Abstract
The mechanism whereby lactic acid bacteria extend the lifespan of Caenorhabditis elegans has previously been elucidated. However, the role of Weissella species has yet not been studied. We show that Weissella koreensis and Weissella cibaria significantly (p < 0.05) extend the lifespan of C. elegans compared with Escherichia coli OP50 and induce the expression of several genes related to lifespan extension (daf-16, aak-2, jnk-1, sod-3 and hif-1). Oral administration of Weissella altered reactive oxygen species (ROS) production and lowered the accumulation of lipofuscin and increased locomotor activity (which translates to a delay in ageing). Moreover, Weissella-fed C. elegans had decreased body sizes, brood sizes, ATP levels and pharyngeal pumping rates compared with E. coli OP50-fed worms. Furthermore, mutations in sod-3, hif-1 or skn-1 did not alter lifespan extension compared with wild-type C. elegans. However, C. elegans failed to display lifespan extension in loss-of-function mutants of daf-16, aak-2 and jnk-1, which highlights the potential role of these genes in Weissella-induced longevity in C. elegans. Weissella species extend C. elegans lifespan by activating DAF-16 via the c-Jun N-terminal kinase (JNK) pathway, which is related to stress response and the AMP-activated protein kinase (AMPK)-pathway that is activated by dietary restriction.
Similar content being viewed by others
Introduction
Weissella species are lactic acid bacteria that have only recently been classified as a new genus. Weissella species are found in fermented foods, including Korean traditional fermented vegetables and kimchi, sugar cane and the intestinal tracts of humans and other animals1. Fermented foods, including kimchi, possess diverse lactic acid bacteria, the composition of which effects fermentation and the sensory properties of kimchi2. Kimchi is a well-known probiotic food, with similar health benefits to probiotic yogurt. Additionally, kimchi has a range of other health benefits including the promotion of brain-, skin- and colorectal-health as well as strengthening the immune system; kimchi has been shown to be effective against cancer, obesity, constipation and high cholesterol; it also has fibrolytic, antioxidative and antiageing properties3. Recently, Weissella species were identified as one of the main fermenters in kimchi2. Weissella species are more resistant to acidic and anaerobic conditions compared with Leuconostoc species4. Weissella also have unusual interpeptide bridges in the peptidoglycan layer that distinguish these bacteria from other lactobacilli5. However, in contrast to other lactic acid bacteria, the possible beneficial effects of Weissella spp. on humans require further study.
Caenorhabditis elegans is a small, free-living soil nematode used in various fields of research. C. elegans is a particularly useful model to study ageing because of its short lifespan and the fact that it is amenable to genetic analyses6. Providing lactic acid bacteria as a food source instead of E. coli OP50 increases the average lifespan of C. elegans7,8,9. Several studies have described the mechanisms whereby lactic acid bacteria extend the lifespan of C. elegans, but the role of Weissella species remains unknown. Lactobacillus rhamnosus extends the lifespan of C. elegans by modulating the DAF-2/DAF-16 signalling pathway10 and facilitates resistance to oxidative stress in C. elegans, as demonstrated by the increased survival of C. elegans upon H2O2-induced stress. Bifidobacterium infantis prolongs the lifespan of C. elegans through activation of skn-1 (which is regulated by the p38 MAPK pathway) in a dose-dependent manner; this effect was not induced by dietary restriction, which means that B. infantis did not promote longevity through the activation of the host defence system via DAF-169. Dietary restriction has been shown to extend the lifespan of animals, including humans11; however, this remains controversial12. It is not clear whether lactic acid bacteria induce dietary restriction, thereby extending the lifespan of C. elegans. Dietary restriction can regulate the lifespan of C. elegans via the insulin/IGF-1 signalling (IIS) and target of rapamycin (TOR) pathways13. These pathways (and others) induce the DAF-16/FOXO transcription factor14, which in turn regulates various genes involved in regulating longevity, stress response, metabolism and development. DAF-16/FOXO is therefore indispensable in stress resistance as well as in lifespan regulation15. Moreover, JNK-1 is associated with stress response in vertebrates and is a positive regulator of DAF-16. In addition, AAK-2, the C. elegans homologue of AMPK, is involved in DAF-16/FOXO activation and promotes longevity during periods of glucose restriction16.
In this study, we investigated whether Weissella species extend the lifespan of C. elegans. We also conducted an attraction assay for C. elegans towards Weissella species and E. coli OP50. To elucidate the mechanism underlying Weissella-mediated lifespan extension in C. elegans, the lifespan of worms was measured using loss-of-function mutants.
Results
Weissella promotes C. elegans longevity
Feeding nematodes on a lawn of W. koreensis or W. cibaria significantly (p < 0.001) increased the mean lifespan (MLS) of worms compared with the group fed on the E. coli OP50 lawn (Table 1). W. koreensis was more effective than W. cibaria in increasing the MLS of worms. The survival rates of the worms were higher in both W. cibaria-fed and W. koreensis-fed worms compared with E. coli OP50-fed worms after 13 days (Fig. 1). The complete lifespan data of wild-type and mutant C. elegans are provided in Table S1.
ROS production
To determine the effect of Weissella on ROS production in C. elegans, total ROS levels of worms fed on E. coli OP50 or Weissella were measured. The two Weissella species showed opposing effects regarding ROS production (Fig. S1), with ROS levels 43.7% lower and 37.5% higher in W. koreensis-fed and W. cibaria-fed C. elegans, respectively, compared with C. elegans fed E. coli OP50.
