Abstract
Copper ions play a vital role in a variety of fundamental physiological processes not only in human beings and plants, but also for extensive insects and microorganisms. In this paper, a novel water-soluble ruthenium(II) complex as a turn-on copper(II) ions luminescent sensor based on o-(phenylazo)aniline was designed and synthesized. The azo group would undergo a specific oxidative cyclization reaction with copper(II) ions and turn into high luminescent benzotriazole, triggering significant luminescent increasements which were linear to the concentrations of copper(II) ions. The sensor distinguished by its high sensitivity (over 80-fold luminescent switch-on response), good selectivity (the changes of the emission intensity in the presence of other metal ions or amino acids were negligible) and low detection limit (4.42 nM) in water. Moreover, the copper(II) luminescent sensor exhibited good photostability under light irradiation. Furthermore, the applicability of the proposed sensor in biological samples assay was also studied and imaged copper(II) ions in living pea aphids successfully.
Similar content being viewed by others
Introduction
As an essential transition metal ion not only for human beings and plants but also for extensive insects and microorganisms, Cu(II) plays a vital role in a variety of fundamental physiological processes including neurotransmission, energy generation, iron transportation, pigmentation and scavenging of free radicals1,2. Moreover, the internal concentrations of Cu2+ in normal organisms are tightly regulated and disruption of the copper homeostasis often cause disease states or pathophysiological conditions3,4. For humans, alterations in the cooper homeostasis are connected to some serious neurodegenerative diseases5,6,7,8 and may cause gastrointestinal disturbance or damages to liver and kidney9. While, for insects such as aphids, excess or deficiency in Cu(II) not only hinders their normal growth and development but also affects their plant responses10,11,12,13, which is closely related to the damage extent with their host plants. In addition, due to their widespread use in industry and agriculture, cupric ions are also considered to be a significant environmental pollutant14. Consequently, developing robust and versatile methods to investigate the biological and environmental roles of copper(II) ions have been attracted extensive attentions.
Among the reported methods for copper ions detection, luminescent probes are extensively employed owing to their distinct advantages in sensitivity and biological imaging15. However, due to the intrinsic fluorescence quenching property of Cu2+ stemming from its paramagnetic nature, most hitherto reported Cu2+ sensors have shown a “turn-off” response via an electron/energy transfer process16,17,18. Although some luminescence “off-on” Cu2+ sensors with high selectivity19,20, nanomolar sensitivity19,20,21,22, good water solubility20,23, excellent photostability24 and long emission wavelength25,26 have been reported, sensors combining all these features are rare up to now15. Furthermore, the probes for Cu2+ detection in biological systems, such as different cancer cells25,27, rat hippocampal slices25,27, zebrafish25,28, human tissues27,29, herb leaves30 have been investigated, however, copper(II) imaging in insects is rarely reported. Definitely, developing new Cu2+-selective turn-on luminescent probes with excellent performance for diverse biological systems is still of importance and necessity.
Ruthenium(II) complexes are one type of potential candidates for environmental and biological Cu2+ probing, due to their good water solubility, high chemical and photostability, intense polarized luminescence, red emission, large Stokes shifts and long lifetimes31. To date, some ruthenium(II) complex based luminescent probes for Cu2+ have been developed24,32,33,34,35,36,37,38,39,40,41. Unfortunately, as far as we know, there is only one example of luminescence enhancement Cu2+ sensor based on a ruthenium(II) complex (Rubp-Ptz) reported by Gopidas's group, which could detect micromolar amounts of Cu2+ in acetonitrile solution24. Herein, in this paper, we focus on the development of a turn-on ruthenium(II) complex based luminescent sensor with superior performance for Cu2+ detection and imaging.
Lee et al. reported a fluorescence turn-on chemodosimeter for Cu2+ based on oxidative cyclization of a non-emissive azoaniline into a highly fluorescent benzotriazole product, which can detect μM-level concentrations of Cu2+ in water at room temperature with the green emission23. Given the relatively high detection limit of the reported sensor, here, we designed and synthesized a novel ruthenium(II) complex RuMAZO (Fig. 1) with o-(phenylazo) aniline group as a turn-on luminescent sensor for Cu2+. The non-emissive RuMAZO in presence of copper(II) ions undergoes oxidative cyclization to form a highly luminescent product RuTAZO (Fig. 1).
