Abstract
Transgenic crops producing Bacillus thuringiensis (Bt) proteins kill key insect pests, providing economic and environmental benefits. However, the evolution of pest resistance threatens the continued success of such Bt crops. To delay or counter resistance, transgenic plant “pyramids” producing two or more Bt proteins that kill the same pest have been adopted extensively. Field populations of the pink bollworm (Pectinophora gossypiella) in the United States have remained susceptible to Bt toxins Cry1Ac and Cry2Ab, but field-evolved practical resistance to Bt cotton producing Cry1Ac has occurred widely in India. Here we used two rounds of laboratory selection to achieve 18,000- to 150,000-fold resistance to Cry2Ab in pink bollworm. Inheritance of resistance to Cry2Ab was recessive, autosomal, conferred primarily by one locus and independent of Cry1Ac resistance. We created a strain with high resistance to both toxins by crossing the Cry2Ab-resistant strain with a Cry1Ac-resistant strain, followed by one selection with Cry2Ab. This multi-toxin resistant strain survived on field-collected Bt cotton bolls producing both toxins. The results here demonstrate the risk of evolution of resistance to pyramided Bt plants, particularly when toxins are deployed sequentially and refuges are scarce, as seen with Bt cotton and pink bollworm in India.
Similar content being viewed by others
Introduction
Insecticidal crystalline proteins from the bacterium Bacillus thuringiensis (Bt) kill some key pests, but are harmless to most non-target organisms including people1,2,3,4. In 2014, the area planted worldwide to genetically engineered cotton, corn and soybean producing Bt proteins grew to 78 million hectares (ha)5. Although Bt crops can increase yield and profit, suppress pests and decrease reliance on conventional insecticides6,7,8,9,10, evolution of resistance by insect pests can reduce these benefits11.
To delay or counter resistance to Bt crops, many growers have switched from transgenic plants that produce one Bt toxin to those producing two or more Bt toxins that kill the same pest12. These combinations of toxins called “pyramids” are especially effective when insects resistant to one toxin are killed by another toxin produced by the same plant13,14. Two conditions favoring success of two-toxin pyramids are: 1) pests are susceptible to both toxins and 2) resistance to one toxin does not cause cross-resistance to the other toxin12.
The most widely used pyramid of Bt cotton (Gossypium hirsutum L.) produces Bt toxins Cry1Ac and Cry2Ab. These toxins bind to different receptors in the midgut of lepidopteran larvae15,16 and cross-resistance between them is usually weak or nil12. In the United States, this two-toxin cotton was first planted in 2003 and reached 3.5 million ha by 2012, equivalent to 69% of the nation’s cotton17,18. Farmers in India first planted this two-toxin cotton in 2006 and its area increased to 11 million ha by 2013, accounting for 91% of India’s cotton19.
The lepidopteran pests targeted by Bt cotton include the pink bollworm, Pectinophora gossypiella (Saunders), which has been a major pest in many countries including the world’s three leading cotton producers (China, India and the United States)20. In the United States, refuges of non-Bt cotton and mass releases of sterile moths have sustained pink bollworm susceptibility to Bt toxins for two decades, helping to bring this invasive insect close to eradication in Arizona and other southwestern states9,21. In India, because farmers have not planted adequate refuges, the efficacy of Bt cotton producing Cry1Ac has been eroded by widespread, field-evolved practical resistance22,23,24,25,26,27. In China, where farmers have not switched to two-toxin Bt cotton, small but significant increases in pink bollworm resistance to Cry1Ac have occurred in northern China, where close to 100% of the three million ha of cotton planted yearly produces Cry1Ac as the only Bt toxin28,29. As far as we know, field-evolved resistance to Cry2Ab has not been documented in pink bollworm.
Here we analyzed eight strains of pink bollworm from Arizona (Table 1) to improve our understanding of resistance to Cry1Ac and Cry2Ab in this global pest. In previous work, laboratory selection with either Cry1Ac only or both Cry1Ac and Cry2Ab yielded pink bollworm strains that survived on Bt cotton producing Cry1Ac, but not on Bt cotton producing both toxins30,31. Whereas some of these strains had >1000-fold resistance to Cry1Ac, the highest previously reported pink bollworm resistance to Cry2Ab was 240-fold11,31. In this study, two rounds of laboratory selection with Cry2Ab generated 18,000- to 150,000-fold resistance to Cry2Ab in a strain named Bt4-R2. After we crossed Bt4-R2 with a Cry1Ac-resistant strain (AZP-R)30,32 and selected once on diet with Cry2Ab, the resulting multi-toxin resistant strain (AZP-R2) survived on bolls of Bt cotton producing both toxins. We used Bt4R-2, AZP-R2 and other strains to assess inheritance and fitness costs of resistance to Cry2Ab, as well as cross-resistance between Cry1Ac and Cry2Ab.
Results
Selection for resistance to Cry2Ab in strain Bt4-R2
The Cry2Ab-resistant Bt4-R2 strain was derived from the Cry1Ac-resistant strain Bt4R33 (Fig. 1). Before selection for resistance to Cry2Ab, a subset of Bt4R, designated Bt4R-P, was selected on cotton plants producing Cry1Ac.
Selection for pink bollworm resistance to Cry1Ac and Cry2Ab.
We created strain Bt4R-P by selecting a subset of larvae from the Cry1Ac-resistant strain Bt4R on bolls of Bt cotton producing Cry1Ac. Next we exposed a total of 10,000 to 20,000 neonates from Bt4R and Bt4R-P to diet containing 3 μg Cry2Ab per mL diet. We started the Cry2Ab-resistant strain Bt4-R2 by pooling the adult survivors from Bt4R (n = 6) and Bt4R-P (n = 7). These 13 adults mated and produced eggs. The next two generations were reared on untreated diet. The F3 larvae of the Bt4-R2 strain were reared on diet containing 5 μg Cry2Ab per mL diet, yielding extremely high resistance to Cry2Ab (Fig. 2 and Tables 2 and 3). We crossed AZP-R (highly resistant to Cry1Ac) with Bt4-R2 to start the AZP-R2 strain. We selected F2 larvae of AZP-R2 on diet containing 10 μg Cry2Ab per mL diet, which yielded high, nearly homogeneous resistance to both Cry1Ac and Cry2Ab in the F4 larvae of AZP-R2 (Table 4). AZP-R2U was a subset of AZP-R2 that was reared without additional selection on Cry2Ab.
In the first selection with Cry2Ab, we exposed approximately 5,000 to 10,000 neonates from Bt4R and a similar number from Bt4R-P to diet containing 3 μg Cry2Ab per mL diet. This yielded six survivors from Bt4R and seven from Bt4R-P for a total of 13 survivors from the 10,000 to 20,000 neonates exposed (ca. 0.1% survival). These 13 survivors mated among themselves to start the Bt4-R2 strain. After rearing Bt4-R2 on untreated diet for two generations (F1 and F2), we conducted the second round of selection by exposing approximately 20,000 to 30,000 neonates of the F3 generation to diet containing 5 μg Cry2Ab per mL, which yielded 877 survivors (ca. 3% survival).
