than HC group scores, which is consistent with the reports of a previous study39. Moreover, similar to previous NIRS studies32,33,37, we found that CFT performance was better than LFT in both groups. It was probably because compared to CFT, LFT is unfamiliar to subjects and more difficult40 and hence requires greater cognitive demands during LFT performance. This point may be supported by previous studies demonstrating that performance of CFT was better than that of LFT, but [oxy-Hb] changes during the LFT were greater than those during the CFT32,33. However, neuropsychological studies in Western populations have suggested that the category version of the VFT is more severely impaired than the letter version of the verbal fluency test in patients with schizophrenia39. Future studies designed to compare performance differences in the LFT and CFT and investigate the relationship with functional outcomes in Taiwanese SZ patients are warranted.
Effects of DUP on brain cortical activity
We failed to provide evidence for a relationship between DUP and cognitive deficits or cortical activity changes during a VFT in FES patients. In a previous NIRS study investigating the effects of DUP on cortical activity, the authors recruited a group of SZ patients with duration of illness < 10 years29. They found no correlations between DUP and brain function in patients with SZ receiving antipsychotic treatment shorter than 6 months, which is similar to our results. We did not find a relationship between DUP and cortical activities in minimally treated FES patients. On the other hand, our findings are consistent with most previous neurocognitive7,8,9,10,11,12 and MRI studies18,19,20,21,22,23 focusing on this topic and confirms that there is little evidence for the neurotoxicity hypothesis. Indeed, our results appear to contradict the hypothesis that schizophrenia is a progressive disease. Alternatively, there is an extensive amount of empirical evidence that functional and structural brain changes occur before psychotic symptom onset41,42,43, suggesting that neurodevelopmental disturbances probably begin pre-morbidly and continue pro-dromally and after the onset of psychotic symptoms24. Therefore, changes in brain structure and cognitive function are independent of the effects of DUP44.
Limitations
Our results should be viewed in light of several limitations. First, given the relatively small number of study participants, we may not have fully detected differences between groups; studies with larger samples and more detailed observation are needed. Second, selection bias must be considered as participants were recruited from the medical centre. Moreover, we had to consider the feasibility of taking measurements when recruiting, so potential selection bias according to participants’ symptoms and severities also needs to be considered. Third, because we employed a cross-sectional design, we could not examine longitudinal causal relationships between DUP and cortical activity. Finally, the effects of medication on cortical activity should also be considered. Although we recruited FES patients who were minimally treated with antipsychotic medication to reduce this confounding effect, recent studies showed that even short-term treatment with antipsychotics was associated with structural brain changes45. We also did not evaluate the effects of different types of antipsychotics used as most study participants received atypical antipsychotics. Future NIRS studies that assess a larger number of subjects with a focus on drug-naïve FES patients are warranted.
Conclusion
In this study, we investigated the relationship between frontotemporal brain region activity and DUP in patients with FES by using two types of VFTs and 52-channel NIRS imaging. We found reduced cortical activities over bilateral frontotemporal regions in FES patients during the LFT but not CFT. Furthermore, we did not find an association between DUP and brain functions over frontotemporal regions. Our findings do not support to the neurotoxicity hypothesis. However, relationship between DUP and long-term clinical outcomes is well established and there might be other consequences of delayed treatment, so these findings should not weaken the rationale for early detection and intervention strategies in SZ patients with a first psychotic episode.
Methods
Study participants
A total of 28 patients (15 males and 13 females) were recruited from the out- and inpatient populations at the Taichung Veterans General Hospital. Patients who met the criteria of Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR)46 for SZ for the study were diagnosed by experienced psychiatrists (P.H.Chou, C.C.Lin, P.S. Hou, T.H. Lan, C.H. Chan and C.C. Hung) and the diagnoses were validated using the Mini International Neuropsychiatric Interview (MINI)47. All patients were experiencing their first episode of psychosis and had received no more than 12 weeks of previous antipsychotic medication treatment19. Nine patients were taking risperidone, 8 paliperidone, 6 olanzapine, 3 aripiprazole, 1 amisulpride and 1 haloperidal.
Twenty-nine healthy individuals (10 males and 19 females) were recruited as control subjects and were screened with the MINI. All study participants were right-handed, which was defined as >70 points in the Edinburgh Inventory48. Subjects who had a history of substance abuse or dependence, mental retardation, neurological disorder, or a medical condition that could have affected brain structure or function were excluded. Controls were excluded if there was a personal history of any axis I or II disorder. This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Taichung Veterans General Hospital (approval No. CF13044). All participants received a complete explanation of the study and provided written informed consent.
