Abstract
The mechanisms behind the shortening-induced force depression commonly observed in skeletal muscles remain unclear, but have been associated with sarcomere length non-uniformity and/or crossbridge inhibition. The purpose of this study was twofold: (i) to evaluate if force depression is present in isolated single sarcomeres, a preparation that eliminates sarcomere length non-uniformities and (ii) to evaluate if force depression is inhibited when single sarcomeres are activated with MgADP, which biases crossbridges into a strongly-bound state. Single sarcomeres (n = 16) were isolated from rabbit psoas myofibrils using two micro-needles (one compliant, one rigid), piercing the sarcomere externally adjacent to the Z-lines. The sarcomeres were contracted isometrically and subsequently shortened, in both Ca2+- and MgADP-activating solutions. Shortening in Ca2+-activated samples resulted in a 27.44 ± 9.04% force depression when compared to isometric contractions produced at similar final sarcomere lengths (P < 0.001). There was no force depression in MgADP-activated sarcomeres (force depression = −1.79 ± 9.69%, P = 0.435). These results suggest that force depression is a sarcomeric property and that is associated with an inhibition of myosin-actin interactions.
Similar content being viewed by others
Introduction
When a shortening is imposed to an activated skeletal muscle, the steady-state force generated after shortening is lower than that produced during isometric contraction at a similar sarcomere length (SL)1,2,3,4. This phenomenon is inconsistent with predictions based on the isometric force-length relationship5 and is commonly referred to as force depression. Force depression has been observed for more than half a century in different muscle preparations, from whole muscles6,7 to single fibres2,8 and myofibrils9,10. Its mechanisms, however, remain unclear.
A non-uniform distribution of the lengths of sarcomeres after shortening has been suggested to explain force depression11,12, as follows: when a muscle is activated and then shortened, the sarcomeres would shorten to different lengths11. A first group of sarcomeres would shorten by a small amount, while another group of sarcomeres would shorten to the ascending limb of the force-length relationship until the force produced by the first group is matched. As a result, the force generated by these two groups of sarcomeres would be lower than that predicted by the average sarcomere length of both populations together12. Although generally accepted, such a mechanism has been challenged by studies demonstrating the existence of force depression in muscle fibres in which the sarcomere lengths were controlled and kept stable both during and after shortening2,13, suggesting a mechanism intrinsic to the sarcomere.
Marechal and Plaghki14 had suggested many years ago that a shortening-induced strain in the actin filaments could cause an inhibition of the crossbridge binding sites on actin’s newly formed overlap zone during shortening, decreasing the probability of crossbridge formation and consequently attenuating force production. A recent study from our laboratory suggests that this mechanism may work at the myofibril level; we observed that force depression was present in myofibrils without a concomitant increase in sarcomere length non-uniformity9. Most tellingly, force depression was partially inhibited by inducing strong binding of crossbridges and actin9, decreasing the crossbridge inhibition that may have been caused by the strain of actin filaments.
In order to clearly elucidate the mechanisms of force depression, it is necessary to isolate the contribution of sarcomeric properties from between-sarcomere properties. According to the sarcomere length non-uniformity theory, at least two sarcomeres in series are necessary to produce residual force depression after shortening. Therefore, the goals of this study were: (i) to examine if force depression was present in single sarcomeres and (ii) to examine if force depression could be prevented by increasing crossbridge binding during/after shortening. In order to reach these goals, we used a single sarcomere preparation developed in our laboratory15, that was activated either with Ca2+ or MgADP – the latter biasing crossbridges into a strong binding state with actin9.
Results
Figure 1 shows a typical contraction produced by a sarcomere during Ca2+ activation, with the corresponding SL and ramp traces. After ~5 s, Ca2+-activating solution was flushed into the experimental chamber and the sarcomere was activated. The force stabilized after ~1 s. In the shortening contraction, the imposed shortening occurred at ~7 s, resulting in a similar final SL in the isometric contraction. Relaxing solution was flushed once a steady-state force was re-established after the imposed shortening, at ~8.5 s. In the isometric contraction, relaxing solution was administered after ~5 s at a maximum steady-state force, at ~10.5 s. The black and grey arrows indicate the timing of solution administration in the isometric and shortening contraction, respectively.
