Abstract
We previously demonstrated that prodynorphin (PDYN) haplotypes and single nucleotide polymorphism (SNP) rs2281285 are associated with alcohol dependence and the propensity to drink in negative emotional states and recent studies suggest that PDYN gene effects on substance dependence risk may be sex-related. We examined sex-dependent associations of PDYN variation with alcohol dependence and related phenotypes, including negative craving, time until relapse after treatment and the length of sobriety episodes before seeking treatment, in discovery and validation cohorts of European ancestry. We found a significant haplotype-by-sex interaction (p = 0.03), suggesting association with alcohol dependence in males (p = 1E-4) but not females. The rs2281285 G allele increased risk for alcohol dependence in males in the discovery cohort (OR = 1.49, p = 0.002), with a similar trend in the validation cohort (OR = 1.35, p = 0.086). However, rs2281285 showed a trend towards association with increased negative craving in females in both the discovery (beta = 10.16, p = 0.045) and validation samples (OR = 7.11, p = 0.066). In the discovery cohort, rs2281285 was associated with time until relapse after treatment in females (HR = 1.72, p = 0.037); in the validation cohort, it was associated with increased length of sobriety episodes before treatment in males (beta = 13.49, p = 0.001). Our findings suggest that sex-dependent effects of PDYN variants in alcohol dependence are phenotype-specific.
Similar content being viewed by others
Introduction
Converging evidence from experimental studies and postmortem human brain research indicate that the dynorphin/kappa-opioid receptor system plays an important role in alcohol and drug dependence1,2,3,4. Genetic findings indicate that sequence variations in PDYN and OPRK1 genes—which encode dynorphins and the kappa-opioid receptor, respectively—are associated with risk for alcohol dependence5,6, as well as opioid and cocaine dependence7,8. We recently demonstrated that PDYN haplotypes are associated with increased risk for alcohol dependence and propensity to use alcohol in order to relieve negative emotions (negative craving)9. We also found that the minor G alleles of rs2281285 in the second intron and rs6132153 downstream of PDYN exhibited trends towards association with both phenotypes9. In a separate study sample, we demonstrated that the rs2281285 G allele is also associated with drinking in order to avoid somatic or emotional discomfort associated with alcohol withdrawal10–another phenotype associated with negative craving11.
Negative (or ‘relief ’) craving is defined as the desire for drinking (craving) in the context of tension or negative emotions, in contrast to positive (or ‘reward’) craving defined as the desire for the rewarding properties of alcohol, or obsessive thoughts about drinking (obsessive or ‘temptation’ craving), conceptualized in a three-pathway psychobiological model11. According to this model, negative/relief craving reflects dysregulation in the glutamate/GABA balance in favor of excitation excess, while positive/reward craving reflects dysregulation in dopamine/opioid neurotransmission and obsessive/temptation craving is attributed to dysregulation in serotonin neurotransmission11. The evolving understanding of the complexity of opioid neurotransmission and the role of dynorphin/kappa-opioid receptor signaling in negative emotions and addiction12,13 and our findings associating PDYN variation with negative craving and alcohol withdrawal9,10, indicate that biological underpinnings of each contextual subtype of craving requires further investigation.
Subtypes and patterns of manifestation of alcohol and substance dependence are also known to be sex-dependent. For example, research findings indicate higher rates of alcohol dependence in males14; however, females report tendency to drink in response to unpleasant emotions more often than males and these associations are mediated by depression severity15. Our data indicate a stronger tendency towards heavy drinking in unpleasant emotional situations in female compared to male alcoholics16. Recently, it has been recognized that sexual dimorphism in disease may arise through evolutionary mechanisms (such as sexual selection and sexual antagonism)17, resulting in sex-dependent genetic architecture18,19; in fact, heritability estimates for alcohol dependence are higher in males than females, indicating that genetic risk may be sex-dependent14.
Importantly, experimental studies indicate sex-related differences in sensitivity to depressive-like effects of the kappa opioid receptor agonist20. Evidence also indicates that sequence variants within the PDYN gene modulate its expression and that effects on transcription differ by brain region, sex and cell type21. Moreover, experimental data indicates that PDYN expression in brain and ovarian tissue is modulated by dihydrotestosterone and gonadal steroid hormones22,23 and that PDYN mRNA levels, as well as cocaine-induced behaviors, may be modulated by estrogen and progesterone24. Therefore, it is possible that effects of PDYN variants on development of alcoholism and related phenotypes may also differ in males and females, resulting in SNP by sex interactions and sex-dependent genetic effects.