Clinical measurements
The Positive and Negative Syndrome Scale (PANSS)49 was used to assess symptoms on the same day as the NIRS measurements. DUP was defined as the time from psychosis onset until the start of adequate treatment. It was calculated from the time period between the onset of first psychotic symptoms and the initiation of antipsychotic treatment based on patient interview, corroborative history from family members and medical records50. Socioeconomic status was assessed using the Hollingshead scale51. Patients’ antipsychotics are presented as chlorpromazine-equivalent doses52.
Cognitive testing
Patients completed 160-s block-design VFTs (both letter and category version). We selected the VFT because previous studies have demonstrated deficits in brain cortical activity over bilateral frontotemporal regions by NIRS measurement in SZ patients during the VFT34,36,37,53,54,55. The 160-s block-design contains three different time periods: a 30-s pre-task period, a 60-s task period and a 70-s post-task period. In the pre- and post-task periods, patients were instructed to fix their gaze at the centre of the screen and repeatedly count from one to five to control for and remove task-related motion artefacts. For the 60-s task period of the LFT, patients were instructed to say as many words as possible that started with a phonological syllable presented as an audible instruction by a computer. The task period comprised three continuous 20-s sub-periods, that were initiated by a single syllable selected from nine possible options (first, /(b)/, /(p)/, or /(d)/; second, /(t)/, /(l)/, or /(n)/; third, /(m)/, /(f)/,or /(dz)/). We chose these syllables because their frequencies of appearance at the beginning of Chinese words are moderate. In the CFT, subjects were asked to produce as many words as possible within a given semantic cue for 20 s each (first: ‘birds,’ ‘fish,’ or ‘insects’; second: ‘sweets,’ ‘fruits,’ or ‘vegetables’; third: ‘vehicles,’ ‘stationery items,’ or ‘home appliances’). Transitions between the 20-s sub-periods were immediate to encourage continuous performance. Before beginning each task session, subjects were given instructions on how to generate correct answers during the task periods by experienced researchers (P.H. Chou and W.H. Lin). Each subject performed three practice trials to ensure that they understood the instructions. We then recorded the total number of correct words generated during the task as an index of VFT performance.
NIRS instrument
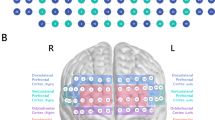
Multichannel NIRS is a widely used functional neuroimaging technology that can measure the haemodynamics in the bilateral frontotemporal cortices. This technique enables spatiotemporal detection of brain function by measuring concentrations of oxy-haemoglobin ([oxy-Hb]) and deoxy-haemoglobin ([deoxy-Hb]), which reflect regional cerebral blood volume as demonstrated by their correlations with fMRI signals56. In the present study, we used a 52-channel NIRS instrument (ETG-4000; Hitachi Medical Co., Tokyo, Japan) to measure changes in [oxy-Hb]. The NIRS probe attachments are thermoplastic 3 × 11 shells set with 52 channels (Fig. 4). The lowest probe line was set along the Fp1–Fp2 line as defined by the international 10–20 system used in electroencephalography. The distance between pairs of source and detector probes was set to 3.0 cm. We defined the measurement area between each probe-set pair as one ‘channel,’ which was sufficient to measure depths between 20 and 30 mm under the scalp, approximately corresponding to the surface of the cerebral cortex.
Probe setting and measurement points for 52-channel NIRS.
(A) The localizations of all 52 channels were positioned according to the international 10–20 system. Red and blue circles indicate near-infrared light emitter and detector positions, respectively. By using the international 10–20 system, the detector 13 was positioned on the F z marker point, while the bottom row of channels was placed on a line between T 3 and T 4. (B) Probes with thermoplastic 3 × 11 shells were placed over bilateral frontotemporal regions. (C) The 52 measuring areas are labelled as ch1 to ch52 from the right posterior to left anterior.