Sample records from a typical experiment displaying superimposed force, SL and ramp traces of a Ca2+-activated sarcomere.
The solid black line represents the isometric contraction. The dashed grey line represents the contraction in which a shortening was imposed. The vertical dotted line represents the time (~7 s) at which the imposed shortening occurred in the shortening condition. The black and grey arrows indicate the time at which the activating and relaxing solutions were flushed into the experimental chamber, in the isometric and shortening contraction, respectively.
Figure 2 shows two superimposed graphs of force, length and ramp traces in MgADP-activated sarcomere. The force plateau of the isometric was time-matched with the force plateau upon force redevelopment in the shortening contraction in order to compare force at the same final SL. In Fig. 2A, in the isometric contraction, activation was induced at 12 s and in the shortening contraction, activation was induced at 7 s – as shown by the black and grey arrows, respectively. The imposed shortening occurred at ~10 s after the force has maximized and we compared the steady-state force generated in both corresponding contractions at ~14.5, which are approximately at the same final SL. Relaxing solution was flushed at 16.5 s, as indicated by the arrows and the force stabilized to zero. Note that that the shortening is imposed slightly before a steady-state force is achieved. It has been shown that a steady-state force is not required prior to active shortening to produce force depression8,12. Due to the longer duration needed to attain maximal force using MgADP9, the duration of experiments was increased prior to shortening. This procedure does not affect the comparisons made between contractions. The traces in Fig. 2B shows another experiment in which MgADP activation was held for 35 s prior to the imposed shortening and the force depression was similar to the experiment depicted in Fig. 2A. Note that the force was still increasing over time, although it was slowly reaching a plateau towards the end of the contraction.
A - Sample records from a typical experiment displaying superimposed force, SL and ramp traces of a MgADP-activated sarcomere. The solid black line represents the isometric contraction. The dashed grey line represents the contraction in which a shortening was imposed. The vertical dotted line represents the time at which the imposed shortening occurred (~10 s) in the shortening condition. The black and grey arrows indicate the time at which the activating and relaxing solutions were flushed into the experimental chamber, in the isometric and shortening contraction, respectively. B - Sample records from a typical experiment displaying superimposed force, SL and ramp traces of a sarcomere that was activated with MgADP for a longer period of time. The solid black line represents the isometric contraction. The dashed grey line represents the contraction in which a shortening was imposed. The vertical dotted line represents the time at which the imposed shortening occurred (~10 s) in the shortening condition. The black and grey arrows indicate the time at which the activating and relaxing solutions were flushed into the experimental chamber, in the isometric and shortening contraction, respectively.
The results from Figs. 1,2 were confirmed statistically as shown in Fig. 3, which illustrates the mean forces produced in all conditions. Post hoc analysis using the Holm-Sidak method indicated that the only significant difference in force existed between Ca2+-activated isometric (43.21 ± 6.08 nN/μm2) and shortening contractions (29.66 ± 6.19 nN/μm2) (t(15) = 3.939, p < 0.001). The final SL and the amount of shortening were not different between Ca2+- and MgADP-activation. The imposed shortening in Ca2+-activated samples resulted in a force depression of 27.44 ± 9.04% whreas force depression was virtually eliminated when the sarcomere was activated by MgADP.
Mean values of the SL-force relation for each experimental condition.
Black circle represents Ca2+-activated, isometric contractions. Black triangle with white cross represents Ca2+-activated, imposed shortening contractions. Grey circle represents MgADP-activated, isometric contractions. Grey triangle represents MgADP-activated, imposed shortening contractions. White cross represents statistically significant force depression relative to isometric counterpart (P  0.05). The dashed line represents the force-length relationship based on the mean isometric force generated by all sarcomeres (both Ca2+- and MgADP-activated samples).
0.05). The dashed line represents the force-length relationship based on the mean isometric force generated by all sarcomeres (both Ca2+- and MgADP-activated samples).