In fact, a sex-specific association between the C allele of downstream PDYN variant rs1022563 and risk of heroin dependence has been reported25,26. According to the 1000 Genomes Project Phase 1 CEU data, in populations of European ancestry, rs1022563 is in high linkage disequilibrium (LD, R2 = 0.81) with rs2281285 and is also in perfect LD (R2 = 1) with rs6132153--SNPs which we found to be associated with alcohol dependence and negative craving in European American subjects9. Therefore, it is possible that these PDYN variants exhibit related sex-dependent effects across substance-dependence and related traits.
To test these hypotheses, we re-analyzed our previously reported data by examining SNP-by-sex interaction effects on alcohol dependence and negative craving. We also conducted additional analyses focused on related phenotypes, including time to relapse after treatment and the length of sobriety episodes before seeking treatment.
Methods
Discovery Sample
This work was approved by the Institutional Review Board of the Mayo Clinic, Rochester, Minnesota and was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants signed informed consent and gave permission for the use of their information in future research of alcohol dependence and related phenotypes. Characteristics of the samples and genotyping procedures have been described previously9,27. In brief, the discovery cohort consisted of 816 alcohol-dependent cases and 1248 controls recruited from alcohol dependent subjects and non-alcohol dependent controls treated in programs affiliated with Mayo Clinic, Rochester, Minnesota9. The Illumina GoldenGate SNP assay was used for genotyping in alcohol dependent subjects (cases) and included 13 PDYN variants (chosen to match the 13 PDYN variants previously genotyped in controls) and 43 ancestry informative markers28 used to verify self-reported race. The genotyping data for control subjects was retrieved from a previously published genome wide association study using the Illumina 660 genome-wide SNP array29. While potential control subjects with a history of alcohol dependence were excluded, controls were not required to have a history of exposure to alcohol. Controls were not matched to cases on age and sex and were older and included more females9.
Because PDYN has undergone positive selection, minor allele frequencies for PDYN variants differ substantially across human subpopulations30, potentially increasing the possibility for spurious associations due to population stratification. To avoid this risk, analyses were restricted to subjects with European ancestry, which were verified with the use of ancestry informative markers. Both discovery and validation cases were recruited from subjects actively enrolled in alcohol treatment programs (see Supplementary Materials for a description of the treatment programs). Clinical and demographic characteristics of the alcohol dependent subjects included in the discovery and validation cohorts are described below and summarized in Table 1.
In the discovery cohort, presence of alcohol dependence was determined in a clinical assessment by board certified addiction psychiatrists and defined based on DSM-IV-TR criteria31. Negative craving was measured by the negative sub-scale of the Inventory of Drug Taking Situations (IDTS)32. Relapse after treatment was defined as a return to any alcohol use following completion of treatment. Time until relapse was measured in the 12 month period following standard treatment. Relapse/sobriety data are collected as a part of clinical follow up at 3, 6, 9 and 12 month intervals after completing standard treatment.
Statistical Analysis
Our previous study indicated that the effects of PDYN on alcohol dependence were driven by rs22812859, so this variant was the primary focus of analysis. In the discovery cohort, we performed single SNP association tests for the previously reported rs2281285 variant. We assumed additive genetic effects, coding SNP genotypes as the number of copies of the minor allele (0–1–2). We evaluated sex-related effects on risk for alcohol dependence of rs2281285 by examining a SNP-by-sex interaction under a logistic regression model, adjusted for age and sex. Similarly, we examined the SNP-by-sex interaction of rs2281285 on negative craving using linear regression (adjusted for age and sex) in a subsample of N = 196 cases with available IDTS data. In addition to phenotypes investigated in our previous publications, we also examined the SNP-by-sex effects of rs2281285 on time until relapse (in months) in a subset of N = 202 case subjects with 12 months of follow-up data after treatment using Cox proportional hazards regression (adjusted for age and sex). Along with tests of SNP-by-sex interaction, analyses were also conducted adjusted for age and sex (without interaction), as well as adjusted for age and stratified by sex. Because the studied phenotypes of interest are highly correlated (and therefore cannot be considered independent tests), no standard multiple testing correction was appropriate; reported p-values are uncorrected. Instead, we used validation in the independent sample (see below) as a way to determine robustness of association findings. All statistical analysis was performed in R Statistical Software, version 2.14.0 (http://www.r-project.org).