Results
The non-emissive RuMAZO in presence of copper(II) ions undergoes oxidative cyclization to form a highly luminescent product RuTAZO (Fig. 1). This cyclization reaction can be triggered by nM-level (4.42 nM) concentrations of Cu2+ in a HEPES (HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer, to exhibit > 80-fold enhancement in a red emission at λmaxem = 599 nm. The chloride salt of RuTAZO exhibits λmaxem value at 599 nm with a quantum yield of 4.7%, using [Ru(bpy)3]2+ (bpy = 2,2-bipyridine) as a standard (see supplementary information part)42. Moreover, this probe has proved to be an appropriate luminescent Cu2+ imaging reagent in live pea aphis. To the best of our knowledge, this is the first report on developing a ruthenium(II) complex-based luminescent sensor for luminescence enhancement detecting Cu2+ in aqueous solution with high selectivity and sensitivity and imaging Cu2+ in insects.
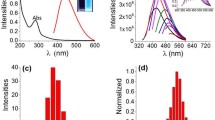
Luminescence enhancement was clearly evident up to 30 min in a HEPES buffer solution (20 mM, pH 7.4, 37°C), then no further significant changes occurred, indicating that the optimal reaction time for Cu2+ detection via oxidative cyclization for this sensor is around 30 min (Fig. S1). In addition, the luminescence properties of RuMAZO were checked under the same conditions. As shown in Fig. S2, after treatment with different concentrations (0–3 equiv.) of Cu2+ at a physiological temperature 37°C, the ligand absorption of RuMAZO (10 μM) at around 263 nm apparently increased and the metal-to-ligand charge transfer (MLCT) absorption at around 452 nm decreased, whereas a new ligand absorption peak at about 294 nm appeared. Correspondingly, within 30 min of reaction under the same conditions, the emission intensity at 599 nm increased to over 80 folds upon excitation at 465 nm with only 1 equiv. of Cu2+ (Fig. 2a). The Stokes shift of RuTAZO is 134 nm. These results indicate that the o-(phenylazo)aniline group of RuMAZO can be efficiently converted into luminescent benzotriazole. Furthermore, the dose-dependent luminescence enhancement followed a good linear relationship with very low Cu2+ concentrations in the range of 0.1–2.0 μM (Fig. 2b) and the limit of detection (LOD) for Cu2+ with RuMAZO (10 μM) was determined to be 4.42 × 10−9 M (see supplementary information part), lower or comparable to those of most previously reported highly sensitive sensors15. Thus, the broad linear range and low detection limit make RuMAZO suitable for environmental or biological copper(II) detection and imaging.
(a) Luminescence intensity of RuMAZO (10 μM) with various concentrations of Cu2+ (0–30 μM) in a HEPES buffer solution (20 mM, pH 7.4); Insert: the changes of luminescence intensity at 599 nm with various concentrations of Cu2+; (b) A linear correlation between emission intensity of RuMAZO at 599 nm and concentrations of Cu2+ (0.1–2.0 μM).
For further biological applications, the cytotoxicity of RuMAZO and Cu2+ to the HeLa cell lines was investigated with an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay after a 24 h treatment (Fig. S10). RuMAZO did not exhibit obvious cytotoxicity towards the HeLa cell lines at the concentrations employed. Confirming RuMAZO can be a suitable luminescence chemosensing probe for Cu2+ detection in vivo.
To investigate the practical applicability of RuMAZO as a Cu2+ sensor in the luminescence imaging of living cells, HeLa cells were incubated with RuMAZO (10 μM) for 2 h at 37°C in a PBS (phosphate buffer solution, pH = 7.4). After washed with PBS to remove the remaining RuMAZO, no obvious luminescence could be observed from the confocal laser scanning microscopy (Fig. S11a). However, the intracellular luminescence showed a clear red luminescence after incubated with Cu2+ (20 μM) and PDTC (pyrrolidine dithiocarbamate, 100 μM) for 2 h at 37°C (Fig. S11b). PDTC43 was used to increase the intracellular level of Cu2+. The results revealed that RuMAZO could be used as an off-on luminescent probe for imaging Cu2+ in living cells.