The effects of Weissella on age-related biomarkers in C. elegans
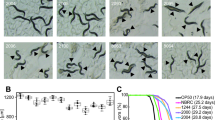
Age-related changes in C. elegans include changes in body movement, pharyngeal pumping rate and body size. Lipofuscin accumulation (a biomarker of ageing) can be determined by autofluorescence, but there is considerable inter-individual variation in lipofuscin levels in age-matched C. elegans17. We found that autofluorescence significantly decreased in Weissella-fed worms compared with E. coli OP50-fed worms (Fig. 2). Although lipofuscin fluorescence was significantly higher on day 14 in the E. coli OP50-fed group compared with either of the Weissella-fed groups, none of the three groups showed large volumes of lipofuscin. By day 16, lipofuscin levels in the W. koreensis and W. cibaria groups were 35.1% and 22.9%, respectively, of the level observed in the E. coli OP50 group. After 18 days, lipofuscin levels in the W. koreensis and W. cibaria groups increased to 86.9% and 75.3%, respectively, of that observed in E. coli OP50.
Lipofuscin accumulation in C. elegans (N2) fed E. coli OP50, W. koreensis and W. cibaria.
(a) Fluorescence of lipofuscin in worms fed E. coli OP50, W. koreensis and W. cibaria on days 14, 16 and 18. Scale bar = 100 μm. (b) Fluorescence was quantified using ImageJ software. The graph depicts the mean percentage in arbitrary units relative to that of control worms fed E. coli OP50 on day 14. Ten worms were used for each measurement; **p < 0.01, ***p < 0.001.
We measured the locomotory rate of C. elegans on days 4, 7, 10, 13 and 16 and found that the proportion of worms displaying sinusoidal locomotion (class A) was higher in Weissella-fed worms compared with worms fed E. coli OP50 (Fig. 3). Body size increased with age in all groups; however, the Weissella-fed worms were smaller than E. coli-fed worms (Fig. 4a). Meanwhile, brood size was smaller in Weissella-fed worms compared with the control group (Fig. 4b). Interestingly, the reproductive periods in the Weissella-fed groups were slightly longer compared with the E. coli OP50-fed group. The mean total brood sizes for each pair of worms were 237, 84 and 74 in worms fed E. coli OP50, W. koreensis and W. cibaria, respectively.
The locomotor activity of C. elegans (N2) fed E. coli OP50 or Weissella.
L4-stage worms fed E. coli OP50 for 3 days after hatching were transferred to fresh mNGM plates with 20 mg of E. coli OP50 or Weissella lawn. Nematodes were classified into four classes based on their locomotion: class (A) normal coordinated sinusoidal locomotion; class (B) uncoordinated and/or sluggish movement; class (C) no movement except head or tail in response to prodding; and class (D) dead worms. The bars indicate the proportion of each class at the designated time.
Influence of Weissella on C. elegans (N2) body size and brood size.
(a) L4-stage C. elegans were transferred to fresh mNGM plates seeded with each bacterial species on day 3 and body size was determined from 20 worms for each bacterial species. (b) Total brood size was determined from 30 animals and values represent the mean for each pair of worms. Significant differences shown are relative to E. coli OP50 (*p < 0.05, ***p < 0.001).
We next determined the level of food intake by measuring the pharyngeal pumping rate. Lowering the pharyngeal pumping rate can induce dietary restriction by limiting feeding (similar to what occurs in eat-2 mutants)18. The pumping rate was measured 30 min after transferring worms to E. coli OP50 or Weissella plates by counting the number of contractions in the terminal bulb of the pharynx (Fig. 5a) for 1 min. The pumping rate of worms grown on the Weissella lawn decreased significantly (p < 0.05) after 3 days (Fig. 5b). The pumping rate was then measured every 24 h from day 4 to day 10, during which the pumping rate of the Weissella group was lower than that of the E. coli group (Fig. 5c). Regardless of the bacterial species, the pumping rate decreased with age.
The pharyngeal pumping rate of C. elegans (N2) fed E. coli OP50 and Weissella.
Pumping rate was measured in the terminal bulb (a). The scale bar represents 50 μm. (b) The pumping rate of 3-day-old worms, measured for 30 min after the worms were transferred to a fresh mNGM plate seeded with each bacterial species. (c) The pumping rate in ageing worms, determined from the mean of 20 worms for each bacterial species. Significant differences shown are relative to E. coli OP50 (*p < 0.05, **p < 0.01, ***p < 0.001).
Because ATP level is related to dietary restriction19 as well as the AMPK pathway, we measured ATP levels and found that worms fed W. koreensis and W. cibaria had a 23.4% and 87.0% decrease in ATP, respectively, compared with the worms fed E. coli OP50 (Fig. S2). Therefore, Weissella significantly affects ATP content in C. elegans, especially W. cibaria, which dramatically decreased ATP production.
C. elegans food preference
C. elegans is known to exhibit preferences for specific bacterial strains20. A chemotaxis assay was performed to prove that the lifespan extension effect of Weissella was not derived from a preference between E. coli OP50 and Weissella. The results showed that C. elegans appeared to prefer E. coli OP50 to Weissella at 30 min after feeding the test strains; however, the numbers of worms in the circle (see Fig. S3) around E. coli OP 50 or Weissella were similar (more than 300 worms) in each test at 60 min after feeding the test strains, which means that C. elegans did not show any preference between E. coli OP50 and Weissella by 60 min (Table S2). Interestingly, many worms appeared to prefer lactic acid (125.0 ± 4.00) at 30 min after feeding on the test compounds and again, the numbers of worms in the circle were similar (more than 300 worms) in each test at 60 min after feeding on lactic acid or 95% ethanol.
Expression of lifespan extension-related genes
We investigated the expression of age-related genes, including those involved in diet restriction in C. elegans, to determine the mechanism of lifespan extension in Weissella-fed C. elegans. Quantitative real-time (qRT)-PCR was performed on the genes shown in Table S3. In Weissella-fed worms, daf-16, sod-3, jkk-1, jnk-1 and aak-2 were significantly overexpressed (Fig. S4), especially in worms fed W. cibaria, where all genes except daf-2 were up-regulated more than two-fold, with daf-16 and sod-3 upregulated 3.9-fold and 2.3-fold, respectively, compared with the E. coli OP50 control group. Meanwhile, in the W. koreensis group, daf-16, sod-3, jkk-1, jnk-1 and aak-2 expression increased, but to a lesser extent than the W. cibaria group and there were no significant increases in daf-2 and hif-1 expression. The other genes observed in this study did not show significant increases compared with the worms fed E. coli OP50 (Fig. S4).