Sensitivity Analyses
As sensitivity analyses, the single SNP analyses of rs2281285 were repeated with the variant rs6132153, which is strongly linked (R2 = 0.82 in controls); this variant is in complete LD with another variant previously associated with opioid dependence25. In the discovery cohort, we also examined previously identified haplotypes9 as sensitivity analyses, because haplotype analyses may be more powerful if an un-genotyped marker in linkage disequilibrium (LD) with rs2281285 is actually causal. Association tests of haplotype-by-sex interaction were adjusted for age and sex. Additionally, haplotype association tests were also performed adjusted for age and stratified by sex. We examined a previously identified PDYN gene-spanning haplotype (rs6045868-rs2235751-rs2281285) associated with alcohol dependence9 using logistic regression. Similarly, we examined the haplotype-by-sex interaction of a previously identified PDYN haplotype (rs6045784-rs910080-rs2235751-rs2281285)9 on negative craving using linear regression and time until relapse using Cox proportional hazards regression. Haplotype association tests were performed using the score statistic proposed by Schaid et al.33 and empirically derived p-values are reported for the global test (4 degrees of freedom for the three SNP haplotype and 5 degrees of freedom for the four SNP haplotype) and for each individual haplotype. Haplotypes were estimated and all haplotype analyses were performed using the R package ‘haplo.stats’ (http://cran.r-project.org/web/packages/haplo.stats/). As the haplotype and analyses and analyses of rs6132153 are highly correlated with the analyses of rs2281285, no additional multiple testing correction was applied.
Validation Sample
The validation cohort has been described in detail in a previous publication27. In brief, patients 18 years and older were consecutively recruited from an inpatient addiction treatment unit of the Department of Psychiatry, Ludwig-Maximilians University, Munich, Germany (see Supplementary Material). All study subjects met DSM IV criteria for alcohol dependence and were assessed with the Semi-Structured Interview for the Assessment on the Genetics of Alcoholism (SSAGA)34,35. All participants provided written informed consent approved by the ethical committee of the Ludwig-Maximilians University. Collected DNA samples were genotyped for rs2281285 only using a TaqMan® SNP Genotyping Assay in 467 alcohol dependent subjects and 431 controls, as described in our previous publication9.
In our previous publications, we examined the effects of rs2281285 on alcohol dependence and negative craving using this sample9,10, although sex-specific effects were not explored. In the present study, we used the validation cohort to examine the sex-dependent effects of rs2281285 on alcohol dependence using logistic regression, adjusted for age. The same measures of negative craving and time to return to drinking used in the discovery sample were not available in the validation sample, limiting the ability for direct replication; however, available measures assessing similar constructs were utilized to validate the findings of the discovery cohort. Specifically, because the IDTS assessment was not performed in this cohort, negative craving was instead assessed with a binary SSAGA item asking if the patient ever ingested alcohol to avoid unwanted emotional or somatic discomfort10; sex-specific effects of rs2281285 were assessed with logistic regression, adjusted for age. Furthermore, data on time until relapse after treatment for alcohol dependence was not available in this sample. Therefore, a related variable of length of sobriety before seeking treatment was utilized to assess association with rs2281285 using age-adjusted linear regression. The choice of this variable was based on an assumption that vulnerability to negative craving associated with PDYN variation may cause earlier return to drinking at any time, not just after treatment.
Results
Descriptive Statistics
Description of demographic characteristics of the study cohorts and investigated alcoholism-related phenotypes are presented in Table 1. Both discovery and validation cohorts were recruited from subjects enrolled in treatment programs and included more males then females. However, the validation cohort was younger (p < 0.0001), had heavier alcohol consumption (p < 0.0001) and a higher percentage of males (p = 0.018); analyses were adjusted for age and sex in both sample sets, but differences in data collection methods prohibited adjustment for consumption in the discovery sample. The discovery cohort had a larger sample size of cases (N = 816) and controls (N = 1248) to test associations between PDYN variants and alcohol dependence compared to the validation cohort (N = 467 and 431, respectively). However, smaller numbers of participants in the discovery cohort were assessed with the negative subscale of IDTS (N = 196) and had available post-treatment relapse data (N = 202). In the validation cohort, response to the SSAGA question about drinking to avoid emotional/physical symptoms of withdrawal (yes/no) and data about maximum length of sobriety before treatment were available for most subjects (N = 417 and 409, respectively).