NIRS measurements were performed with participants sitting in a chair with their eyes open and the probe attachment resting on the head. Participants were instructed to relax and avoid involuntary movements to minimize motion artifacts. The NIRS instrument measures changes in both [oxy-Hb] and [deoxy-Hb] by using two wavelengths (695 and 830 nm) of near-infrared light (indicated as mM) on the basis of the Beer–Lambert law57. We could not measure the absolute path length of each participant from the scalp to the cerebral cortex; therefore, we recorded the haemoglobin concentrations from baseline to activation periods. Relative changes in haemoglobin concentration assessed by NIRS measurements are indicated by mM·mm.
The data sampling rate was 0.1 s using the integral mode. The pre-task baseline was determined as the mean over a 10-s period immediately before the task period and the post-task baseline was determined as the mean over the last 5 s of the post-task period. Linear fitting was applied to the data between these two baselines. A moving average method using a 5-s window width was applied and any short-term motion artefacts were rejected by an automatic artefact-rejection program in the NIRS instrument38. Because we excluded the rejected channels from further analysis, the number of available channels varied among individuals (LFT: FES group: 21–52 [mean, 47.6; SD, 6.9]; control group: 35–52 [mean, 48.1; SD, 5.2]; n.s.; CFT: FES group: 29–52 [mean, 47.8; SD, 5.8]; control group: 38–52 [mean, 48.4; SD, 4.4]; n.s).
The spatial information for each channel was estimated by using data from the Functional Brain Science Laboratory at Jichi Medical University in Japan (http://www.jichi.ac.jp/brainlab/virtual_reg.html);58 According to the LONI Probabilistic Brain Atlas (LPBA40)59, NIRS channels can record functional haemodynamics within the bilateral frontal, temporal and parietal cortices. Similar to previous studies29,38,53, NIRS channels were anatomically labelled only after the LPBA region of highest probability was determined. We used the mean changes in [oxy-Hb] measured during the VFT as an index of brain cortical activity. We chose [oxy-Hb] as an indicator because it better reflects cortical activity and demonstrates stronger correlations with fMRI blood-oxygenation level-dependent signals compared to [deoxy-Hb]56.
Statistical analysis
Each DUP value was transformed to the base 10 logarithm (log10 DUP) to manage skewness and moderate leverage data points2. Then, Spearman’s rank correlation coefficient was used to examine the relationship between log10 DUP and the mean [oxy-Hb] changes measured in each channel during the VFTs. The correction for multiple analyses among 52 channels was made using the false discovery rate method (FDR) (two-tailed; we set the value of q specifying the maximum FDR to 0.05, so that there are no more than 5% false positives on average)60. The correlation between DUP, LFT and CFT performance and clinical parameters were also analyzed. Moreover, to detect any confounding factors, we also investigated the relationship between mean [oxy-Hb] changes and DOI, PANSS positive, negative and general psychopathology scores and daily dosage level of antipsychotic drugs using Spearman’s rank correlation coefficient. The effect of antipsychotic medication on performance of VFTs was also evaluated. Basic characteristics between groups and differences between LFT and CFT scores in each group were compared using Student’s t-tests and paired t-tests, respectively. All statistical analyses were performed with SPSS 18.0 software (IBM Inc., Armonk, NY, USA).
Additional Information
How to cite this article: Chou, P.-H. et al. Duration of Untreated Psychosis and Brain Function during Verbal Fluency Testing in First-Episode Schizophrenia: A Near-Infrared Spectroscopy Study. Sci. Rep. 5, 18069; doi: 10.1038/srep18069 (2015).
References
Marshall, M. et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry 62, 975–983 (2005).
Perkins, D. O., Gu, H., Boteva, K. & Lieberman, J. A. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry 162, 1785–1804 (2005).
Wyatt, R. J. Neuroleptics and the natural course of schizophrenia. Schizophr Bull 17, 325–351 (1991).
Amminger, G. P., Edwards, J., Brewer, W. J., Harrigan, S. & McGorry, P. D. Duration of untreated psychosis and cognitive deterioration in first-episode schizophrenia. Schizophr Res 54, 223–230 (2002).
Lappin, J. M. et al. Duration of untreated psychosis and neuropsychological function in first episode psychosis. Schizophr Res 95, 103–110 (2007).
Zhou, F. C. et al. Characteristics and clinical correlates of prospective memory performance in first-episode schizophrenia. Schizophr Res 135, 34–39 (2012).
Goldberg, T. E. et al. Lack of an inverse relationship between duration of untreated psychosis and cognitive function in first episode schizophrenia. Schizophr Res 107, 262–266 (2009).
Heydebrand, G. et al. Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res 68, 1–9 (2004).