These two rounds of selection with Cry2Ab generated extremely high resistance to Cry2Ab in Bt4-R2 (Tables 2 and 3, Figs 2 and 3). After these two rounds of selection with Cry2Ab, Bt4-R2 was reared without additional exposure to any Bt toxins for the remainder of this study. We calculated the resistance ratio as the concentration of toxin killing 50% of larvae (LC50) for a strain divided by the LC50 for the susceptible strain APHIS-S tested during the same time period. The resistance ratio for Bt4-R2 to Cry2Ab was 150,000 for the F15 generation and 18,000 for the F22 generation (Tables 2 and 3). The highest concentration of Cry2Ab tested, 600 μg Cry2Ab per mL diet, killed only 23% of larvae from the F15 generation of Bt4-R2 (n = 30). Because it was difficult to kill Bt4-R2 with the highest concentrations of Cry2Ab tested, we consider the resistance ratios approximations and cannot determine if the difference in LC50 between the F15 and F22 generations is statistically significant. At the diagnostic concentration of 10 μg Cry2Ab per mL diet, adjusted larval survival ranged from 75 to 100% for Bt4-R2 compared with 0% for all other strains tested (Tables 2 and 3, Figs 2 and 3). The LC50 of Cry2Ab did not differ significantly among Bt4R, Bt4R-P and APHIS-S, indicating that both parent strains of Bt4-R2 were susceptible to Cry2Ab (Table 2).
Responses to Cry2Ab of pink bollworm larvae from a susceptible strain (APHIS-S), a resistant strain (Bt4-R2) and their F1 progeny.
We scored live fourth instars, pupae and adults as survivors after 21 d on diet. Adjusted mortality (%) is 100% minus adjusted survival (%). Adjusted survival (%) is survival on Cry2Ab-treated diet divided by survival on untreated control diet multiplied by 100%. F1A and F1B indicate F1 progeny from mass crosses with male Bt4-R2 × female APHIS-S and female Bt4-R2 × male APHIS-S, respectively.
Responses to Cry2Ab of pink bollworm larvae from single-pair families from a susceptible strain (APHIS-S) (a), a resistant strain (Bt4-R2) (b) and their F1 progeny (c, d).
Survival (%) on control diet (no Bt toxin, green bars) and diet treated with 10 μg Cry2Ab per mL diet (blue bars). Each of the 23 families tested (A-W) was generated by crossing a single male with a single female (n = 8–24 larvae tested per family on each diet; mean = 16 neonates per family on each diet). Asterisks indicate 0% survival on Cry2Ab-treated diet for all 17 families from APHIS-S and F1.
Evaluation of cross-resistance between Cry1Ac and Cry2Ab in Bt4-R2 and its parent strains
The results show little or no cross-resistance occurred between Cry1Ac and Cry2Ab in Bt4-R2 and its parent strains (Table 2). The LC50 of Cry1Ac did not differ significantly between Bt4-R2 and Bt4R and was significantly less for Bt4-R2 than Bt4R-P (Table 2), which indicates that selection with Cry2Ab did not increase resistance to Cry1Ac of Bt4-R2 relative to its parent strains. In addition, selection with Cry1Ac increased the resistance ratio for Cry1Ac to 400 in Bt4R-P without increasing its resistance to Cry2Ab (resistance ratio = 0.58).
Inheritance of resistance to Cry2Ab in strain Bt4-R2
To evaluate inheritance of resistance to Cry2Ab, we used diet bioassays to test Bt4-R2, APHIS-S and their F1 progeny. We generated the F1 progeny with two types of reciprocal crosses: mass crosses and single-pair crosses. Responses of the F1 progeny from both sets of crosses show that the resistance to Cry2Ab in Bt4-R2 was autosomal and recessive (Table 3 and Figs 2 and 3).
Mass crosses
In the mass cross experiment, the LC50 was similar for the progeny from the two reciprocal crosses (Table 3), indicating we did not detect maternal effects or sex linkage, which shows that the inheritance of resistance to Cry2Ab in Bt4-R2 is autosomal. For the F1 pooled from the two reciprocal mass crosses, the LC50 did not differ significantly from the LC50 of the susceptible APHIS-S strain (Table 3), indicating recessive inheritance of resistance. The resistance ratio was 1.6 for the F1 pooled from the mass crosses compared with 18,000 for the F22 of Bt4-R2 used as parents in the reciprocal crosses.
We calculated the dominance parameter h, which varies from zero for completely recessive resistance to one for completely dominant resistance. For the F1 pooled from the mass crosses, survival was 0% yielding h = 0 at both concentrations that were tested against the F1, Bt4-R2 and APHIS-S (1 and 10 μg Cry1Ac per mL diet, Fig. 2).
Single-pair crosses
The results from the single-pair crosses confirm that inheritance of resistance to Cry2Ab was autosomal and recessive (Fig. 3). At the diagnostic concentration of Cry2Ab (10 μg Cry2Ab per mL diet), adjusted survival was 0% for all 11 F1 families, 0% for all six families from APHIS-S and 79 to 100% for six families from Bt4-R2 (Fig. 3). These results yield h = 0 for all 11 F1 families tested, indicating completely recessive inheritance at the diagnostic concentration of Cry2Ab.
AZP-R crossed with Bt4-R2 to create multi-toxin resistant strain AZP-R2
Although the Bt4-R2 strain was extremely resistant to Cry2Ab, it had only 28-fold resistance to Cry1Ac relative to the susceptible APHIS-S strain (Table 2). Conversely, the AZP-R strain had 1,500-fold resistance to Cry1Ac, but only two-fold resistance to Cry2Ab31. To generate a strain with high resistance to both Cry1Ac and Cry2Ab, we crossed AZP-R with Bt4-R2 to create the AZP-R2 strain. To start the AZP-R2 strain, we used two replicates of 50 female pupae from Bt4-R2 pooled with 50 male pupae from AZP-R and two replicates of 50 female pupae from AZP-R pooled with 50 male pupae from Bt4-R2 (total n = 400 pupae). After the adults mated, the F1 eggs from all four replicates were combined as AZP-R2 and the resulting larvae were reared on untreated diet.