Alcohol Dependence
The SNP-by-sex interaction effect of rs2281285 on alcohol dependence did not reach a statistically significant level but showed a trend towards association in the discovery cohort (p = 0.099), indicating that the effect of rs2281285 on risk for alcohol dependence may depend on sex. Subsequent stratified analyses examining the SNP effect separately in males and females yielded consistent results in the discovery and validation cohorts. As shown in Tables 2 and 3, we observed a stronger association between risk for alcohol dependence and the rs2281285 G allele in males in both the discovery (OR = 1.49, p = 0.002) and the validation cohort (OR = 1.35, p = 0.08), but no significant association in females.
Negative Craving
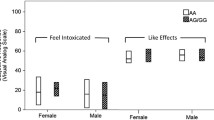
Conversely, sex-stratified analyses of rs2281285 on negative craving showed an association in females but not males (Tables 2 and 3). In females, the G allele of rs2281285 was associated with increased negative craving in the discovery sample (beta = 10.16, p = 0.05; Table 2) and trended towards association with avoidance of unwanted emotional and somatic discomfort in the validation sample (OR = 7.11, p = 0.07; Table 3).
Return to Drinking
Similarly, the rs2281285 G allele was associated with increased risk of post-treatment relapse (OR = 1.42, p = 0.048) in the discovery cohort (Table 2), where the association is driven by females (HR = 1.72, p = 0.037) but not males. In the validation cohort (Table 3), the minor rs2281285 G allele was associated with an increased length of sobriety of 10 months on average (p = 0.02), but the effect is driven by males (beta = 13.5, p = 0.001).
Sensitivity Analyses
Single SNP results for rs6132153 were similar to those for rs2281285 for all phenotypes in the discovery cohort (Supplemental Table 1). Additionally, we found a statistically significant haplotype-by-sex interaction effect on alcohol dependence in the discovery cohort (p = 0.03). In a sex-stratified analysis (Supplemental Table 2), the rs6045868-rs2235751-rs2281285 haplotype was associated with alcohol dependence in males (p = 1E-4) but not females. There was no significant PDYN gene-spanning haplotype-by-sex interaction effect on negative craving or time until relapse after treatment.
Discussion
Results presented here demonstrate that our previously reported findings of association between PDYN SNPs and haplotypes and alcohol dependence may be sex-related and importantly, the sex-dependent effects are also trait (phenotype)-specific. A significant haplotype-by-sex interaction was observed for alcohol dependence and stratified analyses indicated that the effect of the PDYN-spanning haplotype was present in males only. However, the SNP-by-sex interaction for rs2281285 was only marginally significant, indicating that perhaps rs2281285 is not the causal variant, but rather is in LD with a causal variant (or multiple variants) that is also tagged by the PDYN-spanning haplotype. Similarly to the results of the haplotype analysis, the effect of the minor G allele of rs2281285 was stronger in males in both discovery and validation cohort analyses and the male-specific effect observed in the discovery cohort was statistically significant. This effect was consistent in the direction and effect size in the validation cohort, where, despite smaller sample size, we also observed a trend for association of the rs2281285 G allele with increased risk for alcohol dependence in male but not in female alcoholics. Moreover, in sex-specific analyses of the effect of rs2281285 on negative craving, we observed a female-specific trend for association between the rs2281285 minor G allele and increased negative craving in both the discovery and validation cohorts. Similarly, the minor rs2281285 G allele was associated with increased risk of relapse in female subjects in the discovery cohort, while in the validation cohort this same allele was associated with increased length of sobriety before treatment in male alcoholics.
Notably, the genetic effects detected here have not been previously implicated in genome-wide association studies of alcohol dependence36,37,38,39,40. Many of these studies did not investigate SNP-sex interactions or perform sex-stratified analyses; and for prior studies that examined sex strata (or included males only), sample sizes were small. Our results demonstrating sex-specific genetic effects on alcohol related traits suggest that effects for some SNPs may be larger in one sex; by analyzing males and females together, the effects would be reduced and may not be detected. In light of evolutionary mechanisms underlying sex differences in disease risk18, it is likely that interactions between genetic variants and sex exist and ignoring such interactions in analyses can prevent detection of certain genetic effects41.