To examine the applicability of the sensor for visualizing Cu2+ in living organisms, four-day-old pea aphids were selected and divided into three groups. The first two groups were given skin-pop injections at the bottom of the middle legs with Cu2+ (300 nL, 5 mM in a HEPES buffer solution (20 mM, pH 7.4)) or RuMAZO (300 nL, 25 μM in a HEPES buffer solution (20 mM, pH 7.4)) respectively as the control. The third group was given a hypodermic injection of 25 μM RuMAZO and then 50 μM Cu2+ (300 nL, 20 mM HEPES) immediately. All samples were imaged using a Confocal Laser Scanning Microscope with a 488 nm excitation laser after incubation for 6 h. As shown in Fig. 3, pea aphids in the experimental group exhibited distinct luminescence signal over the entire bodies. While no apparent emission was observed in the control groups, illustrating that RuMAZO could detect Cu2+ in vivo without the interference of background signals. Taken together, RuMAZO is proved to be a desired turn on imaging agent for visualizing the distribution of Cu2+ in insects, based on this, a reliable method could be established for investigating the functions of Cu2+ on the plant response of aphids, the work is ongoing now.
Confocal luminescence images of pea aphids given a subcutaneous injection of Cu2+ (a, 300 nL, 5 mM in a HEPES buffer solution (20 mM, pH 7.4)), 25 μM RuMAZO (b, 300 nL, 20 mM HEPES), 25 μM RuMAZO and 50 μM Cu2+ (c, 300 nL, 20 mM HEPES).
Images were taken after incubation for 6 h. Left: Bright field images. Middle: Dark field images. Rigth: Merged images. λex = 488 nm.
Discussion
To investigate the sensing mechanism of RuMAZO to Cu2+, the reaction product of RuMAZO with Cu2+ in ethanol/H2O mixture was isolated and characterized by 1H NMR, 13C NMR and HR-MS (Fig. S19–S21). Furthermore, the isolated product exhibited nearly identical UV-vis and luminescence spectra with those of the testing mixture of RuMAZO and Cu2+ incubated at 37°C for 30 min (Fig. S3). The result of the EDTA (EDTA = ethylene diamine tetraacetic acid) competitive experiment provided further evidence on the non-binding interaction between RuMAZO and Cu2+ (Fig. S4). All these demonstrated the above mentioned proposed mechanism.
To verify the selectivity of RuMAZO towards Cu2+, the influence of other metal ions on the sensing of Cu2+ was determined. As shown in Fig. 4 and Fig. S5, the changes of the emission intensity of RuMAZO in the presence of 10.0 equivalents of other metal ions were negligible. Upon the addition of only 1.0 equivalent of Cu2+ to the 1:10 mixture of RuMAZO and other metal ions, a significant luminescence enhancement was observed, indicating that the existence of those metal ions in testing samples did not interfere copper(II) detection and imaging. Different copper salts (CuSO4, CuCl2, Cu(NO3)2 and Cu(OAc)2) were also tested, not much affection can be observed on the response of RuMAZO to Cu2+ ions with the presence of different counter anions (Fig. S6).
Luminescence changes of RuMAZO (10 μM) upon the addition of various metal ions (100 μM) and 10 μM Cu2+.
Left-hand bars represent the luminescence response towards metal ions (blank, Li+, Na+, K+, Ca2+, Mg2+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+, Ba2+, Pd2+, Pb2+, Cr3+, Al3+, Ag+); right-hand bars represent the subsequent addition of 10 μM Cu2+ to the aforementioned solutions.
RuMAZO was observed to exhibit good photostability under the irradiation of 500 W iodine-tungsten lamp for 2 h (Fig. S7), this is beneficial for long-time luminescence tracking. In addition, the influence of pH on the luminescence of RuMAZO and RuTAZO was examined by luminescence titration under different pH value. As shown in Fig. S8, no obvious signal changes were observed over the pH range of 2–13, confirming that the luminescence of RuMAZO and RuTAZO was independent of pH and expected to work well under physiological conditions.