Effect of Weissella species on nuclear translocation of DAF-16
We analysed the nuclear localization of DAF-16 by using transgenic strains expressing the fusion protein DAF-16::GFP (Fig. 6b). Weissella species induced an increase of nuclear DAF-16::GFP translocation (E. coli OP50: 22.4%; W. koreensis: 73.3%; W. cibaria: 75.0%) and a reduction of the cytosolic DAF-16::GFP fraction (E. coli OP50: 41.5%; W. koreensis: 6.7%; W. cibaria: 9.4%) (Fig. 6c).
Weissella species induced nuclear translocation of DAF-16::GFP.
(a) Depending on the degree of nuclear GFP fluorescence, three different states of translocation of DAF-16::GFP from the cytoplasm into cell nuclei can be distinguished: cytosolic (cyt, left; no nuclear GFP fluorescence), intermediate (int, centre; weak nuclear GFP fluorescence) and nuclear (nuc, right; strong nuclear GFP fluorescence) DAF-16::GFP localization. (b) Representative images of transgenic strain CF1407 fed with E. coli OP50 (left), W. koreensis (centre) and W. cibaria (right). Scale bar = 20 μm. (c) Nuclear localization of DAF-16 by Weissella species. Values shown are the mean ± SD from 90 worms for each bacterial species. Significant differences shown are relative to E. coli OP50 (**p < 0.01, ***p < 0.001).
Lifespan extension in C. elegans mutants
To confirm the role of the aforementioned genes in lifespan extension, C. elegans loss-of-function mutants for each of these genes were fed Weissella or E. coli OP50 and their longevity was assessed. Weissella-fed mutant worms with loss-of-function mutations in daf-16, aak-2 or jnk-1 showed no extension in lifespan compared with the E. coli control group, which suggests an essential role for these genes in the increased longevity of Weissella-fed worms (Table 1, Fig. S5). Unexpectedly, the lifespans of sod-3, jkk-1, skn-1, hif-1 and daf-2 mutants still increased when fed Weissella. The finding that the loss of daf-2 does not affect lifespan extension suggests that the IIS pathway is not involved in Weissella-dependent lifespan extension. Taken together with the qRT-PCR results, these above results suggest that daf-16 may be the key in promoting longevity in Weissella-fed worms.
Discussion
Although W. koreensis and W. cibaria isolated from kimchi might be candidate probiotics, little information, including the lifespan-extension effect of Weissella on C. elegans, is available on Weissella species in general. Previously, biomarkers of ageing such as lipofuscin, body size and locomotor activity have been studied to estimate pro-longevity in C. elegans. Feeding Weissella to C. elegans significantly increased MLS and was negatively associated with biomarkers of ageing. Feeding Weissella to C. elegans reduced lipofuscin accumulation, body size, brood size and pharyngeal pumping rate. Additionally, the locomotor activity of C. elegans fed with Weissella decreased more slowly than in worms fed with E. coli OP50. Body movement can be measured by the locomotory test and the results of locomotory class are predictive of the remaining lifespan of C. elegans after 8 days of age21. Lipofuscin (referred to as age pigment) accumulates in post-mitotic cells and is therefore a marker of ageing in animals such as C. elegans22. Decreases in body size and pumping rate are related not only to ageing but also to dietary restriction, which extends lifespan by reducing brood size23. The inherent ability of nematodes to produce offspring, adult nematodes’ egg-laying activity and the ability of newly hatched nematodes to survive and develop can affect brood size. In this experiment, all C. elegans ate E. coli OP50 during the early larval stage and therefore, the influence of Weissella on the inherent ability of nematodes to produce offspring could be excluded. During the experiment, there were no remarkable differences in the numbers of dead eggs and progeny that failed to develop between E. coli OP50- and Weissella-fed worms. Therefore, Weissella might mainly affect the egg-laying activity of adult nematodes.
Moreover, dietary restriction limits ATP production in C. elegans and ATP levels significantly decreased in Weissella-fed C. elegans compared with E. coli OP50-fed worms. These results suggest that Weissella may extend the lifespan of C. elegans through dietary restriction. According to the attraction assay, C. elegans initially appears to be more attracted to E. coli OP50 than to Weissella; however, C. elegans showed no preference between E. coli OP50 and Weissella by approximately 60 min. These results might be related to the fact that C. elegans rapidly change their behaviours and innate chemosensory preferences24. This means that dietary restriction was not linked to a repulsion of C. elegans towards Weissella.
While the biological effects of ROS on senescence and anti-senescence remain controversial25,26, ROS (by-products of respiration) are generally believed to be harmful to biological processes. However, it has been reported that ROS production induces enzymes that detoxify oxygen radicals, to defend against ROS damage27. In this study, ROS production significantly decreased in worms fed W. koreensis, yet it increased in worms fed W. cibaria compared with controls. This may have occurred because Weissella species might produce different types of ROS that are more or less reactive to 2,7-dichlorofluorescein diacetate (DCF-DA). Other mechanisms could also have affected the ROS results; for example, several types of inhibitors might affect the ROS levels in worms fed W. koreensis or W. cibaria. Furthermore, differences in ROS levels between W. koreensis and W. cibaria might be derived from the limitations of methods using DCF-DA28.