Inheritance of resistance to Cry2Ab in AZP-R2
We hypothesized that the resistance to Cry2Ab in AZP-R2 is recessive, because it was derived from the recessive resistance to Cry2Ab in Bt4-R2. Given that AZP-R was not resistant to Cry2Ab, we hypothesized the F1 offspring of the cross between AZP-R and Bt4-R2 would be heterozygous for Cry2Ab resistance, with a recessive resistance allele from Bt4-R2 and a susceptible allele from AZP-R, yielding no resistant individuals. Consistent with this prediction, adjusted survival of the F1 larvae of AZP-R2 was 0% at a diagnostic concentration of Cry2Ab (Table 4).
If the resistance in AZP-R2 is conferred by a recessive allele at one locus, the expected survival of the F2 of AZP-R2 at a diagnostic concentration of Cry2Ab is 25% based on Mendelian inheritance (i.e., 25% resistant homozygotes). Consistent with this prediction, the adjusted survival of F2 larvae from AZP-R2 at the diagnostic concentration of Cry2Ab was 25% (Table 4). We exposed 1,020 F2 larvae of AZP-R2 to the diagnostic concentration of Cry2Ab and reared the progeny of the survivors (F3) on untreated diet. Adjusted survival of the F4 larvae at the diagnostic concentration of Cry2Ab was 100% (n = 80 larvae, 40 on treated diet and 40 on untreated diet), which is consistent with the expectation that the single selection allowed survival only of the individuals with homozygous resistance to Cry2Ab.
Inheritance of resistance to Cry1Ac in AZP-R2
We hypothesized that the resistance to Cry1Ac in AZP-R2 is conferred by recessive alleles at the cadherin locus PgCad1 because previous work showed that recessive alleles at this locus confer resistance to Cry1Ac in both AZP-R and Bt4R33,34. Previous results also showed that crosses between homozygous Cry1Ac-resistant individuals from these two strains yielded offspring with 100% survival at the diagnostic concentration of Cry1Ac (10 μg Cry1Ac per mL diet)33. Given that resistance of Bt4-R2 to Cry1Ac was derived from Bt4R, we expected that a cross between Cry1Ac-resistant adults from Bt4R-2 and AZP-R would yield AZP-R2 offspring resistant to Cry1Ac. When Bt4-R2 and AZP-R were crossed to generate AZP-R2, concurrent bioassays showed that survival at the diagnostic concentration was 100% for AZP-R and 86.2% for Bt4-R2 (n = 60 larvae per strain, 30 tested at the diagnostic concentration and 30 on control diet). The higher survival of Bt4-R2 in this test conducted at the University of Arizona compared with the 56.5% survival when Bt4-R2 was tested previously at USDA ARS U.S. Arid Land Agricultural Research Center (ALARC) (Table 2) could reflect differences between the two laboratories in bioassay methods, changes in the strain over time, or both.
Assuming Hardy-Weinberg equilibrium and recessive inheritance of resistance conferred by the cadherin locus, the estimated genotype frequency at the cadherin locus when AZP-R2 was generated is 1.0 resistant homozygotes for AZP-R and 0.862 resistant homozygotes and 0.133 heterozygotes for Bt4-R2. Based on these frequencies, we predicted survival of the F1 and subsequent generations of AZP-R2 at the diagnostic concentration of Cry1Ac would be 93% (0.862 + 0.5[0.133] = 0.928). The mean adjusted survival of F1, F2 and F4 larvae of AZP-R2 was 95% (range = 90 to 100%) (Table 4), which does not differ significantly from the predicted 93% (one-sample t-test, t = 0.69, P = 0.56).
In AZP-R2, the percentage of resistant individuals was 90% in the F1 and 100% in the F2 for Cry1Ac, compared with 0% in the F1 and 25% in the F2 for Cry2Ab (Table 4). The lower resistance to Cry2Ab than Cry1Ac in these two generations indicates that the cadherin alleles conferring resistance to Cry1Ac in AZP-R2 conferred little or no cross-resistance to Cry2Ab.
Survival and development rate on Bt cotton bolls producing Cry1Ac and Cry2Ab
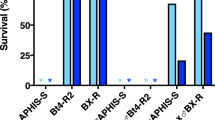
The only strain with survivors on field-collected bolls of two-toxin cotton was AZP-R2, which had survivors in both generations tested (F3 = 3.3% and F4 = 5.6%, Fig. 4 and Table S1). Survival of larvae reared on field-collected bolls producing Cry1Ac and Cry2Ab was 0% for the other four strains tested (Fig. 4 and Table S1): susceptible strain APHIS-SOM, Cry1Ac-resistant strain AZP-R, Bt4-R2, which had moderate resistance to Cry1Ac and high resistance to Cry2Ab (Tables 2 and 3) and AZP-R2U, a subset of AZP-R2 that was not selected with Cry2Ab. Survival on two-toxin bolls was higher for AZP-R2 than AZP-R2U (Fisher’s exact test, total n = 749, P = 0.002), which is consistent with the diet bioassay results (Table 4) indicating that selection of the F2 with Cry2Ab increased the percentage of individuals resistant to Cry2Ab in AZP-R2.
Survival of pink bollworm larvae on field-grown cotton bolls.
Strains were susceptible (APHIS-SOM), resistant to Cry1Ac (AZP-R), moderately resistant to Cry1Ac and highly resistant to Cry2Ab (Bt4-R2), uniformly resistant to Cry1Ac and 25% of larvae resistant to Cry2Ab (AZP-R2U F3) and highly resistant to Cry1Ac and Cry2Ab (AZP-R2 F3 and F4). Survival in the laboratory on bolls collected from field-grown plants of non-Bt cotton (green) and Bt cotton producing Cry1Ac and Cry2Ab (blue). Asterisks indicate 0% survival on Bt cotton bolls producing Cry1Ac and Cry2Ab for all larvae tested except AZP-R2 F3 and F4, which both had survival of 0.17 on two-toxin cotton relative to non-Bt cotton (see Table S1 and Methods for details).
Relative to survival on field-collected bolls of non-Bt cotton, the proportion of larval survival on bolls producing both toxins was 0.17 for both F3 and F4 generations of AZP-R2 (Fig. 4 and Table S1), indicating incomplete resistance35. For AZP-R2, the percentage of survivors reaching pupation by the end of the bioassays was 0% on two-toxin bolls versus 93% on non-Bt bolls in the F3 (Fisher’s exact test, total n = 20, P = 0.0004) and 8.0% on two-toxin bolls versus 69% on non-Bt bolls in the F4 (Fisher’s exact test, total n = 51, P < 0.0001). The slower development of AZP-R2 on two-toxin bolls relative to non-Bt cotton bolls also indicates incomplete resistance.