Hoff, A. L. et al. Lack of association between duration of untreated illness and severity of cognitive and structural brain deficits at the first episode of schizophrenia. Am J Psychiatry 157, 1824–1828 (2000).
Leeson, V. C. et al. The relationship between IQ, memory, executive function and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull 36, 400–409 (2010).
Rapp, C. et al. Duration of untreated psychosis and cognitive functioning. Schizophr Res 145, 43–49 (2013).
Rund, B. R. et al. Neurocognition and Duration of Psychosis: A 10-year Follow-up of First-Episode Patients. Schizophr Bull, 10.1093/schbul/sbv083 (2015).
Crespo-Facorro, B. et al. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res 91, 87–96 (2007).
Keshavan, M. S. et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 32, 161–167 (1998).
Lappin, J. M. et al. Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res 83, 145–153 (2006).
Malla, A. K., Bodnar, M., Joober, R. & Lepage, M. Duration of untreated psychosis is associated with orbital-frontal grey matter volume reductions in first episode psychosis. Schizophr Res 125, 13–20 (2011).
Guo, X. et al. Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naive schizophrenia. PLoS One 8, e83679 (2013).
Crespo-Facorro, B. et al. Reduced thalamic volume in first-episode non-affective psychosis: correlations with clinical variables, symptomatology and cognitive functioning. Neuroimage 35, 1613–1623 (2007).
Fannon, D. et al. Features of structural brain abnormality detected in first-episode psychosis. Am J Psychiatry 157, 1829–1834 (2000).
Hietala, J. et al. Regional brain morphology and duration of illness in never-medicated first-episode patients with schizophrenia. Schizophr Res 64, 79–81 (2003).
Ho, B. C., Alicata, D., Mola, C. & Andreasen, N. C. Hippocampus volume and treatment delays in first-episode schizophrenia. Am J Psychiatry 162, 1527–1529 (2005).
Ho, B. C. et al. Untreated initial psychosis: relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry 160, 142–148 (2003).
Xiao, Y. et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 41, 201–210 (2015).
Rund, B. R. Does active psychosis cause neurobiological pathology? A critical review of the neurotoxicity hypothesis. Psychological medicine 44, 1577–1590 (2014).
Anderson, K. K. et al. Minimal evidence that untreated psychosis damages brain structures: a systematic review. Schizophr Res 162, 222–233 (2015).
McGlashan, T. H. Is active psychosis neurotoxic? Schizophr Bull 32, 609–613 (2006).
Guo, W. et al. Decreased resting-state interhemispheric coordination in first-episode, drug-naive paranoid schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48, 14–19 (2014).
Guo, W. et al. Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry 49, 16–20 (2014).
Chou, P. H. et al. Distinct effects of duration of untreated psychosis on brain cortical activities in different treatment phases of schizophrenia: a multi-channel near-infrared spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry 49, 63–69 (2014).
Hafner, H., Hambrecht, M., Loffler, W., Munk-Jorgensen, P. & Riecher-Rossler, A. Is schizophrenia a disorder of all ages? A comparison of first episodes and early course across the life-cycle. Psychological medicine 28, 351–365 (1998).
Hurford, I. M., Marder, S. R., Keefe, R. S., Reise, S. P. & Bilder, R. M. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull 37, 538–545 (2011).
Ehlis, A. C., Herrmann, M. J., Plichta, M. M. & Fallgatter, A. J. Cortical activation during two verbal fluency tasks in schizophrenic patients and healthy controls as assessed by multi-channel near-infrared spectroscopy. Psychiatry Res 156, 1–13 (2007).
Ikezawa, K. et al. Impaired regional hemodynamic response in schizophrenia during multiple prefrontal activation tasks: a two-channel near-infrared spectroscopy study. Schizophr Res 108, 93–103 (2009).
Koike, S. et al. Different hemodynamic response patterns in the prefrontal cortical sub-regions according to the clinical stages of psychosis. Schizophr Res 132, 54–61 (2011).
Kubota, Y. et al. Prefrontal activation during verbal fluency tests in schizophrenia–a near-infrared spectroscopy (NIRS) study. Schizophr Res 77, 65–73 (2005).
Takizawa, R. et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res 99, 250–262 (2008).
Marumo, K. et al. Functional abnormalities in the left ventrolateral prefrontal cortex during a semantic fluency task and their association with thought disorder in patients with schizophrenia. Neuroimage 85 Pt 1, 518–526 (2014).