In C. elegans, dietary restriction resulted in low-level ROS production and increased lifespan29. Although the factor(s) involved in elevating ROS levels in W. cibaria-fed C. elegans remain unknown, several positive effects have been reported regarding ROS and longevity in C. elegans. First, ROS induces the expression of superoxide dismutase (SOD), which detoxifies ROS30,31. Second, ROS promote the expression of hif-1, which also regulates lifespan. Although it is still not clear how HIF-1 is activated under low-oxygen conditions and which downstream genes facilitate lifespan extension, an increase in ROS levels extends the lifespan of C. elegans by stabilizing HIF-132. Third, high levels of ROS induce phosphorylation and the subsequent activation of JNKs33, which may also occur in C. elegans. It still remains controversial whether dietary restriction extends lifespan by reducing ROS production or by increasing ROS defences and repair34. Likewise, ROS levels in worms fed with W. cibaria and W. koreensis increased and decreased, respectively, compared with the worms fed with E. coli OP50. ROS production in C. elegans fed with W. cibaria was not consistent with the results for lipofuscin, which is a complex mixture of oxidized and cross-linked macromolecules including proteins, lipids and carbohydrates. Lipofuscin levels in worms fed with both Weissella species were significantly lower than in worms fed with E. coli OP50. Metabolic and detoxification activities change with age in C. elegans35. In addition, ROS levels were measured using 4-day-worms that fed on Weissella species for 24 h, while lipofuscin levels were measured using 14-, 16- and 18-day worms, which may have caused the inconsistency between ROS level and lipofuscin accumulation in W. cibaria-fed C. elegans. We could therefore not conclude what the effects of dietary restriction on ROS levels were from our data.
The results obtained using W. koreensis or W. cibaria were slightly different. In this study, W. koreensis did not affect hif-1 gene expression. However, W. cibaria induced overexpression of hif-1 and still increased the lifespan of hif-1 mutants. DAF-16/FOXO might be actively relocalized in hif-1-mutants; it has been shown that hif-1 mutants live longer than wild-type worms because knocking down hif-1 triggers nuclear relocalization of DAF-16/FOXO36. The expression of sod-3 increased in both Weissella groups, but to a lesser extent in the W. koreensis group. The lifespan of sod-3 mutants fed W. koreensis and W. cibaria was extended compared with that of sod-3 mutants fed E. coli. The results suggest that SOD-3 is not an essential target of either Weissella species for extending the lifespan of C. elegans. However, the overexpression of sod-3 in the W. cibaria-fed worms might be related to the increase of ROS production in this group.
Pro-longevity related to dietary restriction is associated with many pathways, such as the TOR pathway, the AMPK pathway, the IIS pathway and sirtuins14. Among these pathways, DAF-16, which functions as a transcription factor, plays a major role in lifespan extension37 by regulating longevity, fat metabolism, stress response, free-radical detoxification and pathogen resistance. Meanwhile, suppression of DAF-2, which is related to the IIS pathway, extends lifespan by negatively regulating DAF-1638. In this study, however, daf-16 expression increased in Weissella-fed C. elegans despite increased expression of daf-2. Therefore, lifespan extension was apparently not related to the IIS pathway. The stress-related genes sod-3 and hif-1 can also affect C. elegans lifespan30,31. The genes jkk-1 and jnk-1 are members of the JNK pathway39 and aak-2 activates the AMPK pathway16. We used C. elegans loss-of-function mutants to investigate the relevant contributions of longevity-associated pathways to C. elegans lifespan; jnk-1 and aak-2 mutants were used to investigate the role of the JNK and AMPK pathways, respectively. We found that several age-related genes increased in Weissella-fed worms and both W. koreensis and W. cibaria failed to extend lifespan in C. elegans daf-16, aak-2 or jnk-1 mutants, which suggests that these genes are essential to lifespan extension in Weissella-fed C. elegans. JNK-1 and AAK-2 modulate DAF-16 activity by phosphorylation; specifically, the JNK family, a subgroup of the MAPK superfamily, is a part of the signal transduction cascade activated by exposure to environmental stress and cytokines40. In C. elegans, JNK-1 directly interacts with DAF-16 to mediate nuclear translocation of DAF-16, which triggers upregulation of several stress- and damage-response genes39. Similarly, AAK-2, which is one of the two α-catalytic subunits of AMPK, has recently been found to increase worm longevity41. AMPK is an important mediator of dietary restriction on longevity that acts via DAF-16/FOXO16 and is independent of the IIS pathway because DAF-16 is directly phosphorylated by AMPK16. The aak-2 gene encodes low-energy-sensing AMPK and therefore, it is linked to dietary restriction. AMPK/aak-2 is necessary to mediate lifespan extension via dietary restriction42. The lifespan of the C. elegans aak-2 mutant was not extended and the expression of the aak-2 gene significantly increased with feeding on Weissella. In Weissella-fed C. elegans, ATP levels significantly decreased, which is consistent with the activation of the AMPK pathway. Our results demonstrate that Weissella species promote longevity in C. elegans by increasing the expression of daf-16 via the JNK and AMPK pathways.
The mechanism of lifespan extension of Weissella-fed C. elegans was different from those of worms fed B. infantis9 and L. rhamnosus10. Based on qRT-PCR and mutant survival data, we predicted the pathways involved in lifespan extension of Weissella-fed C. elegans (Fig. 7). Briefly, Weissella promoted C. elegans longevity by inducing dietary restriction and stress response and, consequently, downstream expression of daf-16 via the AMPK and JNK pathways. We do not know if Weissella is lower in calories or less nutritious compared with E. coli OP50. Interestingly, although brood size decreased, the brooding period increased in Weissella-fed C. elegans. In this study, C. elegans was allowed to feed freely on the food source (E. coli OP50 or Weissella); thus, dietary restriction was not artificially induced. Dietary restriction in C. elegans was not derived from a repulsion to Weissella and was not associated with lactic acid produced by Weissella.
Mechanism predicted to be involved in the lifespan extension effect of Weissella.
JNK-1 and AAK-2 activate DAF-16 through the JNK and AMPK pathways, respectively. Activated DAF-16 affected sod-3, hif-1 and other genes related to longevity. The insulin/IGF-1 receptor, DAF-2, was not related to the lifespan extension of C. elegans by Weissella.