Amino acids were also examined as potential interfering factors for bioimaging applications of the probe. The result demonstrated that the presence of amino acids had no interference with the sensitive detection of Cu2+ by RuMAZO (Fig. S9). All these proved that RuMAZO is appropriate for biological Cu2+ sensing and imaging.
In summary, a fully water-soluble ruthenium(II) complex (RuMAZO) with o-(phenylazo)aniline group as reactive site has been developed as a turn on copper(II) luminescence sensor. Under a physiological environment (20 mM HEPES buffer solution, pH 7.4; 37°C), non-emissive RuMAZO can be efficiently transformed into high luminescent RuTAZO by an oxidative cyclization reaction with Cu2+ within 30 min, which can be triggered by nM-level (4.42 nM) concentration of Cu2+ with excellent selectivity. Moreover, the probe has been employed to image Cu2+ in live pea aphids with a turn-on luminescence signal.
Methods
All solvents and chemical reagents employed for synthesis were analytical grade and purchased from commercial suppliers. The solutions of EDTA and metal ions were prepared from either their chloride or their nitrate salts. Deionized water was used as solvent. HEPES buffered aqueous solution (20 mM, pH = 7.4) was prepared in double-distilled water. Pea aphids, four-day-old, were obtained from Key Laboratory of Applied Entomology of Northwest A&F University. 1H NMR and 13C NMR spectra were recorded on a Bruker 500 AVANCE III spectrometer with chemical shifts reported in ppm at room temperature. Mass spectra were obtained with Thermo Fisher LCQ Fleet mass spectrometer (USA) and a LC/Q-Tof MS spectrometry (USA). The pH of the testing systems was determined by a PHS-3C pH Meter (China). Absorption spectra were collected by using a Shimadzu 1750 UV-visible spectrometer (Japan). Emission spectra were measured with a Shimadzu RF-5301 fluorescence spectrometer (Japan). Microinjection experiments were carried out by using a Drummond Nanoject II™ Auto-Nanoliter Injector (USA). Images of pea aphids were performed on an Olympus FV1000 confocal microscope (Japan).
Compound 1 was prepared according to the literature44. The MAZO ligand was prepared through the coupling reaction of compound 1 with phenyl diazonium salt45. The ruthenium(II) complex was obtained in a satisfactory yield (89%) through direct reaction of MAZO with the appropriate molar ratios of cis-[Ru(phen)2Cl2] in ethanol46.
MAZO
Aniline (186 mg, 2.0 mmol) was dissolved in 2 mL concentrated hydrochloric acid, then 8 mL cold solution of NaNO2 (138 mg, 2.0 mmol) was added. The mixture was stirring under 0°C for 1 h. Then it was added into 36 mL 5-amino-1,10-phenanthroline (400 mg, 2.05 mmol) acetate buffer (3.0 g sodium acetate and 6 mL acetate) in dropwise in 30 minutes. After the addition was complete, the mixture was stirred for 24 h. Then mixture was filtered and the filtrate was suspended in 50 mL 3% ammonia. Stirred overnight and then filtered, washed with pure water, recrystallized in absolute ethanol. The yield was 0.548 g, 89.4%. 1H NMR (500 MHz, DMSO-d6): δ (ppm) 9.16 (dd, J = 3.1, 1.6 Hz, 1H), 9.15 (s, 1H), 9.07 (dd, J = 8.4, 1.5 Hz, 1H), 8.84 (dd, J = 4.2, 1.7 Hz, 1H), 7.99 (dd, J = 8.3, 1.0 Hz, 2H), 7.85 (dd, J = 8.4, 4.3 Hz, 1H), 7.70 (dd, J = 8.4, 4.2 Hz, 1H), 7.59 (t, J = 8.0 Hz, 2H), 7.46 (t, J = 7.3 Hz, 1H). 13C NMR (125 MHz, DMSO-d6): δ (ppm) 153.37, 152.28, 147.91, 146.74, 140.94, 137.73, 133.38, 130.67, 129.93, 129.88, 129.67, 124.43, 123.48, 122.33, 122.00, 121.68. ESI-MS: 300.16, [M + H]+; 322.11, [M + Na]+.