The findings of this study are intriguing, as the minor alleles of the PDYN SNPs rs2281285 and rs6132153 seem to have different effects on risk for alcohol dependence, negative craving and the length of sobriety before and after treatment for males and females. Specifically, the minor G alleles of these genetic variants convey increased risk for alcohol dependence in males, while increasing vulnerability to negative craving and post-treatment relapse in alcohol dependent females. One possible explanation for these findings is that the above mentioned genetic variants are associated with an intermediate phenotype, which is more common in alcoholic males compared to non-alcoholic males and even more common in alcoholic females. Increased stress vulnerability as well as comorbidity of alcohol dependence with depression and/or anxiety are among potential candidates for such an intermediate phenotype(s). In fact, clinical and epidemiological studies indicate that comorbid depression or anxiety are more common in alcoholics compared to the general population and are also more common in alcoholic females than males42,43,44, while alcohol consumption in the context of negative emotional states (negative craving) is also known to be associated with female sex15,16.
Importantly, the presence of comorbid mood and anxiety disorders in alcohol dependent subjects is reported among empirically described subtypes of alcohol dependence, which are also characterized by persistence of symptoms, treatment seeking and worse mental health status45,46. Moreover, evidence indicates that comorbidity of alcohol dependence with mood and anxiety disorders appears to be attributable to factors shared among these disorders47. Although speculative at this point, it is possible that the sex-related effects of the PDYN variants reported here are narrowing in on the genetic underpinnings of these particular subtypes of alcohol dependence. Similarly, other traits relevant to alcohol dependence that may differ between males and females should also be considered, as additional factors may be involved in higher-order (perhaps endophenotypic) interactions with SNP and sex effects on alcohol dependence and may define important sex-associated subtypes. For example, negative craving is associated with length of time abstinent from drinking48 and our data suggests that both sex and PDYN variants may modify these relationships. These hypotheses regarding the role of comorbidities and how they may have impacted the observed associations, should be investigated in the future studies.
Several limitations to the current study need to be acknowledged. First, sample size limited power to detect gene-sex interactions and sex-specific effects. Fewer female subjects in both the discovery and validation cohorts resulted in lower power for female-specific compared to male-specific analyses and because the discovery and validation samples were selected with alcohol dependence as the primary phenotype of interest, sample sizes and power to detect associations with the secondary case-only phenotypes were reduced. Furthermore, the validation sample was smaller than the discovery sample with respect to the primary phenotype, which also limited power.
Second, the controls in the discovery cohort were previously ascertained from a sample of control subjects representing the general population. This has become a common strategy in study of genetic epidemiology49. However, because of this, cases and controls were genotyped separately (although subjects genotyped in both sets were 100% concordant) and controls were not matched to cases on either age or sex. To mitigate this difference, analyses were adjusted for age and sex to eliminate potential biases due to these factors. Although control subjects with a history of alcohol dependence were excluded, controls were not required to have a history of exposure to alcohol. We do not expect this approach to induce an ascertainment bias, but perhaps further reduce power, because the control population may include subjects with high genetic risk who may have developed alcohol dependence after exposure50.
Third, because the phenotypes investigated here are highly correlated, the analyses we conducted were highly dependent. Therefore, multiple testing correction methods (e.g. Bonferroni correction or false discovery rate methods) commonly used to correct for the number of independent tests were not utilized. In the absence of such a correction, we relied on validation in the independent sample to support the robustness of our findings. Due to the large dependence among tests, the findings reported here likely represent a single underlying phenomenon and subsequent studies will be necessary to gain a better understanding of these observations.
Finally, the differences between the discovery and validation samples should also be noted. Key characteristics such as age, sex and alcohol consumption levels differed between the study samples, suggesting that both samples may represent different populations of alcoholics. Moreover, identical measures for negative craving and return to drinking were not available in the validation cohort, limiting our ability to directly replicate the findings in the discovery cohort. Yet, consistent findings acquired in samples differing in those characteristics and with different measured assessments of the same underlying phenotypes can be interpreted as validation, rather than direct replication of our findings; this is true particularly regarding the phenotypes of alcohol dependence and negative craving. Regarding return to drinking, length of sobriety before treatment was chosen as a surrogate measure for time until relapse, under the assumption that PDYN variation may be associated with earlier return to drinking at any time. It seemed reasonable to assume that these phenotypes would be correlated, although, in fact, these phenotypes are targeting events (sobriety) before and after treatment, which may potentially be driven by different mechanisms. Therefore the differences in association found between the rs2281285 G allele and length of sobriety in male and female alcoholics before and after treatment may be indicative of a different underlying biology. Further studies are needed to investigate whether these results are due to the fact the phenotypes are distinct, or caused by differences in clinical characteristics between the discovery and validation samples (e.g. differences in levels of alcohol consumption), or simply due to false positive associations. However, despite the differences in study samples and the smaller size of the validation sample, we were able to validate the magnitude and direction of many of the discovered associations regarding the alcohol dependence and negative craving phenotypes. This consistency of results across samples from populations with differing clinical characteristics highlights the robustness of these findings.