Metchnikoff hypothesized that lactic acid bacteria are important for human health and longevity on the basis of the longevity of Bulgarians who eat plenty of yogurt43. It is possible that similar mechanisms of pro-longevity exist in C. elegans. Additionally, dietary restriction is known to extend lifespan and to retard age-related health declines in a number of different species, including rodents, worms, yeast and possibly primates44. It is unclear whether dietary restriction affects other animals in the same way, but it is possible that the mechanisms identified in this study may apply to other species including humans. Finally, the JNK and AMPK pathways stimulate autophagy under dietary restriction and therefore, further studies on the possible relation between autophagy in lifespan extension of C. elegans by Weissella are required. In addition, in the present study, a subset of genes known to extend lifespan were investigated; thus, further studies are needed to investigate the whole genome of C. elegans to reveal other possible factors and/or pathways contributing to the lifespan extension of C. elegans by Weissella.
Methods
Bacterial strains and culture conditions
W. koreensis KACC 11853 and W. cibaria KACC 11845 were obtained from the Korean Agricultural Culture Collection (KACC) and used as test food sources for nematodes. E. coli OP50 was provided by the Caenorhabditis Genetics Center, University of Minnesota (CGC) and used as a control food source. E. coli OP50 was grown in Luria-Bertani (LB) broth (Difco, Detroit, MI, USA) at 37 °C for 18−24 h with shaking. Weissella strains were grown at 30 °C for W. koreensis and 37 °C for W. cibaria in de Man, Rogosa and Sharpe (MRS) broth (Difco) for 24 h without shaking. Bacteria were harvested by centrifugation at 3,000 × g for 10 min and washed in sterile M9 buffer. Then, bacteria were adjusted to a final concentration of 0.1 mg (wet weight) per microlitre in M9 buffer8.
Nematodes and growth conditions
C. elegans Bristol strain N2 (wild-type) and mutant strains were provided by the CGC. Bristol strain N2 was used for all measurements except the DAF-16 localization assay and the longevity assay with the mutant strains. The mutants used for lifespan measurements were CF1038 daf-16 (mu86), RB754 aak-2 (ok524), EU1 skn-1 (zu67), GA186 sod-3 (tm760), VC8 jnk-1 (gk7), ZG596 hif-1 (ia7), CB1370 daf-2 (e1370) and KU2 jkk-1 (km2). CF1407 daf-16 (mu86) I; muIs 71 [pKL99(daf-16Ap::GFP::daf-16A(bKO)) + pRF4(rol-6)] was used for the DAF-16 localization assay. Nematodes were maintained and propagated at 25 °C according to standard techniques45. The bacterial suspension was spread on peptone-free modified nematode growth medium (mNGM) in 90-mm diameter plates to feed worms. To exclude the possibility of nematocidal effects of nutrients in the bacterial growth medium, every experiment was performed on mNGM plates9. Eggs were obtained from adult worms after being exposed to a sodium hypochlorite-sodium hydroxide solution as previously described46. The egg suspension in M9 buffer was incubated overnight at 25 °C to allow eggs to hatch and the suspension of L1 stage worms was centrifuged at 1,200 × g for 2 min. After removing the supernatant, the remaining larvae were transferred onto fresh mNGM plates seeded with E. coli OP50 and incubated at 25 °C for two days to synchronize pubescence. All experiments were conducted with 3-day-old young-adult (day 1 of adulthood) wild-type worms (except for mutant survival tests).
Longevity assay
For the longevity assay, mNGM/FUdR plates were produced by supplementing with 5-fluoro-2′-deoxyuridine (FUdR, Sigma Aldrich) (50 μM)47. E. coli OP50, W. koreensis and W. cibaria cells in M9 buffer (20 mg wet weight) were spread on mNGM/FUdR plates (90-mm diameter). The longevity assay was started at the L4 stage of N2 nematodes and mutants, after which the worms were transferred to plates with a platinum wire. For each assay, 30 worms were assayed on three plates (10 worms per plate) for each bacterial species. The plates were incubated at 25 °C and live and dead worms were counted every 24 h. A worm was considered dead when it failed to respond to a gentle touch with a platinum wire picker. Worms showing abnormal death, such as hatched progeny inside the body, vulva explosion, or death as a result of adhering to the wall of the plate were excluded from the lifespan analysis. During the assay, worms being tested were transferred to fresh mNGM plates every day for the first 3 days and once a week for the rest of experiment to maintain a sufficient food source. The longevity assay was performed at least three times independently. The mean lifespan was estimated using Eq. (1)48:

where j is the age (day), dj is the number of worms that died in the age interval (x j, x j+1) and N is the total number of worms. The standard error (SE) of the estimated mean lifespan (MLS) was calculated using Eq. (2):

Quantification of ROS in C. elegans
Total ROS levels were quantified in whole worms using 2,7-dichlorodihydro-fluorescein-diacetate (H2-DCF-DA) (Sigma-Aldrich). ROS levels were determined according to previously performed protocols with slight modifications49. Briefly, worms were fed on each bacterial lawn for 24 h and collected with M9 buffer. Bacteria were removed by washing three times with M9 buffer and worms were resuspended in M9 buffer. To avoid overestimation of ROS levels, whole worms were used50. A 50-μl aliquot of the suspension was transferred into each well of a black 96-well plate and a 50-μl aliquot of the freshly prepared 100 μM H2-DCF-DA solution was added, resulting in a final concentration of 50 μM. Four wells were used per sample. There were control wells containing nematodes from each bacterial lawn without H2-DCF-DA and containing H2-DCF-DA without nematodes. After the addition of H2-DCF-DA, the basal fluorescent signal from each well was immediately measured with a fluorescence microplate reader (SpectraMAX GEMINI EM, Molecular Devices) at excitation and emission wavelengths of 485 nm and 520 nm, respectively. After the initial reading, the plate was incubated at 25 °C for 1 h and the fluorescence intensity was measured at the same wavelength. The change in fluorescence was determined by subtracting the initial value from the final value for each well including control wells. One millilitre of the initial worm suspension from each sample was used for protein quantification to normalize the fluorescence signal. Three independent experiments were performed.