Evaluation of fitness costs associated with increased survival on two-toxin Bt cotton
To check for fitness costs, we compared performance on non-Bt cotton bolls for the F3 and F4 of AZP-R2 (which both had survival on two-toxin bolls) relative to AZP-R2U, as well as APHIS-SOM, AZP-R, Bt4-R2 (which all had 0% survival on two-toxin bolls). The specific comparison between the F3 of AZP-R2 and AZP-R2U is especially relevant because these two sets of larvae were tested simultaneously, had a similar genetic background and differed only in that the parents of AZP-R2, but not AZP-R2U, were selected with Cry2Ab. Larval survival on non-Bt cotton bolls for the F3 was not lower for AZP-R2 (20%) than AZP-R2U (16%) (Fig. 4 and Table S1). These results imply that a fitness cost affecting survival on non-Bt cotton was not associated with the increased resistance to Cry2Ab and increased survival on two-toxin bolls of AZP-R2 versus AZP-R2U. Larval survival on non-Bt cotton bolls was not lower for the F3 and F4 of AZP-R2 relative to any of the other strains tested (Fig. 4 and Table S1), yet these more general comparisons may be affected by differences between strains that are not directly related to resistance.
The percentage of F3 survivors reaching pupation after 21 days on non-Bt bolls was not lower for AZP-R2 (93%) than AZP-R2U (92%). As described above for survival on non-Bt bolls, these results indicate that no major fitness cost affecting the percentage of survivors pupating after 21 days on non-Bt bolls was associated with the increased resistance to Cry2Ab of AZP-R2 relative to AZP-R2U. The percentage of F3 survivors that were adults after 21 days on non-Bt bolls was 0% for AZP-R2 versus 17% for AZP-R2U, but this difference suggesting a potential fitness cost is not statistically significant (Fisher’s exact test, total n = 27, P = 0.19). In addition, the percentage of survivors that were adults after 21 days on non-Bt bolls did not differ significantly between the F3 and F4 of AZP-R2 pooled (0%) and the four other strains pooled (7.9% for APHIS-SOM, AZP-R, Bt4-R2 and AZP-R2U; Fisher’s exact test, total n = 79, P = 0.11). Nonetheless, more extensive testing might reveal fitness costs affecting development rate or other traits.
Discussion
We started with pink bollworm strains that were susceptible to Cry2Ab and achieved 18,000- to 150,000-fold resistance to Cry2Ab in strain Bt4-R2 after only two generations of laboratory selection (Fig. 1, Tables 2 and 3). To create the AZP-R2 strain with high levels of resistance to both toxins, we crossed Bt4R-2 with the AZP-R strain, which was highly resistant to Cry1Ac but not to Cry2Ab31. The results indicate inheritance of resistance to Cry2Ab in Bt4-R2 and AZP-R2 is autosomal, recessive and probably conferred by a single locus (Figs 2 and 3, Tables 3 and 4). In boll bioassays conducted after one additional selection of AZP-R2 with Cry2Ab, the proportion of larval survival on Bt cotton producing Cry1Ac and Cry2Ab relative to non-Bt cotton was 0.17 for AZP-R2 (Fig. 4 and Table S1). The lower survival and slower development of AZP-R2 on two-toxin cotton relative to non-Bt cotton indicate incomplete resistance35.
As far as we know, AZP-R2 is the first pink bollworm strain with documented survival on Bt cotton bolls producing both Cry1Ac and Cry2Ab. In the tests here, survival on two-toxin Bt cotton was 0% for AZP-R, AZP-R2U and Bt4-R2 (Fig. 4). These results are consistent with previous results for AZP-R, indicating that larvae from this Cry1Ac-resistant strain were killed by the Cry2Ab in two-toxin cotton31,36. Analogously, we infer that the Cry2Ab in two-toxin Bt cotton killed larvae of AZP-R2U, a subset of AZP-R2 that was not selected with Cry2Ab after AZP-R2 was created. Conversely, the Cry1Ac in two-toxin cotton probably killed larvae of Bt4-R2, because it was highly resistant to Cry2Ab, but had only 28-fold resistance to Cry1Ac (Tables 2 and 3).
In previous work, pink bollworm strain BX-R1 had 0% survival on Bt cotton bolls producing Cry1Ac and Cry2Ab, despite its high survival on Bt cotton bolls producing Cry1Ac, 420-fold resistance to Cry1Ac and 240-fold resistance to Cry2Ab31. Because the concentration of toxin is 160 times higher for Cry2Ab than Cry1Ac in bolls of two-toxin cotton37, the minimum LC50 value required for survival on these bolls is expected to be much higher for Cry2Ab than Cry1Ac. We conclude that survival on two-toxin cotton bolls was higher for AZP-R2 than BX-R1 primarily because of the higher resistance of AZP-R2 to Cry2Ab.
With some assumptions, we can estimate the initial frequency of Cry2Ab resistance alleles in Bt4R-P and Bt4R. If 1 to 13 of the survivors from the approximately15,000 larvae screened initially with Cry2Ab were homozygous resistant, the frequency of homozygous resistant individuals in these strains was roughly 0.00007 to 0.0009. Assuming Hardy-Weinberg equilibrium, the estimated initial Cry2Ab resistance allele frequency is about 0.008 to 0.03. The lower limit is almost certainly an underestimate, because it is unlikely that just one of the 13 survivors of the initial selection was resistant to Cry2Ab. Although alleles conferring high resistance to Cry2Ab were not extremely rare in the Bt4R and Bt4R-P strains, such alleles were not detected in other strains of pink bollworm, including the BX-R1, BX-R2 and BX-R strains that were selected extensively with Cry2Ab31. For comparison, the estimated Cry1Ac resistance allele frequency for Arizona field populations of pink bollworm was 0.16 in 1997, the second year of Bt cotton cultivation32. The estimated Cry2Ab resistance allele frequency for Helicoverpa punctigera in Australia was 0.006 in non-cropping areas and 0.008 in cropping areas before cotton producing Cry2Ab was commercialized38.
Consistent with previous results from lab- and field-selected strains of pink bollworm from Arizona and India, respectively22,25,26,30,31, the results here show that pink bollworm resistance to Cry1Ac does not confer strong cross-resistance to Cry2Ab. For example, Bt4R-P had 400-fold resistance to Cry1Ac, yet was susceptible to Cry2Ab (Table 2). Also, survival at a diagnostic toxin concentration for the F1 generation of AZP-R2 was 90% for Cry1Ac and 0% for Cry2Ab (Table 4).
We also found here that selection for resistance to Cry2Ab did not cause strong cross-resistance to Cry1Ac. For example, relative to its parent strains Bt4R and Bt4R-P, selection of Bt4R-2 with Cry2Ab increased resistance to Cry2Ab >150,000-fold, but resistance of Bt4R-2 to Cry1Ac was only 28-fold, which is between the resistance to Cry1Ac of its two parent strains (11- and 400-fold for Bt4R and Bt4R-P, respectively, Table 2). This pattern differs from our previous results with the BX-R1 and BX-R2 strains of pink bollworm, where selection with Cry2Ab produced up to 420-fold cross-resistance to Cry1Ac31. While the mechanism of the previously reported “asymmetrical” cross-resistance between Cry2Ab and Cry1Ac remains unknown, the results here and previously31,33 demonstrate that the pink bollworm mutations conferring resistance to Cry1Ac by disrupting a Cry1Ac-binding cadherin protein do not confer cross-resistance to Cry2Ab. Furthermore, given that Cry1A and Cry2A bind to different sites in the larval midgut of pink bollworm15,16, it is unlikely that mutations affecting a single toxin-binding protein can confer resistance to both toxins in this pest31. In Spodoptera exigua, however, both Cry1Ac and Cry2Ab bind to midgut cadherin39.