RuMAZO
cis-[Ru(phen)2Cl2]·2H2O (0.284 g, 0.5 mmol) and MAZO (0.150 g, 0.5 mmol) were dissolved in 50 mL anhydrous ethanol, then the mixture was refluxed for 10 h under nitrogen. The mixture was concentrated to 2 mL; the residue was dropped to NH4PF6 solution and stirred for 30 minutes. Orange precipitate was filtered and washed with cold water. The crude product was purified by column chromatography on alumina with CH2Cl2/CH3CH2OH (100:1, v/v) as the eluent. Yield: 0.525 g, 89%. 1H NMR (500 MHz, Acetone-d6): δ (ppm) 9.38 (dd, J = 8.6, 1H), 9.22 (dd, J = 8.5, 1H), 8.88–8.75 (m, 4H), 8.57 (dd, J = 5.3, 1H), 8.53 (dd, J = 5.3, 1H), 8.47 (dd, J = 5.3, 1H), 8.43 (dt, J = 4.0, 4H), 8.41 (dd, J = 5.2, 1H), 8.38 (dd, J = 5.2, 1H), 8.12–8.04 (m, 3H), 7.92–7.85 (m, 2H), 7.84–7.78 (m, 3H), 7.68 (dt, J = 20.3, 1H), 7.61 (dd, J = 10.5, 2H), 7.52 (t, J = 7.3, 1H). 13C NMR (125 MHz, Acetone-d6): δ (ppm) 154.96, 154.09, 154.01, 153.86, 153.75, 150.68, 149.62, 148.86, 148.82, 148.78, 148.76, 143.34, 138.77, 137.80, 134.31, 133.86, 131.94, 131.89, 130.66, 131.11, 130.29, 129.05, 127.38, 127.12, 127.06, 127.03, 126.30, 122.82. HR-MS: 906.1203, [M-PF6−]+; 380.5777, [M-2PF6-]2+.
RuMAZO was then converted to the chloride salt by dissolving in a minimum amount of acetone and then dropped to a saturated solution of tetrabutylammonium chloride in acetone, stirred for 15 minutes. The chloride salt was filtered, washed with acetone and dried under vacuum. Yield: 0.340 g, 92%. ESI-MS: 796.00, [M-Cl−]+; 380.97, [M-2Cl−]2+.
RuTAZO
RuMAZO (53 mg, 0.05 mmol) was dissolved in 10 mL ethanol and water (2/3, V/V), then CuSO4·5H2O (25 mg, 0.1 mmol) was added to the mixture. It was refluxed for 1 h. Then the mixture was concentrated and purified by chromatography to get red orange solid 50 mg, 94.7%. 1H NMR (500 MHz, Acetone-d6): δ (ppm) 9.17 (dd, J = 8.2, 1.1 Hz, 2H), 8.91–8.76 (m, 4H), 8.62–8.54 (m, 2H), 8.46 (ddd, J = 7.7, 6.0, 1.6 Hz, 4H), 8.44 (s, 4H), 8.40 (dd, J = 5.2, 1.1 Hz, 2H), 7.91 (dd, J = 8.2, 5.4 Hz, 2H), 7.86 (dd, J = 8.3, 5.3 Hz, 2H), 7.82 (dd, J = 8.3, 5.2 Hz, 2H), 7.76 (t, J = 8.0 Hz, 2H), 7.66 (t, J = 7.4 Hz, 1H). 13C NMR (125 MHz, Acetone-d6): δ 153.46, 153.20, 153.08, 150.10, 148.01, 147.92, 140.61, 139.83, 137.16, 137.15, 131.80, 131.17, 130.05, 129.80, 128.26, 127.34, 126.30, 126.27, 124.55, 124.55, 120.08. HR-MS: 904.1257, [M-PF6−]+; 379.5697, [M-2PF6-]2+.
References
Peña, M. M. O., Lee, J. & Thiele, D. J. A Delicate Balance: Homeostatic Control of Copper Uptake and Distribution. J. Nutr. 129, 1251–1260 (1999).