Lipofuscin accumulation assay
The autofluorescence of intestinal lipofuscin was measured as an index of senescence between days 10 and 18 of adulthood. Randomly selected worms from the plate lawned with E. coli OP50 or Weissella species were washed three times with M9 buffer. Worms were then placed onto a 5% agar pad coated with 10 mM sodium azide (Junsei Chemical, Tokyo, Japan) in M9 buffer to induce anaesthesia. Lipofuscin autofluorescence images were taken using blue excitation light (405−488 nm), a DAPI (4′,6-diamidino-2-phenylindole) channel of laser confocal scanning microscope (Olympus Ix81-FV1000, Japan)51. Fluorescence was quantified on days 14, 16 and 18 using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to determine the lipofuscin levels. Three independent experiments were performed with over 30 worms for each bacterial species on each day.
Locomotory scoring
The locomotor activity of worms at different ages was examined using a scoring method described in previous reports21,52. Worms were classified as class “A” when they showed spontaneous movement or vigorous locomotion in response to prodding; class “B” worms did not move unless prodded or appeared to have uncoordinated movement; class “C” worms moved only their head and/or tail in response to prodding; class “D” worms were dead. Experiments were repeated three times independently and at least 100 worms were scored for each bacterial species.
Measurement of body size
Three-day-old adult worms (L4 stage) were transferred to mNGM plates (60 mm diameter) covered with 5 mg (wet weight) Weissella or E. coli OP50 cells in M9 buffer. The plates were incubated at 25 °C and the body size of live worms was measured every 24 h until 7 days of age. Five worms per bacterial species were assayed using five plates (one worm per plate). Images of worms were taken with a stereomicroscope (Olympus SZ61) and a ToupCam (UCMOS05100KPA). Images were analysed using ImageJ software. In this system, the area of a worm’s projection was estimated automatically and used as indices of body size7. Three independent experiments were performed with 20 worms for each bacterial species.
Brood size
L4-stage worms were transferred to mNGM plates coated with Weissella or E. coli OP50 and incubated at 25 °C. The parental animals were transferred daily to fresh mNGM plates (60 mm diameter) until the end of the reproductive period. The progeny of each animal were counted at the L2 or L3 stage. Ten worms per bacterial species were assayed using five plates (two worms per plate) and the test was performed three times9.
Pharyngeal pumping rate
Pumping assays were performed on mNGM plates with bacterial lawns at room temperature. After 30 min, L4-stage worms (3 days old) were transferred to bacteria-seeded mNGM plates and the number of contractions in the terminal bulb of the pharynx was counted for 1 min using an Olympus CKX41 inverted microscope (×400). The worms were incubated at 25 °C and the worms’ pumping rate on each bacterial lawn was measured every 24 h8. Three independent experiments were performed with 20 worms for each bacterial species on each day.
Quantification of ATP in C. elegans
Four-day-old adult worms fed on each bacterial lawn for 24 h were collected and washed three times in M9 buffer. Worm pellets were treated with three freeze/thaw cycles and boiled for 15 min to release ATP and destroy ATPase activity. After that, worms were centrifuged at 12,000 × g for 10 min at 4 °C and ATP levels were quantified with the ATPlite kit according to the manufacturer’s instructions (PerkinElmer, USA). Luminescence was measured with a fluorescence microplate reader (Molecular Devices, SpectraMAX GEMINI EM). The soluble protein concentration of the same preparation was measured using the Bradford assay and the ATP content value was normalized against the protein level53. Three independent experiments were performed.
Attraction assay
To examine the chemotactic activity of worms towards Weissella, 95% ethanol (Samchun chemical, Korea), lactic acid (Samchun chemical) and M9 buffer, we designed a chemotaxis assay with slight modification of the methods of Bargmann54 and Beale55. Bacterial food or other material was spotted onto the centre of the 90-mm diameter mNGM plates (Fig. S3) and over 1,000 worms were placed 10 mm from the sides of the plates. After 30 min and 60 min of incubation at 25 °C, the number of worms in the 30 mm-diameter-circle in the centre of each plate, including each bacterial lawn, was counted. Three independent experiments were performed.
RNA isolation and quantitative real-time polymerase chain reaction (RT-PCR)
Worms fed on E. coli OP50 or Weissella for 24 h were collected in M9 buffer and total RNA was isolated from whole worms as previously reported56. Total RNA was converted to cDNA using the RevertAid First Strand cDNA Synthesis kit according to the manufacturer’s instructions (Thermo Scientific), followed by quantitative RT-PCR (qRT-PCR) using the SYBR green (KAPA Biosystems, USA) and StepOnePlus real-time PCR system (Applied Biosystems). Primers were designed using Primer 3 software57. The primer sequences are shown in Table S3. Reactions were initiated at 95 °C, followed by 40 cycles of 95 °C for 20 s, 56 °C for 20 s and 72 °C for 30 s, followed by melt curve analysis. Relative expression levels were calculated using the 2−ΔΔCT method58. The control gene act-2 was used to normalize gene expression data59. Three independent experiments were performed.
DAF-16 localization assay
The transgenic strain CF1407 daf-16 (mu86) I; muIs 71 [pKL99(daf-16Ap::GFP::daf-16A(bKO)) + pRF4(rol-6)] was used to detect the intracellular localization of GFP-tagged DAF-16 protein. Randomly selected 4-day-old worms that were fed on plates lawned with E. coli OP50 or Weissella species for 24 h were washed three times with M9 buffer. Worms were then placed onto 5% agar pads coated with 10 mM sodium azide (Junsei Chemical, Tokyo, Japan) in M9 buffer to induce anaesthesia. GFP images were taken using green excitation light (460−495 nm), a GFP (green fluorescence protein) channel of a laser confocal scanning microscope (Olympus Ix81-FV1000, Japan). Localization of DAF-16 GFP from the cytoplasm into the cell nuclei was examined by analysing the degree of nuclear GFP fluorescence. Three different states can easily be distinguished (no, weak, or strong nuclear GFP fluorescence), which are related to a cytoplasmic, intermediate, or nuclear location of DAF-16-GFP60. The degree of nuclear translocation of DAF-16 was evaluated by counting the number of worms showing either weak or strong nuclear GFP fluorescence. Three independent experiments were performed with over 90 worms for each bacterial species.