We previously hypothesized that two or more loci confer resistance to Cry2Ab in the BX-R strains31. If true, this would differ from the apparent single-locus control of resistance to Cry2Ab in Bt4-R2 and AZP-R2 seen here. Because resistance to Cry1Ac is recessive in the BX-R strains31 as well as in AZP-R2 and Bt4R-2, interstrain complementation tests should be useful for determining if the Cry2Ab resistance alleles in the BX-R strains and those found here occur at the same locus.
Although survival on Bt cotton producing Cry1Ac and Cry2Ab was not reported previously for pink bollworm, it is documented for two other major lepidopteran pests: Helicoverpa zea in independent field, greenhouse and laboratory experiments17,40,41 and Trichoplusia ni reared for seven days in the laboratory on cotton leaves, then transferred to whole cotton plants in the greenhouse42. The results with T. ni are strikingly similar to the results here with pink bollworm, including high levels of resistance to both toxins, incomplete resistance to two-toxin Bt cotton, little or no cross-resistance between the two toxins and autosomal, recessive inheritance to each toxin conferred by a different locus42,43. The resistance of T. ni to Cry2Ab was not linked with cadherin, nor several other candidate resistance genes including the ATP-binding cassette transporter gene ABCC2, which is linked with resistance to Cry1A toxins in T. ni and several other lepidopterans44,45.
Although incomplete resistance to two-toxin Bt cotton was also seen in the GA-R strain of H. zea17, other aspects of its resistance differ markedly from those of pink bollworm and T. ni, including non-recessive inheritance of resistance to Cry1Ac, less than five-fold resistance to Cry2Ab and selection with Cry1Ac that increased survival on two-toxin cotton17. Direct interspecific comparisons between susceptible strains show that intrinsic susceptibility is much lower for H. zea than pink bollworm for both Cry1Ac (72-fold) and Cry2Ab (485-fold)37. Field-evolved resistance has been documented in populations of H. zea, T. ni and pink bollworm to Cry1Ac, but only for H. zea to Cry2Ab11,22,23,24,25,26,45,46,47.
The pink bollworm survival on Bt cotton producing Cry1Ac and Cry2Ab reported here has implications for managing this pest in the field, particularly in China and India. Given that our multi-toxin resistant strain was generated from relatively small populations selected in the laboratory, the billions of pink bollworm larvae in field populations exposed to Bt cotton in China and India are also expected to harbor alleles for resistance to both toxins. Indeed, pink bollworm populations in India have diverse mutations in cadherin genes associated with field-evolved resistance to Bt cotton producing Cry1Ac23. The risk of resistance is high in both countries, because neither has abundant refuges of non-Bt cotton or other pink bollworm host plants24,25,26,28. For populations resistant to Cry1Ac, only the Cry2Ab in two-toxin cotton is effective. In northern China, where a low, but significant increase in the percentage of pink bollworm resistant to Cry1Ac has been reported28, the two-toxin cotton is likely to be more durable if it is adopted before resistance to Cry1Ac becomes more common. In India, where resistance to Cry1Ac is already widespread and grower compliance with the refuge strategy is low, we expect rapid evolution of resistance to Cry2Ab. The genetically modified toxins Cry1AbMod and Cry1AcMod were effective in the laboratory against pink bollworm with resistance to Cry1Ac and Cry2Ab11, yet their efficacy in the field remains to be tested.
Methods
Insects
We used eight strains of pink bollworm from Arizona: APHIS-S, APHIS-SOM, Bt4R, Bt4R-P, Bt4-R2, AZP-R, AZP-R2 and AZP-R2U (Table 1 and Fig. 1). APHIS-S is a susceptible strain that had been reared in the laboratory for more than 30 years without exposure to Bt toxins49,50. APHIS-SOM is a susceptible strain derived by crossing APHIS-S with a susceptible field strain, collected from Somerton, Arizona in 200733. Bt4R is a laboratory-selected strain with moderate resistance to Cry1Ac and previously demonstrated survival on Bt cotton producing Cry1Ac Bt33,51,52. Bt4R-P was derived from Bt4R by greenhouse selection of larvae on Bollgard® DP 449 BG/RR cotton bolls in July 2010 (Fig. 1). Survivors of selection on 3 μg Cry2Ab per mL diet from both Bt4R (n = 6) and Bt4R-P (n = 7) were pooled as pupae and eggs collected from adults were used to start the Bt4-R2 strain in Dec. 2010 (Fig. 1). A second selection of Bt4-R2 on 5 μg Cry2Ab per mL diet was done before performing concentration-response and crossing experiments. AZP-R is a Cry1Ac-resistant strain that was started by pooling survivors of exposure to Cry1Ac in diet from 10 populations derived in 1997 from Arizona cotton fields53. In June 2012, we crossed Bt4-R2 with AZP-R to create the AZP-R2 strain (Fig. 1). We selected the F2 progeny of AZP-R2 on diet with 10 μg Cry2Ab per mL (Fig. 1). The crosses to generate AZP-R2 and all subsequent experiments were conducted at the University of Arizona in Tucson. All other experiments were conducted at the USDA ARS U.S. ALARC in Maricopa, Arizona.
Diet Bioassays
We used 21-d diet incorporation bioassays to evaluate susceptibility to Cry1Ac and Cry2Ab31,33,36,53. Newly hatched neonates were placed individually in wells of bioassay trays (BIO-BA-128, Pitman, NJ) containing approximately 1.5 mL of diet and covered with Pull N’ Peel tab tray covers (BIO-CU-16, Pitman, NJ). We tested five to eight concentrations of each toxin ranging from 0–1,000 μg toxin per mL diet. After 21 d at 26 °C and a photoperiod of 14 light:10 dark, we scored live fourth instar larvae, pupae and adults as survivors.