Discussion
The current study is the first to show that the shortening-induced force depression commonly observed in skeletal muscles is present at the single sarcomere level with Ca2+ activation. Furthermore, this study shows that force depression is abolished when samples are activated with MgADP, which biases crossbridges into a strongly-bound state. These findings suggest that force depression is caused partly by crossbridge binding site inhibition. Such a mechanism would be consistent with an actin distortion in the newly formed overlapped zone during shortening9,14.
When single sarcomeres were activated with Ca2+ and subsequently shortened, force was noticeably depressed in comparison to the isometric contraction at the corresponding final SL. One suggested mechanism for force depression is sarcomere length non-uniformities11,12 – a result of strength differences between sarcomeres in series. The heterogeneity in sarcomere length shortening results in some sarcomeres being longer, lying on the descending limb of the force-length relationship and sarcomeres on the ascending limb matching the force produced by the other group of sarcomeres. The actual force produced is thus less than that predicted during isometric contractions. While the observation of force depression in Ca2+-activated samples in our study is consistent with previous studies using muscle fibres and myofibrils2,7,9, this study is the first to assess the magnitude of force depression when removing the contribution of non-uniformity between sarcomeres.
Previously, we observed that force depression was greatly reduced when myofibrils were activated with MgADP9. In the present study, we showed a full abolishment of force depression when single sarcomeres were activated in MgADP solution. The difference may reside in the fact that in myofibril preparations, some degree of sarcomere length non-uniformities may contribute to the overall force depression.
The absence of force depression in MgADP-activated samples supports a mechanism of force depression, intrinsic to the sarcomere, that has been suggested many years ago; a shortening-induced inhibition of myosin-actin attachment in the newly formed overlap zone during shortening14. Such an inhibition may be caused by a stress-deformation of actin, hindering its myosin-binding sites. Activation via MgADP happens when ADP enters the myosin nucleotide-binding pocket, inducing some crossbridges to transit into a strongly-bound state16. The strong binding of myosin-actin in turn may introduce conformational changes in the tropomyosin filaments, cooperatively exposing adjacent myosin-binding sites on actin, ultimately leading to further force-generating crossbridge attachments17,18. Furthermore, the strong binding from MgADP-activation has been suggested to compress the myofilament lattice19, pulling actin and myosin closer together.
It is tempting to speculate that MgADP activation allows the sarcomere to counteract and overcome the shortening-induced force depression by improving the alignment and resultant probability of actomyosin formation. MgADP induces strong binding between myosin crossbridges and actin in a cooperative fashion. Studies performed in solution with reconstituted filaments show that strong biding of myosin crossbridges to actin filament induces the binding of adjacent crossbridges20,21. Such strongly-bound crossbridges may cause conformational changes of the thin filaments that may in fact enhance Pi release or other steps during isomerization of the actin-myosin–ADP–Pi complex. Another effect of strong binding between crossbridges and actin is the compression of the myofilament lattice space upon muscle activation19, approximating thick and thin filaments. A decreased lattice space will likely increase the probability of crossbridge attachment to actin, which could counteract some of the shortening induced depression effects on force. Finally, the rigidity of the actin filament is reduced upon strong binding of myosin crossbridges22, which would release the stress caused by activation and shortening to the thin filaments. Altogether, these observations as well as our results indicate that the main difference between sarcomeres activated with Ca2+ or MgADP is the myosin–actin binding probability. When myofibrils are activated and shortened with continuous flow of MgADP, the strong binding of crossbridges will be enhanced, increasing myosin cooperativity while decreasing the lattice spacing and the rigidity of the actin filaments. The newly formed overlap zone after shortening will be better aligned for myosin binding to actin.
In summary, this study was the first to provide direct support for a purely sarcomeric mechanism of force depression. While between-sarcomere mechanisms cannot be discounted in myofibril preparations, the observation that MgADP-activation abolishes force depression suggests that force depression is caused by an inhibition of myosin-actin interactions in the newly formed overlap zone during shortening.