In conclusion, our findings support growing evidence that the dynorphin-kappa-opioid system is a critical component linking negative emotions with substance dependence51,52,53. Our findings expend contemporary knowledge by demonstrating that PDYN effects on alcohol use disorders differ between males and females and this pattern of this difference appears to depend on phenotype. Although the exact mechanisms of the reported associations are yet unclear, the results of this study suggest the following directions for future research. First, a search for intermediate phenotypes associated with alcohol dependence subtypes and relevant comorbidities are needed, using meta-analyses and/or combined data sets of subjects with genome wide data to allow increased sample size. Second, PDYN sequencing in alcoholics with the proper clinical assessments and treatment outcome data will provide more comprehensive coverage of potentially causal variants and allow testing for association with the above mentioned phenotypes. Last but not least, functional analyses of biological pathways and potential cis and trans regulatory mechanisms involving PDYN brain expression are necessary to disentangle the meaning and examine potential causal mechanisms of the differential sex association.
Additional Information
How to cite this article: Winham, S. J. et al. Associations of prodynorphin sequence variation with alcohol dependence and related traits are phenotype-specific and sex-dependent. Sci. Rep. 5, 15670; doi: 10.1038/srep15670 (2015).
References
Wee, S. & Koob, G. F. The role of the dynorphin-κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology 209, 121–135 (2010).
Sirohi, S., Bakalkin, G. & Walker, B. M. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5, 95, 10.3389/fnmol.2012.00095 (2012).
Butelman, E. R., Yuferov, V. & Kreek, M. J. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci 35, 587–596, 10.1016/j.tins.2012.05.005 (2012).
Bazov, I. et al. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol 18, 161–169, 10.1111/j.1369-1600.2011.00366.x (2013).
Xuei, X. et al. Association of the kappa-opioid system with alcohol dependence. Molecular psychiatry 11, 1016–1024 (2006).
Edenberg, H. J. et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet 17, 1783–1789, 10.1093/hmg/ddn068 (2008).
Wei, S. G. et al. Association between heroin dependence and prodynorphin gene polymorphisms. Brain Res Bull 85, 238–242, 10.1016/j.brainresbull.2011.02.010 (2011).
Yuferov, V. et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology 34, 1185–1197, 10.1038/npp.2008.187 (2009).
Karpyak, V. M. et al. Association of the PDYN gene with alcohol dependence and the propensity to drink in negative emotional states. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 16, 975–985, 10.1017/S1461145712001137 (2013).
Preuss, U. W. et al. PDYN rs2281285 Variant Association with Drinking to Avoid Emotional or Somatic Discomfort. PLoS One 8, e78688, 10.1371/journal.pone.0078688 (2013).
Verheul, R., van den Brink, W. & Geerlings, P. A three-pathway psychobiological model of craving for alcohol. Alcohol and Alcoholism 34, 197–222, 10.1093/alcalc/34.2.197 (1999).
Carlezon, W. A., Jr., Beguin, C., Knoll, A. T. & Cohen, B. M. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol. Ther. 123, 334–343, 10.1016/j.pharmthera.2009.05.008 (2009).
Shippenberg, T. S., LeFevour, A. & Chefer, V. I. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS & neurological disorders drug targets 7, 442–453 (2008).
Ehlers, C. L. et al. Age at regular drinking, clinical course and heritability of alcohol dependence in the San Francisco family study: a gender analysis. Am J Addict 19, 101–110, 10.1111/j.1521-0391.2009.00021.x (2010).
Lau-Barraco, C., Skewes, M. C. & Stasiewicz, P. R. Gender differences in high-risk situations for drinking: are they mediated by depressive symptoms? Addict Behav 34, 68–74, 10.1016/j.addbeh.2008.09.002 (2009).
Abulseoud, O. A. et al. A retrospective study of gender differences in depressive symptoms and risk of relapse in patients with alcohol dependence. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions 22, 437–442, 10.1111/j.1521-0391.2013.12021.x (2013).
Morrow, E. H. The evolution of sex differences in disease. Biol Sex Differ 6, 5, 10.1186/s13293-015-0023-0 (2015).