Bt Toxins
The source of Cry1Ac was MVP II (Dow Agrosciences, San Diego, CA), a liquid formulation containing a hybrid protoxin produced in and encapsulated by Pseudomonas fluorescens30,54. The protoxin amino sequence is 98.5% identical between MVP II and Cry1Ac. The first 1,067 of 1,182 amino acids in MVP II protoxin, including the entire active toxin (domains I, II and III), are identical to the holotype Cry1Ac protoxin and are encoded by part of the Cry1Ac gene from B. thuringiensis subsp. kurstaki HD-7354. We used Cry2Ab protoxin from Luke Masson and Jie Zhang that was produced using a recombinant acrystalliferous strain of Bt subspecies kurstaki (HD73 cry-) that was transformed with the cry2Ab gene from strain HD1 of Bt subspecies kurstaki as described previously55,56. Although pink bollworm responses to Cry2Ab from the two sources were similar, we used Cry2Ab from one source for each experiment so that comparisons among strains and the progeny from crosses within experiments were not affected by potential differences between the two sources of Cry2Ab. One exception is the multi-generational data for AZP-R2 reported in Table 4, where the F1 was tested with Cry2Ab from Masson and the F2 and F4 were tested with Cry2Ab from Zhang.
Mass crosses
We obtained and determined the sex of 120 Bt4-R2 pupae and 120 APHIS-S pupae and set up four reciprocal mass crosses including 30 ♂ Bt4-R2 × 30 ♀ APHIS-S, 30 ♀ Bt4-R2 × 30 ♂ APHIS-S, 30 ♀ APHIS-S × 30 ♂ APHIS-S and 30 ♀ Bt4-R2 × 30 ♂ Bt4-R2 in paper cups (350 mL). Adults were allowed to mate and deposit eggs onto oviposition paper. Newly hatched F1 neonates were transferred to individual wells of bioassay trays containing various concentrations of Cry2Ab and maintained at 26 °C (14:10 L:D). After 21 days, live individuals that developed beyond the third instar were scored as survivors49. For all bioassays containing F1 neonates resulting from crosses for which at least one of the parents was APHIS-S, we used concentrations of 0, 0.03, 0.1, 0.3, 1 and 10 μg Cry2Ab per mL diet. Concentrations used in bioassays on F1 neonates resulting from the Bt4-R2 intrastrain mass cross were 0, 1, 10, 30 and 100 μg Cry2Ab per mL diet. We tested a total of 736 neonates including 32 neonates at each concentration for all four mass crosses.
Single-pair crosses
To bolster evaluation of dominance, maternal effects and sex linkage and to generate informative families for future biphasic genetic linkage analysis34,57, we performed single-pair reciprocal crosses between Bt4-R2 and APHIS-S. We obtained and tested progeny from five or six families from each of the four types of single-pair cross: ♂ Bt4-R2 × ♀ APHIS-S, ♀ Bt4-R2 × ♂ APHIS-S, ♀ APHIS-S × ♂ APHIS-S and ♀ Bt4-R2 × ♂ Bt4-R2. The progeny from these crosses were tested for survival on control diet without any Bt toxin or on diet with 10 μg Cry2Ab per mL diet. After 14 days at 26 °C (14:10 L:D), we collected all surviving fourth instars and pupae.
Boll bioassays
We evaluated survival and development rate in the laboratory using bolls collected from cotton planted on 23 April 2012 in heavy loam soil at the Campus Agricultural Center of the University of Arizona in Tucson, Arizona. Plants were grown using standard agronomic practices, flood irrigated and not treated with insecticide. We used Bt cotton cultivar DP 164 B2RF, which produces Cry1Ac and Cry2Ab and non-Bt cotton cultivar DP 5415.
We conducted two sets of boll bioassays. In the first set, we tested five strains: APHIS-SOM, AZP-R, Bt4-R2, AZP-R2 and AZP-R2U. In the second set, we tested only AZP-R2 to verify its survival on two-toxin Bt cotton that was seen in the first set. In the first set, bolls were collected on 20 August 2012 and infested on 22 August 2012. In the second set, bolls were collected and infested on 18 September 2012.
In both sets of bioassays, fruiting branches with bolls (about four to six weeks old) and leaves were cut from plants, put in plastic bags under cool conditions and brought to the lab (6 km from the field). Bolls were briefly rinsed with dilute bleach (5%), washed with running water and blotted dry. Leaves of plants from which the bolls were used were frozen for subsequent testing for Bt toxins (see below).
In the first assays, bolls with bracteoles removed were stored at 4 °C for two days before infestation. We put 15 neonates on the carpel surface of each boll using a fine brush. For each of the five strains tested, we infested 10 bolls of Bt cotton and five bolls of non-Bt cotton (total n = 75 bolls infested with 1,025 larvae). Before infestation, the mean fresh weight for the 75 bolls was 19 g (range = 15 to 23 g). We put each boll in a 150-mL plastic cup sealed with a plastic lid and put the cups in an incubator at 29 °C (16:8 L:D). After two days, we loosened the lid, lined each cup with Kimwipe tissue paper and transferred all cups to 1.5-L plastic buckets that were lined with paper towels and had lids with fine mesh windows. After two to four days in the buckets, lids were removed to increase ventilation and reduce mold growth. After 21 days, we cut bolls open and recorded survivors inside bolls and on the tissue paper in the buckets.
In the second assays, bolls were infested by putting a piece of paper towel (ca. 1 × 1 cm) bearing pink bollworm eggs under the bracteoles of each boll. We infested 30 Bt cotton bolls and 5 non-Bt cotton bolls using eggs from AZP-R2. We put each boll in a 150-mL plastic cup with a plastic lid and put the cups in an incubator at 29 °C (16:8 L:D). After two to four days, for each boll, we counted pink bollworm larval entry holes and removed the bracteoles and pieces of paper bearing eggs. We excluded 10 bolls of Bt cotton and one of non-Bt cotton from further consideration because mold prevented accurate identification of entry holes. For the remaining 20 bolls of Bt cotton and four bolls of non-Bt cotton, we transferred each boll to a new cup lined with tissue paper and held the cups in the 1.5-L plastic buckets as described above. After 22–24 days, we checked for survivors as described above.
In both sets of bioassays, we scored live fourth instars and pupae as survivors. In the first set, we calculated survival as the number of survivors divided by the total number of larvae placed on the bolls. In the second set, we calculated survival as the number of survivors divided by the number of pink bollworm entry holes.
To check for Cry1Ac and Cry2Ab in leaves, we used the QuickStix Combo Kit for Cry1A and Cry2A Leaf & Seed (Envirologix, Portland, Maine) according to the manufacturer’s instructions. In the first set, we tested 12 leaves from Bt cotton plants (including leaves from all four plants from which bolls yielded AZP-R2 survivors) and 10 leaves from non-Bt cotton plants. In the second set, we tested 18 leaves from Bt cotton plants (including leaves from 15 of 16 plants from which bolls yielded AZP-R2 survivors) and 2 leaves from non-Bt cotton plants. All Bt cotton leaves tested positive for Cry1A and Cry2A (30 of 30) and all non-Bt cotton leaves tested negative for both toxins (12 of 12).