Festa, R. A. & Thiele, D. J. Copper: An essential metal in biology. Curr. Biol. 21, R877–R883.
Kim, H., Wu, X. & Lee, J. SLC31 (CTR) family of copper transporters in health and disease. Molecular Aspects of Medicine 34, 561–570 (2013).
Arguello, J. M., Raimunda, D. & Padilla-Benavides, T. Mechanisms of Copper Homeostasis in Bacteria. Front. Cell. Infect. Microbiol. 3, 1–14 (2013).
Bush, A. I. Metals and neuroscience. Curr. Opin. Chem. Biol. 4, 184–191 (2000).
Barnham, K. J., Masters, C. L. & Bush, A. I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214 (2004).
Bruijn, L. I., Miller, T. M. & Cleveland, D. W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (2004).
Siggs, O. M. et al. Disruption of copper homeostasis due to a mutation of Atp7a delays the onset of prion disease. PANS 109, 13733–13738 (2012).
Georgopoulos, P. G. et al. Environmental copper: Its Dynamics and Human Exposure Issues. J. Toxicol. Environ. Health B Crit. Rev. 4, 341–394 (2001).
Auclair, J. L. & Srivastava, P. N. Some mineral requirements of the pea aphid, acyrthosiphon pisum (homoptera: aphididae). Can. Entomol. 104, 927–936 (1972).
Crawford, L. A., Hodkinson, I. D. & Lepp, N. W. The Effects of Elevated Host-Plant Cadmium and Copper on the Performance of the Aphid Aphis fabae (Homoptera: Aphididae). J. Appl. Ecol. 32, 528–535 (1995).
Görür, G. Developmental instability in cabbage aphid (Brevicoryne brassicae) populations exposed to heavy metal accumulated host plants. Ecological Indicators 6, 743–748 (2006).
Giordanengo, P. et al. Compatible plant-aphid interactions: How aphids manipulate plant responses. Cr. Biol. 333, 516–523 (2010).
Ghrefat, H. & Yusuf, N. Assessing Mn, Fe, Cu, Zn and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65, 2114–2121 (2006).
Pal, S., Chatterjee, N. & Bharadwaj, P. K. Selectively sensing first-row transition metal ions through fluorescence enhancement. RSC Advances 4, 26585–26620 (2014).
Bergonzi, R., Fabbrizzi, L., Licchelli, M. & Mangano, C. Molecular switches of fluorescence operating through metal centred redox couples. Coordin. Chem. Rev. 170, 31–46 (1998).
Yu, M. et al. Highly Sensitive and Fast Responsive Fluorescence Turn-On Chemodosimeter for Cu2+ and Its Application in Live Cell Imaging. Chem. Eur. J. 14, 6892–6900 (2008).
Ruan, Y., Li, C., Tang, J. & Xie, J. Highly sensitive naked-eye and fluorescence “turn-on” detection of Cu2+ using Fenton reaction assisted signal amplification. Chem. Commun. 46, 9220–9222 (2010).
Wu, Q. & Anslyn, E. V. Catalytic Signal Amplification Using a Heck Reaction. An Example in the Fluorescence Sensing of Cu(II). J. Am. Chem. Soc. 126, 14682–14683 (2004).
Liu, J. & Lu, Y. A DNAzyme Catalytic Beacon Sensor for Paramagnetic Cu2+ Ions in Aqueous Solution with High Sensitivity and Selectivity. J. Am. Chem. Soc. 129, 9838–9839 (2007).
Xu, Z., Xiao, Y., Qian, X., Cui, J. & Cui, D. Ratiometric and Selective Fluorescent Sensor for CuII Based on Internal Charge Transfer (ICT). Org. Lett. 7, 889–892 (2005).
Wen, Z., Yang, R., He, H. & Jiang, Y. A highly selective charge transfer fluoroionophore for Cu2+. Chem. Commun. 1, 106–108 (2006).