Takizawa, R. et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 85 Pt 1, 498–507 (2014).
Bokat, C. E. & Goldberg, T. E. Letter and category fluency in schizophrenic patients: a meta-analysis. Schizophr Res 64, 73–78 (2003).
Martin, A., Wiggs, C. L., Lalonde, F. & Mack, C. Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia 32, 1487–1494 (1994).
Bora, E. & Murray, R. M. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull 40, 744–755 (2014).
Nenadic, I. et al. Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia and healthy controls: a VBM study. Schizophr Res 161, 169–176 (2015).
Tognin, S. et al. Reduced parahippocampal cortical thickness in subjects at ultra-high risk for psychosis. Psychological medicine 44, 489–498 (2014).
Rund, B. R. et al. The course of neurocognitive functioning in first-episode psychosis and its relation to premorbid adjustment, duration of untreated psychosis and relapse. Schizophr Res 91, 132–140 (2007).
Lesh, T. A. et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry 72, 226–234 (2015).
American Psychiatric Association . Diagnostic and statistical manual-text revision (DSM-IV-TRim, 2000). (American Psychiatric Association, 2000).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33;quiz 34-57 (1998).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13, 261–276 (1987).
Esterberg, M. & Compton, M. Family history of psychosis negatively impacts age at onset, negative symptoms and duration of untreated illness and psychosis in first-episode psychosis patients. Psychiatry Res 197, 23–28 (2012).
Hollingshead, A. B., 1965. Two-Factor Index of Social Position. Yale University Press, New Haven, USA.
Inada, T. & Inagaki, A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci, 10.1111/pcn.12275 (2015).
Chou, P. H. et al. Similar age-related decline in cortical activity over frontotemporal regions in schizophrenia: a multichannel near-infrared spectroscopy study. Schizophr Bull 41, 268–279 (2015).
Pu, S. et al. Association between subjective well-being and prefrontal function during a cognitive task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res 149, 180–185 (2013).
Suto, T., Fukuda, M., Ito, M., Uehara, T. & Mikuni, M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biological psychiatry 55, 501–511 (2004).
Sato, H. et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage 83, 158–173 (2013).
Jobsis, F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977).
Tsuzuki, D. et al. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage 34, 1506–1518 (2007).
Shattuck, D. W. et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39, 1064–1080 (2008).
Singh, A. K. & Dan, I. Exploring the false discovery rate in multichannel NIRS. Neuroimage 33, 542–549 (2006).
Acknowledgements
We thank Kai-Dih Juang and Yu-Chen Lin for their assessments of clinical information of study participants. This study was supported in part by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-1034001A, TCVGH-1044002B and TCVGH-YM1040101).
Author information
Authors and Affiliations
Contributions
P.H.C. conducted data analysis and wrote the manuscript. C.C.H. and C.P.L. contributed to project management and supervision. P.H.C. and W.H.L. conducted NIRS measurements. P.H.C. coordinated the entire research design and took responsibility for the management of this study. P.H.C., C.C.L., P.S.H., W.R.L., T.H.L. and C.H.C. contributed to the clinical assessment, critical revision and approval of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chou, PH., Lin, WH., Lin, CC. et al. Duration of Untreated Psychosis and Brain Function during Verbal Fluency Testing in First-Episode Schizophrenia: A Near-Infrared Spectroscopy Study. Sci Rep 5, 18069 (2016). https://doi.org/10.1038/srep18069
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18069
- Springer Nature Limited
This article is cited by
-
The two-back task leads to activity in the left dorsolateral prefrontal cortex in schizophrenia patients with predominant negative symptoms: a fNIRS study and its implication for tDCS
Experimental Brain Research (2024)
-
Functional near-infrared spectroscopy in developmental psychiatry: a review of attention deficit hyperactivity disorder
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
Cortical haemodynamic response during the verbal fluency task in patients with bipolar disorder and borderline personality disorder: a preliminary functional near-infrared spectroscopy study
BMC Psychiatry (2021)
-
Validating a functional near-infrared spectroscopy diagnostic paradigm for Major Depressive Disorder
Scientific Reports (2020)
-
Perceived Occupational Stress is associated with Decreased Cortical Activity of the Prefrontal Cortex: A Multichannel Near-infrared Spectroscopy Study
Scientific Reports (2016)