Data analysis
In diet bioassays, larval survival (%) was calculated as number of fourth instars, pupae, or adults alive at 21 d divided by the initial number of neonates times 100%. For cotton boll assays, we divided survival on two-toxin Bt cotton bolls by the survival on non-Bt cotton bolls (as described above) to calculate the proportional survival on two-toxin Bt cotton relative to non-Bt cotton. We estimated the concentration of toxin killing 50% of larvae (LC50) and its 95% fiducial limits from diet bioassays using SAS PROC PROBIT58. Resistance ratios were calculated by dividing the concentration of toxin in diet killing 50% of larvae (LC50) of a strain by the LC50 of the susceptible strain APHIS-S. We estimated dominance (h), which varies from zero for completely recessive resistance to one for completely dominant resistance, as previously described59.
Additional Information
How to cite this article: Fabrick, J. A. et al. Multi-Toxin Resistance Enables Pink Bollworm Survival on Pyramided Bt Cotton. Sci. Rep. 5, 16554; doi: 10.1038/srep16554 (2015).
References
Mendelsohn, M., Kough, J., Vaituzis, Z. & Matthews, K. Are Bt Crops Safe? Nat. Biotechnol. 21, 1003–1009 (2003).
Bravo, A., Likitvivatanavong, S., Gill, S. S. & Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41, 423–431 (2011).
Sanahuja, G., Banakar, R., Twyman, R. M., Capell, T. & Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 9, 283–300 (2011).
Nicolia, A., Manzo, A., Vironessi, F. & Rosselini D. An overview of the last 10 years of genetically engineered crop safety research. Critical Rev. Biotechnol. 34, 77–88 (2014).
James, C. Global status of commercialized biotech/GM crops. ISAAA Briefs 49 (ISAAA, Ithaca, NY, 2014) (2014).
National Research Council. The impact of genetically engineered crops on farm sustainability in the United States. (National Academies Press, Washington, D.C., 2010) (2010).
Carpenter, J. E. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 28, 319–321 (2010).
Hutchison, W. D. et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330, 222–225 (2010).
Tabashnik, B. E. et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat. Biotechnol. 28, 1304–1307 (2010).
Lu, Y., Wu, K., Jiang, Y., Guo, Y. & Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365 (2012).
Tabashnik, B. E. et al. Efficacy of genetically modified Bt toxins alone and in combinations against pink bollworm resistant to Cry1Ac and Cry2Ab. PLoS ONE 8(11), e80496. doi:10.1371/journal.pone.0080496 (2013).
Carrière, Y., Crickmore, N. & Tabashnik, B. E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 33, 161–168 (2015).
Roush, R. T. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Phil. Trans. Roy. Soc. London B: Biological Sciences 353, 1777–1786 (1998).
Gould, F. Simulation models for predicting durability of insect-resistant germ plasm: A deterministic diploid, two-locus model. Environ. Entomol. 15, 1–10 (1986).
Karim, S., Riazuddin, S., Gould, F. & Dean, D. H. Determination of receptor binding properties of Bacillus thuringiensis delta-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pestic. Biochem. Phys. 67, 198–216 (2000).
Gonzalez-Cabrera, J., Escriche, B., Tabashnik, B. E. & Ferre, J. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gossypiella). Insect Biochem. Mol. Biol. 33, 929–935 (2003).
Brévault, T. et al. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA 110, 5806–5811 (2013).
USDA National Agricultural Statistics Service. Crop Production 2014 Summary. http://www.usda.gov/nass/PUBS/TODAYRPT/cropan15.pdf (2015).
Choudhary, B. & Gaur, K. Bt Cotton in India: A Country Profile. ISAAA Series of Biotech Crop Profiles (ISAAA, Ithaca, NY, 2013).
Henneberry, T. J. & Naranjo, S. E. Integrated management approaches for pink bollworm in the Southwestern United States. Integr. Pest Manage. Rev. 3, 31–52 (1998).
Tabashnik, B. et al. Sustained susceptibility of pink bollworm to Bt cotton in the United States. GM Crops Food 3(3), 1–7 (2012).
Dhurua, S. & Gujar, G. T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 67, 898–903 (2011).
Fabrick, J. A. et al. Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PLoS ONE 9(5), e97900. 10.1371/journal.pone.0097900 (2014).
Ojha, A. et al. Analysis of resistance to Cry1Ac in field-collected pink bollworm, Pectinophora gossypiella (Lepidoptera:Gelechiidae), populations. GM Crops Food 5(4), 280–286 (2014).
Nair, R., Kamath, S. P., Mohan, K. S., Head, G. & Sumerford, D. V. Inheritance of field-relevant resistance to the Bacillus thuringiensis protein Cry1Ac in Pectinophora gossypiella (Lepidoptera: Gelechiidae) collected from India. Pest Manag. Sci. In press. 10.1002/ps.4023 (2015).
Mohan, K. S., Ravi, K. C., Suresh, P. J., Sumerford, D. & Head, G. P. Field resistance to the Bacillus thuringiensis protein Cry1Ac expressed in Bollgard® hybrid cotton in pink bollworm, Pectinophora gossypiella (Saunders), populations in India. Pest Manag. Sci. In press. 10.1002/ps.4047 (2015).
Tabashnik, B. E., Mota-Sanchez, D., Whalon, M. E., Hollingworth, R. M. & Carrière, Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 107, 496–507 (2014).
Wan, P. et al. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE 7(1), e29975. doi:10.1371/journal.pone.0029975 (2012).
Jin, L. et al. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 33, 169–174 (2015).
Tabashnik, B. E. et al. Inheritance of resistance to Bt toxin Cry1Ac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 95, 1018–1026 (2002).
Tabashnik, B. E. et al. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Natl. Acad. Sci. USA 16, 11889–11984 (2009).
Tabashnik, B. E. et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc. Natl. Acad. Sci. USA 97, 12980–12984 (2000).
Fabrick, J. A. & Tabashnik, B. E. Similar genetic basis of resistance to Bt toxin Cry1Ac in boll-selected and diet-selected strains of pink bollworm. PLoS ONE 7(4), e35658. 10.1371/journal.pone.0035658 (2012).
Morin, S. et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100, 5004–5009 (2003).
Carrière, Y. & Tabashnik, B. E. Reversing insect adaptation to transgenic insecticidal plants. Proc. Roy. Soc. B: Biol. Sci. 268, 1475–1480 (2001).
Tabashnik, B. E. et al. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Microbiol. 68, 3790–3794 (2002).
Sivasupramaniam, S. et al. Toxicity and characterization of cotton expressing Bacillus thuringiensis Cry1Ac and Cry2Ab2 proteins for control of lepidopteran pests. J. Econ. Entomol. 101, 546–554 (2008).
Downes, S., Parker, T. & Mahon, R. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II® cotton. PLoS ONE 5(9), e12567 (2010).