Ober, C., Loisel, D. A. & Gilad, Y. Sex-specific genetic architecture of human disease. Nat Rev Genet 9, 911–922, 10.1038/nrg2415 (2008).
Gilks, W. P., Abbott, J. K. & Morrow, E. H. Sex differences in disease genetics: evidence, evolution and detection. Trends Genet 30, 453–463, 10.1016/j.tig.2014.08.006 (2014).
Russell, S. E. et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76, 213–222, 10.1016/j.biopsych.2013.07.042 (2014).
Babbitt, C. C. et al. Multiple Functional Variants in cis Modulate PDYN Expression. Molecular Biology and Evolution 27, 465–479 (2010).
Kaynard, A. H., Low, K. G. & Melner, M. H. Differential regulation of anterior pituitary prodynorphin and gonadotropin-subunit gene expression by steroid hormones. Mol Cell Endocrinol 88, 67–75 (1992).
Kaynard, A. H., McMurray, C. T., Douglass, J., Curry, T. E., Jr. & Melner, M. H. Regulation of prodynorphin gene expression in the ovary: distal DNA regulatory elements confer gonadotropin regulation of promoter activity. Mol Endocrinol 6, 2244–2256 (1992).
Jenab, S. et al. Effects of cocaine on c-fos and preprodynorphin mRNA levels in intact and ovariectomized Fischer rats. Brain Res Bull 58, 295–299 (2002).
Clarke, T. K., Krause, K., Li, T. & Schumann, G. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addict Biol 14, 366–370, 10.1111/j.1369-1600.2009.00151.x (2009).
Clarke, T. K. et al. Genetic association analyses of PDYN polymorphisms with heroin and cocaine addiction. Genes, brain and behavior 11, 415–423, 10.1111/j.1601-183X.2012.00785.x (2012).
Preuss, U. W. et al. Association of ADH4 genetic variants with alcohol dependence risk and related phenotypes: results from a larger multicenter association study. Addiction biology 16, 323–333, 10.1111/j.1369-1600.2010.00236.x (2011).
Kosoy, R. et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat 30, 69–78, 10.1002/humu.20822 (2009).
Heit, J. A. et al. Genetic variation within the anticoagulant, procoagulant, fibrinolytic and innate immunity pathways as risk factors for venous thromboembolism. J Thromb Haemost 9, 1133–1142, 10.1111/j.1538-7836.2011.04272.x (2011).
Rockman, M. V. et al. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol 3, e387, 10.1371/journal.pbio.0030387 (2005).
First, M., Spitze, R., Williams, J. & Gibbon, M. Structured Clinical Interview for DSM-IV (SCID). (American Psychiatric Association, 1995).
Turner, N. E., Annis, H. M. & Sklar, S. M. Measurement of antecedents to drug and alcohol use: psychometric properties of the Inventory of Drug-Taking Situations (IDTS). Behav Res Ther 35, 465–483 (1997).
Schaid, D., Rowland, C., Tines, D., Jacobson, R. & Poland, G. Score tests for association between traits and haplotypes when linkage phase is ambiguous. American Journal of Human Genetics 70, 425–434 (2002).
Bucholz, K. K. et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55, 149–158 (1994).
Hesselbrock, M., Easton, C., Bucholz, K. K., Schuckit, M. & Hesselbrock, V. A validity study of the SSAGA--a comparison with the SCAN. Addiction (Abingdon, England) 94, 1361–1370 (1999).
Bierut, L. J. et al. A genome-wide association study of alcohol dependence. Proceedings of the National Academy of Sciences of the United States of America 107, 5082–5087 (2010).
Treutlein, J. et al. Genome-wide Association Study of Alcohol Dependence. Arch Gen Psychiatry 66, 773–784, 10.1001/archgenpsychiatry.2009.83 (2009).
Frank, J. et al. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addiction biology 17, 171–180, 10.1111/j.1369-1600.2011.00395.x (2012).
Wang, J. C. et al. A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Molecular psychiatry 18, 1218–1224, 10.1038/mp.2012.143 (2013).
Zuo, L. et al. Genome-wide association study of alcohol dependence implicates KIAA0040 on chromosome 1q. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 37, 557–566, 10.1038/npp.2011.229 (2012).
Winham, S. J. & Biernacka, J. M. Gene-environment interactions in genome-wide association studies: current approaches and new directions. J Child Psychol Psychiatry 54, 1120–1134, 10.1111/jcpp.12114 (2013).