Methods
Muscle Preparation and Single Sarcomere Isolation
Sections of rabbit psoas muscles (3-4 cm) were dissected and subject to a standard permeabilization process23. For four hours, the muscles were bathed in rigor solution (pH = 7.0), after which the samples were placed in a rigor:glycerol (50:50) solution overnight. The samples were then transferred to a new rigor:glycerol solution with an additional mixture of protease inhibitors (Roche Diagnostics, USA). The muscles were frozen (−20 °C) for at least seven days. The protocol was approved by the McGill University Animal Care Committee and complied with the guidelines of the Canadian Council on Animal Care.
Small sections of a muscle sample were cut and placed in a 2 mL Eppendorf tube containing fresh rigor solution. The sample was thawed for one hour in the fridge (4 °C) and then transferred to approximately 5 mL of rigor solution in a 10 mL test tube. This sample was then homogenized using the following procedure: twice for 5 s at 18,000 rpm, twice for 5 s at 26,000 rpm and once for 3 s at 34,000 rpm. The homogenate, containing single myofibrils, was pipetted onto a glass cover-slip (thickness: 0.15 mm), which was vacuum grease-sealed to an experimental chamber positioned on the stage of an inverted microscope (NIKON Eclipse TE 2000U). Surrounding the chamber, a circulating cooling solution controlled the temperature at 10 °C. After 10 minutes, the homogenate was replaced with a relaxing solution (pH 7.0), which was subsequently used to fill the chamber, minimizing the appearance of floating debris. A myofibril was chosen for mechanical experimentation based on its striation pattern appearance, upon which a single sarcomere could be selected for isolation.
Solutions
The rigor solution (pH 7.0) was composed of (in mM): 50 Tris, 100 KCl, 2 MgCl2 and 1 EGTA. The relaxing solution (pH 7.0) was composed of (in mM): 0.01372 CaCl2, 7.2 EGTA, 20.27 Imidazole, 5.41 MgCl2, 68.678 KCl, 6.1 ATP and 18.97 creatine phosphate. Two activating solutions were used in this investigation. The Ca2+-activating solution (pH 7.0) was composed of (in mM): 7 CaCl2, 7.2 EGTA, 20.27 Imidazole, 5.41 MgCl2, 52.31 KCl, 6.169 ATP and 18.97 creatine phosphate. The MgADP-activating solution (20 mM) (pH 7.0) was composed of (in mM): 2 MgCl2, 20 MOPS, 4 EGTA, 4 ATP and 20 ADP.
Micro-needle production and calibration
A vertical pipette puller (KOPF 720, David Kopf Instruments) was used to make two glass micro-needles. Calibration of the micro-needles was done using a cross-bending method using a pair of micro-fabricated cantilevers of known stiffness (51.8 and 52.6 nN/μm)24. For each experiment, a thin, compliant micro-needle (stiffness varied between 30 and 110 nN/μm) and a thick, rigid micro-needle were used.
Mechanical isolation, visualization and force measurement of single sarcomeres
Using the two pre-calibrated micro-needles controlled by micromanipulators (Narishige NT-88-V3, Tokyo, Japan), single sarcomeres were pierced externally adjacent to Z-lines. The sarcomeres were raised from the glass coverslip by 0.5-1.0 μm. Under high magnification provided by an oil immersion phase-contrast lens (Nikon plan-fluor, X100, numerical aperture 1.30), the images of the single sarcomeres were further magnified X1.5 by an internal microscope function. A video was taken throughout sarcomere isolation and mechanical experimentation.
After the mechanical isolation of the sarcomere, a contraction was initiated by a computer-controlled release of the activating solution through a double-barreled pipette connected to a multichannel perfusion system (VC-6M, Harvard Apparatus)15,25. To avoid contamination within the perfusion system, each side of the double-barrel was equipped to flush its own, independent activating solution contacting Ca2+ or MgADP, as well as relaxing solution.
The contrast between the micro-needles produces a pattern of light intensity peaks that allow for tracking of their centroids frame-by-frame using a particle-tracker algorithm. The force produced during activation of the single sarcomere was obtained by measuring the displacement of the micro-needles, as previously described15.