Qui, L. et al. Cadherin is involved in the action of Bacillus thuringiensis toxins Cry1Ac and Cry2Aa in the beet armyworm, Spodoptera exigua. J. Invertebr. Pathol. 127, 47–53 (2015).
Jackson, R. E., Bradley, J. R. & van Duyn, J. W. Performance of feral and Cry1Ac-selected Helicoverpa zea (Lepidoptera: Noctuidae) strains on transgenic cottons expressing one or two Bacillus thuringiensis ssp. kurstaki proteins under greenhouse conditions. J. Entomol. Sci. 39, 46–55 (2004).
Brévault, T., Tabashnik, B. E. & Carrière, Y. A seed mixture increases dominance of resistance to Bt cotton in Helicoverpa zea. Sci. Rep. 5, 1–7 (2015).
Kain, W. et al. Resistance of Trichoplusia ni populations selected by Bacillus thuringiensis sprays to pyramided Bt cotton plants expressing Cry1Ac and Cry2Ab. Appl. Environ. Microbiol. 81(5), 1884–1890 (2015).
Song, X., Kain, W., Cassidy, D. & Wang, P. Resistance to Bacillus thuringiensis toxin Cry2Ab in Trichoplusia ni is conferred by a novel genetic mechanism. Appl. Environ. Microbiol. In press. 10.1128/AEM.00593-15 (2015).
Baxter, S. W. et al. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics 189, 675–679 (2011).
Heckel, D. G. Learning the ABCs of Bt: ABC transporters and insect resistance to Bacillus thuringiensis provide clues to a crucial step in toxin mode of action. Pest. Biochem. Physiol. 104, 103–110 (2012).
Janmaat, A. F. & Myers, J. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc. Roy. Soc. London – Biol. Sci. 270, 2263–2270 (2003).
Ali, M. I., Luttrell, R. G. & Young, S. Y. Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J. Econ. Entomol. 99, 164–175 (2006).
Ali, M. I. & Luttrell, R. G. Susceptibility of bollworm and tobacco budworm (Lepidoptera: Noctuidae) to Cry2Ab2 insecticidal protein. J. Econ. Entomol. 100, 921–931 (2007).
Liu, Y. B. et al. Effects of Bt cotton and Cry1Ac toxin on survival and development of pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 94, 1237–1242 (2001).
Bartlett, A. C. Resistance of the pink bollworm to Bt transgenic cotton. In Proceedings, Beltwide Cotton Conferences. National Cotton Council of America, Memphis, TN. pp 766–768 (1995).
Henneberry, T. J., Forlow-Jech, L. & Maurer, J. Effects of pink bollworm larval feeding on ‘NuCOTN 33b’ cotton bolls and pollen and tolerance to Cry1Ac toxin in artificial diet bioassays. Southwest. Entomol. 31, 169–185 (2006).
Fabrick, J. A., Forlow Jech, L. & Henneberry, T. J. Novel pink bollworm resistance to the Bt toxin Cry1Ac: Effects on mating, oviposition, larval development and survival. J. Insect Sci. 9, 8 pp (2009).
Tabashnik, B. E. et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc. Natl. Acad. Sci. USA 97, 12980–12984 (2000).
Gilroy, T. E. & Wilcox, E. R. Hybrid Bacillus thuringiensis gene, plasmid and transformed Pseudomonas fluorescens. U. S. patent 5,128,130 (1992).
Bah, A., Van Frankenhuyzen, K., Brousseau, R. & Masson, L. The Bacillus thuringiensis Cry1Aa toxin: Effects of trypsin and chymotrypsin site mutations on toxicity and stability. J. Invert. Pathol. 85, 120–127 (2004).
Wei, J. et al. Cross-resistance and interactions between Bt toxins Cry1Ac and Cry2Ab against the cotton bollworm. Sci. Rep. 5, 7714 10.1038/srep07714 (2015).
Heckel, D. G., Gahan, L. J., Liu, Y. B. & Tabashnik, B. E. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc. Natl. Acad. Sci. USA 96, 8373–8377 (1999).
SAS Institute. 2012. PROC user’s manual 9.3. SAS Institute, Cary, NC.
Liu, Y. B. & Tabashnik, B. E. Inheritance of resistance to the Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl. Environ. Microbiol. 63, 2218–2223 (1997).
Acknowledgements
We thank Barbara Hefner for technical support and Dale Spurgeon for comments that improved the paper. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
J.A.F., Y.C. and B.E.T. designed the study. J.A.F., G.C.U., A.J.Y. and B.D. performed the experiments. J.A.F. and B.E.T. analyzed the data. J.A.F. and B.E.T. wrote the manuscript. L.M. and J.Z. provided toxins. All authors reviewed the manuscript.
Ethics declarations
Competing interests
This is a cooperative investigation between USDA ARS, the University of Arizona and DuPont-Pioneer with J.A.F., B.E.T. and Y.C. receiving partial funding from DuPont-Pioneer to support this work (agreement #58-3K95-4-1666). J.A.F. is coauthor of a patent "Cadherin Receptor Peptide for Potentiating Bt Biopesticides" (patent numbers: US20090175974A1, US8354371, WO2009067487A2, WO2009067487A3). B.E.T. is a coauthor of a patent on modified Bt toxins, "Suppression of Resistance in Insects to Bacillus thuringiensis Cry Toxins, Using Toxins that do not Require the Cadherin Receptor" (patent numbers: CA2690188A1, CN101730712A, EP2184293A2, EP2184293A4, EP2184293B1, WO2008150150A2, WO2008150150A3). Bayer CropScience, Dow AgroSciences, Monsanto and Syngenta did not provide funding to support this work, but may be affected financially by publication of this paper and have funded other work by some of the authors.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fabrick, J., Unnithan, G., Yelich, A. et al. Multi-Toxin Resistance Enables Pink Bollworm Survival on Pyramided Bt Cotton. Sci Rep 5, 16554 (2015). https://doi.org/10.1038/srep16554
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16554
- Springer Nature Limited
This article is cited by
-
Impact of numerous larval diets on the biology of pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) under laboratory conditions
Journal of Cotton Research (2024)
-
Genome-wide analysis reveals distinct global populations of pink bollworm (Pectinophora gossypiella)
Scientific Reports (2023)
-
Frequency of Cry1Ac and Cry2Ab resistance alleles in pink bollworm, Pectinophora gossypiella Saunders from Andhra Pradesh, India
Phytoparasitica (2023)
-
Impact of various oviposition substrates on biology of pink bollworm Pectinophora gossypiella (Lepidoptera: Gelechiidae) under laboratory conditions
International Journal of Tropical Insect Science (2022)
-
Pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) survival on transgenic cotton in India
Egyptian Journal of Biological Pest Control (2021)