Grant, B. F. et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61, 807–816, 10.1001/archpsyc.61.8.807 (2004).
Dawson, D. A., Goldstein, R. B., Moss, H. B., Li, T. K. & Grant, B. F. Gender differences in the relationship of internalizing and externalizing psychopathology to alcohol dependence: likelihood, expression and course. Drug Alcohol Depend 112, 9–17, 10.1016/j.drugalcdep.2010.04.019 (2010).
Bucholz, K. K., Helzer, J. E., Shayka, J. J. & Lewis, C. E. Comparison of alcohol dependence in subjects from clinical, community and family studies. Alcohol Clin Exp Res 18, 1091–1099 (1994).
Moss, H. B., Chen, C. M. & Yi, H. Y. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91, 149–158, 10.1016/j.drugalcdep.2007.05.016 (2007).
Moss, H. B., Chen, C. M. & Yi, H. Y. Prospective follow-up of empirically derived Alcohol Dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol And Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior, health status and treatment seeking. Alcohol Clin Exp Res 34, 1073–1083, 10.1111/j.1530-0277.2010.01183.x (2010).
Hasin, D. S., Stinson, F. S., Ogburn, E. & Grant, B. F. Prevalence, correlates, disability and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64, 830–842, 10.1001/archpsyc.64.7.830 (2007).
Schneekloth, T. D. et al. Alcohol craving as a predictor of relapse. Am J Addict 21 Suppl 1, S20–26, 10.1111/j.1521-0391.2012.00297.x (2012).
Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678, 10.1038/nature05911 (2007).
Edwards, B. J., Haynes, C., Levenstien, M. A., Finch, S. J. & Gordon, D. Power and sample size calculations in the presence of phenotype errors for case/control genetic association studies. BMC Genet 6, 18, 10.1186/1471-2156-6-18 (2005).
Knoll, A. T. & Carlezon, W. A., Jr. Dynorphin, stress and depression. Brain Res 1314, 56–73, 10.1016/j.brainres.2009.09.074 (2010).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238, 10.1038/npp.2009.110 (2010).
Bruchas, M. R., Land, B. B. & Chavkin, C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314, 44–55, 10.1016/j.brainres.2009.08.062 (2010).
Acknowledgements
This study was supported by grants from the St. Marys Hospital Sponsorship Award (VMK), Samuel C. Johnson Genomics of Addiction Program (VMK, JMB), NIH/NIAAA P20 AA17830Z (VMK, JMB), NIH K12 HD65987 (SJW) and the Swedish Council for Working Life and Social Research, Swedish Science Research Council and Swedish Research Council FORMAS(GB). Controls were recruited and genotyped as part of the GWAS of Venous Thrombosis study (NIH/NHGRI grant HG04735, PI J.A. Heit). We thank the Mayo Clinic Cancer Center for the use of the Genotyping Core, which provided genotyping services. Mayo Clinic Cancer Center is supported in part by an NCI Cancer Center Support Grant 5P30 CA15083-37. This project was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The datasets used for the eQTL analyses described in this manuscript were obtained from the database of Genotypes and Phenotypes (dbGaP) found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000417.v2.p1. Submission of this data (phs000417.v2.p1) to dbGaP was provided by Drs. Barbara Lipska and Joel Kleinman. Collection of the data was through a collaborative study sponsored by the NIMH Intramural Research Program. Initial report on this dataset is from Colantuoni, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature, (2011).
Author information
Authors and Affiliations
Contributions
V.M.K. and S.J.W. conceived of the study and design. S.J.W. and J.R.G. performed statistical analyses. S.J.W., J.M.B. and V.M.K. drafted the manuscript. S.J.W., U.W.P., J.R.G., P.Z., J.A.H., G.B., J.M.B. and V.M.K. reviewed and approved the final version of the manuscript.
Ethics declarations
Competing interests
Dr. Preuss has received research support, consultancy, or lecture fees from Pfizer, Astra-Zeneca, Eli-Lilly, Janssen-Cilag, Novartis and Probiodrug in the past 3 years. The remaining authors have no conflicts of interest.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Winham, S., Preuss, U., Geske, J. et al. Associations of prodynorphin sequence variation with alcohol dependence and related traits are phenotype-specific and sex-dependent. Sci Rep 5, 15670 (2015). https://doi.org/10.1038/srep15670
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15670
- Springer Nature Limited