Statistical analysis
The nematode survival rate was calculated by the Kaplan−Meier method and the significance of survival differences was tested using the Log-rank test8. Differences in lipofuscin levels were assessed using the Mann−Whitney U test9. One-way analysis of variance (ANOVA) with the post hoc Tukey test was used to compare the effect of Weissella on pharyngeal pumping rates and ANOVA with Duncan’s test was used to compare chemotaxis towards different food sources. Results were considered significant if p < 0.05. In other experiments, the means of the E. coli OP50 and Weissella group values were determined using Student’s t-test. P-values ≤ 0.05 were considered statistically significant and error bars depict the standard deviation.
Additional Information
How to cite this article: Lee, J. et al. Elucidating the Mechanism of Weissella-dependent Lifespan Extension in Caenorhabditis elegans. Sci. Rep. 5, 17128; doi: 10.1038/srep17128 (2015).
References
Lee, K. W. et al. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 18, 96–102, doi: 10.1016/j.anaerobe.2011.12.015 (2012).
Jung, J. Y., Lee, S. H. & Jeon, C. O. Kimchi microflora: history, current status and perspectives for industrial kimchi production. Appl Microbiol Biotechnol 98, 2385–2393, doi: 10.1007/s00253-014-5513-1 (2014).
Park, K. Y., Jeong, J. K., Lee, Y. E. & Daily, J. W., 3rd . Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food 17, 6–20, doi: 10.1089/jmf.2013.3083 (2014).
Chang, J. Y. & Chang, H. C. Improvements in the quality and shelf life of kimchi by fermentation with the induced bacteriocin-producing strain, Leuconostoc citreum GJ7 as a starter. J Food Sci 75, M103–110, doi: 10.1111/j.1750-3841.2009.01486.x (2010).
Stiles, M. E. & Holzapfel, W. H. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol 36, 1–29 (1997).
Riddle, D. L., Blumenthal, T., Meyer, B. J. & Priess, J. R. In C. elegans II (eds D. L. Riddle, T. Blumenthal, B. J. Meyer & J. R. Priess ) (1997).
Ikeda, T., Yasui, C., Hoshino, K., Arikawa, K. & Nishikawa, Y. Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against salmonella enterica serovar enteritidis. Appl Environ Microbiol 73, 6404–6409, doi: 10.1128/aem.00704-07 (2007).
Zhao, Y. et al. Lactobacillus salivarius strain FDB89 induced longevity in Caenorhabditis elegans by dietary restriction. J Microbiol 51, 183–188, doi: 10.1007/s12275-013-2076-2 (2013).
Komura, T., Ikeda, T., Yasui, C., Saeki, S. & Nishikawa, Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology 14, 73–87, doi: 10.1007/s10522-012-9411-6 (2013).
Grompone, G. et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS One 7, e52493, doi: 10.1371/journal.pone.0052493 (2012).
Yu, B. P. Why calorie restriction would work for human longevity. Biogerontology 7, 179–182, doi: 10.1007/s10522-006-9009-y (2006).
Shanley, D. P. & Kirkwood, T. B. Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology 7, 165–168, doi: 10.1007/s10522-006-9006-1 (2006).
Lapierre, L. R. & Hansen, M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab 23, 637–644, doi: 10.1016/j.tem.2012.07.007 (2012).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Murphy, C. T. et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283, doi: 10.1038/nature01789 (2003).
Greer, E. L. et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17, 1646–1656, doi: 10.1016/j.cub.2007.08.047 (2007).
Pincus, Z. & Slack, F. J. Developmental biomarkers of aging in Caenorhabditis elegans. Dev Dyn 239, 1306–1314, doi: 10.1002/dvdy.22224 (2010).
Onken, B. & Driscoll, M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1 and SKN-1. PLoS One 5, e8758, doi: 10.1371/journal.pone.0008758 (2010).
Sagi, D. & Kim, S. K. An engineering approach to extending lifespan in C. elegans. PLoS Genet 8, e1002780, doi: 10.1371/journal.pgen.1002780 (2012).
Abada, E. A. et al. C. elegans behavior of preference choice on bacterial food. Mol Cells 28, 209–213, doi: 10.1007/s10059-009-0124-x (2009).
Hosono, R., Sato, Y., Aizawa, S. I. & Mitsui, Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol 15, 285–289 (1980).
Hosokawa, H. et al. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mech Ageing Dev 74, 161–170 (1994).
Bishop, N. A. & Guarente, L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549, doi: 10.1038/nature05904 (2007).
Lee, J., Jee, C. & McIntire, S. L. Ethanol preference in C. elegans. Genes Brain Behav 8, 578–585, doi: 10.1111/j.1601-183X.2009.00513.x (2009).
Cannizzo, E. S., Clement, C. C., Sahu, R., Follo, C. & Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics 74, 2313–2323, doi: 10.1016/j.jprot.2011.06.005 (2011).
Ristow, M. & Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic Biol Med 51, 327–336, doi: 10.1016/j.freeradbiomed.2011.05.010 (2011).
Kharade, S. V., Mittal, N., Das, S. P., Sinha, P. & Roy, N. Mrg19 depletion increases S. cerevisiae lifespan by augmenting ROS defence. FEBS Lett 579, 6809–6813, doi: 10.1016/j.febslet.2005.11.017 (2005).
Forman, H. J. et al. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radical Biology and Medicine 78, 233–235 (2015).
Walker, G., Houthoofd, K., Vanfleteren, J. R. & Gems, D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev 126, 929–937, doi: 10.1016/j.mad.2005.03.014 (2005).
Prasad, K. N. & Bondy, S. C. Evaluation of role of oxidative stress on aging in Caenorhabditis elegans: a brief review. Curr Aging Sci 6, 215–219 (2013).
Back, P., Braeckman, B. P. & Matthijssens, F. ROS in aging Caenorhabditis elegans: damage or signaling? Oxid Med Cell Longev 2012, 608478, doi: 10.1155/2012/608478 (2012).