Jo, J. et al. Reactivity-Based Detection of Copper(II) Ion in Water: Oxidative Cyclization of Azoaromatics as Fluorescence Turn-On Signaling Mechanism. J. Am. Chem. Soc. 134, 16000–16007 (2012).
Ajayakumar, G., Sreenath, K. & Gopidas, K. R. Phenothiazine attached Ru(bpy)32+ derivative as highly selective “turn-ON” luminescence chemodosimeter for Cu2+. Dalton Trans. 7, 1180–1186 (2009).
Li, P. et al. A near-infrared fluorescent probe for detecting copper(ii) with high selectivity and sensitivity and its biological imaging applications. Chem. Commun. 47, 7755–7757 (2011).
Ballesteros, E. et al. A New Selective Chromogenic and Turn-On Fluorogenic Probe for Copper(II) in Water−Acetonitrile 1:1 Solution. Org. Lett. 11, 1269–1272 (2009).
Kang, D. E. et al. Two-Photon Probe for Cu2+ with an Internal Reference: Quantitative Estimation of Cu2+ in Human Tissues by Two-Photon Microscopy. Anal. Chem. 86, 5353–5359 (2014).
Swamy, K. M. K. et al. Boronic acid-linked fluorescent and colorimetric probes for copper ions. Chem. Commun. 45, 5915–5917 (2008).
Zhou, L. et al. Molecular Engineering of a TBET-Based Two-Photon Fluorescent Probe for Ratiometric Imaging of Living Cells and Tissues. J. Am. Chem. Soc. 136, 9838–9841 (2014).
Yao, J. et al. Efficient Ratiometric Fluorescence Probe Based on Dual-Emission Quantum Dots Hybrid for On-Site Determination of Copper Ions. Anal. Chem. 85, 6461–6468 (2013).
Balzani, V., Bergamini, G., Marchioni, F. & Ceroni, P. Ru(II)-bipyridine complexes in supramolecular systems, devices and machines. Coordin. Chem. Rev. 250, 1254–1266 (2006).
Bolletta, F. et al. A [RuII(bipy)3]-[1,9-diamino-3,7-diazanonane-4,6-dione] two-component system, as an efficient ON-OFF luminescent chemosensor for Ni2+ and Cu2+ in water, based on an ET (energy transfer) mechanism. Dalton Trans. 9, 1381–1386 (1999).
Comba, P., Kramer, R., Mokhir, A., Naing, K. & Schatz, E. Synthesis of New Phenanthroline-Based Heteroditopic Ligands-Highly Efficient and Selective Fluorescence Sensors for Copper(II) Ions. Eur. J. Inorg. Chem. 2006, 4442–4448 (2006).
Gei German Sz Ligature Er, B. & Alsfasser, R. Probing the aqueous copper(ii) coordination chemistry of bifunctional chelating amino acid ligands with a luminescent ruthenium chromophore. Dalton Trans. 4, 612–618 (2003).
Li, X. et al. A New Luminescent Ruthenium(II) Polypyridine-derived Dipicolylamine Complex as a Sensor for Cu2+ Ions. Chinese J. Chem. 29, 1947–1950 (2011).
Lin, Q., Pei, L., Xu, W., Chao, H. & Ji, L. [Ru(bpy)2(pipdpa)]2+ as a highly sensitive and selective luminescent chemosensor for Cu2+ in aqueous solution. Inorg. Chem. Commun. 16, 104–106 (2012).
Muegge, B. D. & Richter, M. M. Electrochemiluminescent Detection of Metal Cations Using a Ruthenium(II) Bipyridyl Complex Containing a Crown Ether Moiety. Anal. Chem. 74, 547–550 (2001).
Patra, S. Boricha, V. P. Sreenidhi, K. R. Suresh, E. & Paul, P. Luminescent metalloreceptors with pendant macrocyclic ionophore: Synthesis, characterization, electrochemistry and ion-binding study. Inorg. Chim. Acta. 363, 1639–1648 (2010).