Experimental Protocol
Single sarcomeres (n = 16) were first activated isometrically and relaxed after force reached a steady-state for several seconds. Activation was induced in separate contractions using either Ca2+ or MgADP solutions. Subsequently, the same sarcomere was passively stretched to a longer sarcomere length and activated. After force reached a steady-state (Ca2+ activation) or a quasi-steady-state (MgADP activation), a computer-controlled shortening with an amplitude of 0.40 ± 0.03 μm at a speed of 203.14 ± 14.45 nm.s−1 was imposed by the rigid micro-needle. In order to clearly identify force depression, the potential difference in forces was always investigated at the same final SL’s. Relaxing solution was flushed into the experimental bath in order to stop the contractions. It is known that, during MgADP activation, the rate of force development is significantly slower than that produced during Ca2+ activation9 and it may take up to a few minutes. Therefore, shortening was not always imposed during steady-state force in MgADP-activated sarcomeres and in some cases force was still rising. However, it has been shown that force depression is present when shortening is imposed in the force rising phase, at levels that are qualitatively similar to those observe when shortening is imposed during the plateau of the force response in fully activated muscle fibres8,9,10,11,12.
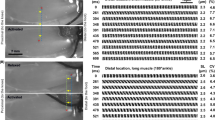
The order of different activations (Ca2+ and MgADP) and contraction types (isometric and shortening) was randomized to account for confounding effects. Figure 4 shows the needle movement through each phase of a typical MgADP-activated, shortening contraction.
Needle movement through each phase of an MgADP-activated, shortening contraction.
The white dashed lines represent the midpoint of the needle at each phase of the contraction. The right needle (which imposes the computer-controlled shortening) is ~10x stiffer than the left needle, which moves in response to any sarcomere length changes (activation, relaxation and imposed shortening). A) Baseline – represents the initial position of both needles; B) Activation – the sarcomere is activated and contracts; C) Shortening – a shortening is imposed by the thick needle, decreasing the sarcomere length while moving the thin needle in the process; D) Re-development – the sarcomere contracts at this new, shorter sarcomere length as crossbridges begin to reattach; E) Relaxation – the relaxing solution is flushed into the preparation.
Data Analysis
The amount of force depression was measured by calculating the difference between the isometric force and the force produced after the imposed shortening in each sarcomere. The values taken for the calculation were averaged over 1 second in both contractions.
The results are presented as means ± standard error (SEM). Since the data were not normally distributed, a two-way ANOVA for repeated measures on ranks (n = 16) was used to identify potential differences in force and final SL between the four experimental conditions between groups. The Holm-Sidak post-hoc test was used to locate these differences when they were present. A level of significance (P) was set at 0.05. Since there is a relationship between the amount of shortening and active force depression, a student’s t-test (n = 12) was used to compare the amount of shortening between the two activating solutions.
Additional Information
How to cite this article: Trecarten, N. et al. Residual force depression in single sarcomeres is abolished by MgADP-induced activation. Sci. Rep. 5, 10555; doi: 10.1038/srep10555 (2015).
References
Edman, K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J. Physiol. 246, 255–275 (1975).
Granzier, H. L. & Pollack, G. H. Effect of active pre-shortening on isometric and isotonic performance of single frog muscle fibres. J. Physiol. 415, 299–327 (1989).
Morgan, D. L., Claflin, D. R. & Julian, F. J. Tension in frog single muscle fibers while shortening actively and passively at velocities near Vu. Biophys. J. 57, 1001–1007 (1990).
Herzog, W., Leonard, T. R. & Wu, J. Z. Force depression following skeletal muscle shortening is long lasting. J. Biomech. 31, 1163–1168 (1998).
Gordon, A. M., Huxley, A. F. & Julian, F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170–192 (1966).
Abbott, B. C. & Aubert, X. The force exerted by active striated muscle during and after change of length. J. Physiol. 117, 77–86 (1952).
Herzog, W. & Leonard, T. R. Depression of cat soleus-forces following isokinetic shortening. J. Biomech. 30, 865–872 (1997).
Edman, K. A., Caputo, C. & Lou, F. Depression of tetanic force induced by loaded shortening of frog muscle fibres. J. Physiol. 466, 535–552 (1993).