Hwang, A. B. & Lee, S. J. Regulation of life span by mitochondrial respiration: the HIF-1 and ROS connection. Aging 3, 304–310 (2011).
Lee, H. S., Hwang, C. Y., Shin, S. Y., Kwon, K. S. & Cho, K. H. MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species. Sci Signal 7, ra52, doi: 10.1126/scisignal.2005260 (2014).
Sinclair, D. A. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126, 987–1002, doi: 10.1016/j.mad.2005.03.019 (2005).
Dallaire, A. et al. Down regulation of miR-124 in both Werner syndrome DNA helicase mutant mice and mutant Caenorhabditis elegans wrn-1 reveals the importance of this microRNA in accelerated aging. Aging 4, 636–647 (2012).
Leiser, S. F., Begun, A. & Kaeberlein, M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell 10, 318–326, doi: 10.1111/j.1474-9726.2011.00672.x (2011).
Mukhopadhyay, A., Oh, S. W. & Tissenbaum, H. A. Worming pathways to and from DAF-16/FOXO. Exp Gerontol 41, 928–934, doi: 10.1016/j.exger.2006.05.020 (2006).
Kaletsky, R. & Murphy, C. T. The role of insulin/IGF-like signaling in C. elegans longevity and aging. Dis Model Mech 3, 415–419, doi: 10.1242/dmm.001040 (2010).
Oh, S. W. et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci USA 102, 4494–4499, doi: 10.1073/pnas.0500749102 (2005).
Davis, R. J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Apfeld, J., O’Connor, G., McDonagh, T., DiStefano, P. S. & Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev 18, 3004–3009, doi: 10.1101/gad.1255404 (2004).
Greer, E. L., Banko, M. R. & Brunet, A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Annals of the New York Academy of Sciences 1170, 688–692, doi: 10.1111/j.1749-6632.2009.04019.x (2009).
Metchnikoff, E. The prolongation of life. (Putnam, 1908).
Sohal, R. S. & Weindruch, R. Oxidative stress, caloric restriction and aging. Science 273, 59–63 (1996).
Stiernagle, T. Maintenance of C. elegans. WormBook, 1–11, doi: 10.1895/wormbook.1.101.1 (2006).
Sulston, J. & Hodgkin, J. in Methods (ed. W. B. Wood ) 587–606 (1988).
Gruber, J., Ng, L. F., Poovathingal, S. K. & Halliwell, B. Deceptively simple but simply deceptive–Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett 583, 3377–3387, doi: 10.1016/j.febslet.2009.09.051 (2009).
Wu, D., Rea, S. L., Yashin, A. I. & Johnson, T. E. Visualizing hidden heterogeneity in isogenic populations of C. elegans. Exp Gerontol 41, 261–270, doi: 10.1016/j.exger.2006.01.003 (2006).
Schulz, T. J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–293, doi: 10.1016/j.cmet.2007.08.011 (2007).
Fong, S., Gruber, J. & Halliwell, B. Measuring reactive oxygen species in C. elegans using DCFDA–a word of caution. Worm Breeder’s Gaz 18, 11 (2010).
Neumann-Haefelin, E. et al. SHC-1/p52Shc targets the insulin/IGF-1 and JNK signaling pathways to modulate life span and stress response in C. elegans. Genes Dev 22, 2721–2735, doi: 10.1101/gad.478408 (2008).
Herndon, L. A. et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419, 808–814, doi: 10.1038/nature01135 (2002).
Yang, W. & Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8, e1000556, doi: 10.1371/journal.pbio.1000556 (2010).
Bargmann, C. I., Hartwieg, E. & Horvitz, H. R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527 (1993).
Beale, E., Li, G., Tan, M. W. & Rumbaugh, K. P. Caenorhabditis elegans senses bacterial autoinducers. Appl Environ Microbiol 72, 5135–5137, doi: 10.1128/aem.00611-06 (2006).
Burdine, R. D. & Stern, M. J. Easy RNA isolation from C. elegans: a TRIZOL based method. Worm Breed. Gaz 14, 10 (1996).
Untergasser, A. et al. Primer3–new capabilities and interfaces. Nucleic acids research 40, e115–e115 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, doi: 10.1006/meth.2001.1262 (2001).
Sivamaruthi, B. S. et al. Caenorhabditis elegans as a model for studying Cronobacter sakazakii ATCC BAA-894 pathogenesis. J Basic Microbiol 51, 540–549, doi: 10.1002/jobm.201000377 (2011).
Wolf, M., Nunes, F., Henkel, A., Heinick, A. & Paul, R. J. The MAP kinase JNK‐1 of Caenorhabditis elegans: Location, activation and influences over temperature‐dependent insulin‐like signaling, stress responses and fitness. Journal of cellular physiology 214, 721–729 (2008).
Acknowledgements
Supported by Korea University Grant (K1508391).
Author information
Authors and Affiliations
Contributions
Y.H.L. and J.L. designed the research and J.L., G.K. and Y.H.L. performed the experiments. Y.H.L. and J.L. wrote the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, J., Kwon, G. & Lim, YH. Elucidating the Mechanism of Weissella-dependent Lifespan Extension in Caenorhabditis elegans. Sci Rep 5, 17128 (2015). https://doi.org/10.1038/srep17128
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17128
- Springer Nature Limited
This article is cited by
-
Geroprotective potential of microbiome modulators in the Caenorhabditis elegans model
GeroScience (2023)
-
Lacticaseibacillus rhamnosus Probio-M9 extends the lifespan of Caenorhabditis elegans
Communications Biology (2022)
-
5′-Hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone extends longevity mediated by DR-induced autophagy and oxidative stress resistance in C. elegans
GeroScience (2021)
-
Probiotics Interactions and the Modulation of Major Signalling Pathways in Host Model Organism Caenorhabditis elegans
Indian Journal of Microbiology (2021)
-
Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy
Nature Communications (2019)