Rawle, S. C., Moore, P. & Alcock, N. W. Synthesis and coordination chemistry of 1-(2[prime or minute],2[double prime]-bipyridyl-5[prime or minute]-yl-methyl)-1,4,8,11-tetraazacyclotetra- decane L1. Quenching of fluorescence from [Ru(bipy)2(L1)]2+ by coordination of Ni or Cu in the cyclam cavity (bipy = 2,2[prime or minute]-bipyridine; cyclam = 1,4,8,11-tetra-azacyclotetra- decane). J. Chem. Soc. Chem. Commun. 9, 684–687 (1992).
Zhang, P. et al. A Dinuclear Ruthenium(II) Complex as a One- and Two-Photon Luminescent Probe for Biological Cu2+ Detection. Chem. Eur. J. 19, 15494–15503 (2013).
Zhang, R. et al. Development of a heterobimetallic Ru(II)–Cu(II) complex for highly selective and sensitive luminescence sensing of sulfide anions. Anal. Chim. Acta. 691, 83–88 (2011).
Nakamaru, K. Synthesis, Luminescence Quantum Yields and Lifetimes of Trischelated Ruthenium(II) Mixed-ligand Complexes Including 3,3′-Dimethyl-2,2′-bipyridyl. B. Chem. Soc. Jpn. 55, 2697–2705 (1982).
Verhaegh, G. W. Richard, M. & Hainaut, A. P. Regulation of p53 by Metal Ions and by Antioxidants: Dithiocarbamate Down-Regulates p53 DNA-Binding Activity by Increasing the Intracellular Level of Copper. Mol. Cell. Biol. 10, 5699–5706 (1997).
Ji, S. et al. A Highly Selective OFF-ON Red-Emitting Phosphorescent Thiol Probe with Large Stokes Shift and Long Luminescent Lifetime. Org. Lett. 12, 2876–2879 (2010).
Slouka, J., inventor; Czech, assignee. 5-Amino-6-(arylazo)-1,10-phenanthroline dyes for wool, silk and leather. Czech patent CS 255, 194. 1988 Feb 15.
Liu, J. et al. Polypyridyl ruthenium(II) complexes containing intramolecular hydrogen-bond ligand: syntheses, characterization and DNA-binding properties. J. Inorg. Biochem. 76, 265–271 (1999).
Acknowledgements
This work was supported by the National Science Foundation of China (No. 21206137, 21272030, 201205095), the Scientific Research Foundation of Northwest A&F University (Z111021103 and Z111021107), Shaanxi Province Science and Technology (No.2013K12-03-23), the Open Project Program of Key Laboratory of ECO-Textiles (Jiangnan University), the Ministry of Education (No. KLET1102).
Author information
Authors and Affiliations
Contributions
S.G.S. supervised and interpreted the research. Y.F.Z. and Z.L.L. performed the measurements and wrote the manuscript. K.Y. performed the cells imaging and Y.Z. performed pea aphids imaging. Y.Q.X., H.J.L., C.X.W. and A.P.L. helped with interpreted data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Y., Liu, Z., Yang, K. et al. A ruthenium(II) complex as turn-on Cu(II) luminescent sensor based on oxidative cyclization mechanism and its application in vivo. Sci Rep 5, 8172 (2015). https://doi.org/10.1038/srep08172
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08172
- Springer Nature Limited
This article is cited by
-
A Ruthenium Bipyridyl Molecular Dye Sensitizer and an Excited-State Intermolecular Proton Transfer-Active Colorimetric Probe for Anions, with High Affinity Towards CN− in DMSO
Chemistry Africa (2022)
-
Determination and Imaging of Small Biomolecules and Ions Using Ruthenium(II) Complex-Based Chemosensors
Topics in Current Chemistry (2022)
-
Investigation of fluorenyl-thioic-based ditopic as a functional colorimetric probe for heavy metal cations and anions with higher selectivity towards Cu2+ followed by Zn2+, displaying logic functions: Experimental and computational studies
Chemical Papers (2021)
-
Geometric, optical, and phosphorescent properties of cationic Ir(III) and Rh(III) complexes with cyclometalated ligands: DFT/TDDFT investigations
Monatshefte für Chemie - Chemical Monthly (2021)
-
A dinuclear ruthenium(II) complex as turn-on luminescent probe for hypochlorous acid and its application for in vivo imaging
Scientific Reports (2016)