Pun, C., Syed, A. & Rassier, D. E. History-dependent properties of skeletal muscle myofibrils contracting along the ascending limb of the force-length relationship. Proc. R. Soc B 277, 475–484, 10.1098/rspb.2009.1579 (2010).
Joumaa, V. & Herzog, W. Force depression in single myofibrils. J. Appl. Physiol. 108, 356–362, 10.1152/japplphysiol.01108.2009 (2010).
Julian, F. & Morgan, D. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J. Physiol 293, 379–392 (1979).
Morgan, D., Whitehead, N., Wise, A., Gregory, J. & Proske, U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J. Physiol. 522, 503–513 (2000).
Sugi, H. & Tsuchiya, T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol. 407, 215–229 (1988).
Marechal, G. & Plaghki, L. The deficit of the isometric tetanic tension redeveloped after a release of frog muscle at a constant velocity. J. Gen. Physiol. 73, 453–467 (1979).
Minozzo, F. C., Baroni, B. M., Correa, J. A., Vaz, M. A. & Rassier, D. E. Force produced after stretch in sarcomeres and half-sarcomeres isolated from skeletal muscles. Sci. Rep. 3, 2320, 10.1038/srep02320 (2013).
Kintses, B. et al. Reversible movement of switch 1 loop of myosin determines actin interaction. EMBO J. 26, 265–274 (2007).
Shimizu, H., Fujita, T. & Ishiwata, S. i. Regulation of tension development by MgADP and Pi without Ca2+. Role in spontaneous tension oscillation of skeletal muscle. Biophys. J. 61, 1087–1098 (1992).
Gordon, A. M., Homsher, E. & Regnier, M. Regulation of contraction in striated muscle. Physiol Rev. 80, 853–924 (2000).
Brenner, B. & Yu, L. C. Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys. J. 48, 829–834 (1985).
Bremel, R. D. & Weber, A. Cooperation within actin filament in vertebrate skeletal muscle. Nat. New Biol. 238, 97–101 (1972).
Greene, L. E. & Eisenberg, E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc. Natl. Acad. Sci. USA 77, 2616–2620 (1980).
Yanagida, T., Nakase, M., Nishiyama, K. & Oosawa, F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature 307, 58–60 (1984).
Minozzo, F. C., Hilbert, L. & Rassier, D. E. Pre-power-stroke cross-bridges contribute to force transients during imposed shortening in isolated muscle fibers. PloS ONE 7, e29356, 10.1371/journal.pone.0029356 (2012).
Pavlov, I., Novinger, R. & Rassier, D. E. The mechanical behavior of individual sarcomeres of myofibrils isolated from rabbit psoas muscle. Am. J. Physiol - Cell Ph. 297, C1211–C1219 (2009).
Rassier, D. E. & Pavlov, I. Force produced by isolated sarcomeres and half-sarcomeres after an imposed stretch. Am. J. Physiol - Cell Ph. 302, C240–248, 10.1152/ajpcell.00208.2011 (2012).
Acknowledgements
This study was supported by the ‘Natural Sciences and Engineering Research Council’ of Canada (NSERC). NT is supported by an NSERC Alexander Graham Bell Scholarship as part of the Canada Graduate Scholarships-Master’s (CGS M) Program. FL is the recipient of a CNPq Scholarship from the Brazilian National Council for Scientific and Technological Development.
Author information
Authors and Affiliations
Contributions
N.T. wrote the main manuscript text which was reviewed and edited by all authors. N.T., F.M. and F.S. contributed to the experimental setup. N.T. performed all the experiments. N.T. and F.M. prepared figures and analyzed the results. All authors contributed to the critical discussion of the scientific process. D.R. contributed to the design of the study and supervised the experimental setup, data collection and data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Trecarten, N., Minozzo, F., Leite, F. et al. Residual force depression in single sarcomeres is abolished by MgADP-induced activation. Sci Rep 5, 10555 (2015). https://doi.org/10.1038/srep10555
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10555
- Springer Nature Limited
This article is cited by
-
Force–length relation of skeletal muscles: from sarcomeres to myofibril
Biomechanics and Modeling in Mechanobiology (2018)