Abstract
A generic and intuitive model for coherent energy transport in multiple minima systems coupled to a quantum mechanical bath is shown. Using a simple spin-boson system, we illustrate how a generic donor-acceptor system can be brought into resonance using a narrow band of vibrational modes, such that the transfer efficiency of an electron-hole pair (exciton) is made arbitrarily high. Coherent transport phenomena in nature are of renewed interest since the discovery that a photon captured by the light-harvesting complex (LHC) in photosynthetic organisms can be conveyed to a chemical reaction centre with near-perfect efficiency. Classical explanations of the transfer use stochastic diffusion to model the hopping motion of a photo-excited exciton. This accounts inadequately for the speed and efficiency of the energy transfer measured in a series of recent landmark experiments. Taking a quantum mechanical perspective can help capture the salient features of the efficient part of that transfer. To show the versatility of the model, we extend it to a multiple minima system comprising seven-sites, reminiscent of the widely studied Fenna-Matthews-Olson (FMO) light-harvesting complex. We show that an idealised transport model for multiple minima coupled to a narrow-band phonon can transport energy with arbitrarily high efficiency.
Similar content being viewed by others
Introduction
In this paper, we use the principle of minimum design to develop a general mechanism whereby energy can be transported with near-perfect efficiency through a generic system with multiple energy minima. Spectroscopists have been measuring wavelike electronic quantum coherences in photosynthetic light-harvesting protein complexes1,2,3,4,5,6,7, including at the single-molecule level8, since 2004. The molecules capture photons from sunlight and transport their energy via photoexcited excitons to a chemical reaction center with near-perfect efficiency, unmatched by artificial systems, by computing the fastest path to the reaction centre. These observations add weight to the prediction articulated by the founders of quantum physics, Werner Heisenberg and Erwin Schrödinger, that a new picture of biology would emerge when quantum theory would be applied to its study9,10,11,12.
Considering that primitive photosynthetic cells (Stromatolites) appeared over three billion years before any other lifeform, we can speculate that nature assembled a photosynthetic mechanism with minimal resources and evolved it to near-perfection under the action of natural selection. Land-based plant life appeared much later, around 400 million years ago and is a recent development in the evolutionary history of photosynthesis. Seen in this light, the high efficiency of energy-transfer in light-harvesting is perhaps less surprising.
From the perspective of quantum physics, biomolecules are large and hot objects, so at first sight, it seems surprising that quantum mechanics might play a useful role. It is beyond the scope of this paper to speculate on the functional value of quantum processes in biology, however, we can look to the timescales of biological processes for insights to inform our analysis. Typically, we have four distinct timescales, each separated by around three orders of magnitude. The fastest biological processes occur on the timescale of optical excitations, typically femtoseconds (10−15 s). The next timescale covers the coherent hopping of excitons, of the order of picoseconds (10−12 s). This is followed by chemical reactions that deliver the excitonic energy to say the ATP cycle, which then occurs on timescales of microseconds. Finally, there are the macroscopic timescales from milliseconds to seconds, in processes like neurological response.
It follows from a heuristic argument that quantum coherence can realistically only be present within the first two timescales13. Consider a typical molecule of at least one hundred thousand atoms, which absorbs a photon that projects the molecule into a conformal quantum superposition of different spatially arranged states. Such conformal changes are ubiquitous in living molecules. How long can this superposition survive? Analysis suggests that the ratio of decoherence time to dissipation time is  , where m is the mass of the molecule, x, is the coherence length, a few nanometers and comparable to the molecular size and T is the temperature, ~300 K. The dissipation, then, is typically of the order of seconds to milliseconds, which in turn leads to decoherence times of nano- to pico-seconds. A more detailed and rigorous analysis leads to the same conclusion13. So only processes on similar or shorter timescales can survive the environmental interaction and persist longer than what might be expected of processes that decohere before they affect the dynamics. These two timescales, in the femto- and picosecond domains, are exactly those relevant to the process responsible for the efficient transfer of energy in the LHC.
, where m is the mass of the molecule, x, is the coherence length, a few nanometers and comparable to the molecular size and T is the temperature, ~300 K. The dissipation, then, is typically of the order of seconds to milliseconds, which in turn leads to decoherence times of nano- to pico-seconds. A more detailed and rigorous analysis leads to the same conclusion13. So only processes on similar or shorter timescales can survive the environmental interaction and persist longer than what might be expected of processes that decohere before they affect the dynamics. These two timescales, in the femto- and picosecond domains, are exactly those relevant to the process responsible for the efficient transfer of energy in the LHC.
A widely used tool for calculating energy transfer rates between molecules is given by Förster theory15. This yields a rate for the energy transport from the overlap between the absorption and emission spectra of donor and acceptor states. In the semi-classical picture, as presented by Förster theory, energy is transferred by the incoherent hopping of photoexcited excitons down an energy ladder towards a chemical reaction center.
The nano-meter dimensions and dense packing of the LHC pigment sites, where inter-molecular separation is of the order of the size of the molecules, allow us to postulate that the protein environment could indeed host strong correlations associated with quantum phenomena. The picture that emerges then is quantum mechanical in that excitons display a wavelike character and exist in a quantum superposition of states. This allows an exciton to enter simultaneaously different energy pathways before finding the fastest route to the reaction center, so that no energy is lost. That is the picture suggested by the recent experiments on LHCs1,2,3,4,5,6,7,8. This is at odds with Förster theory, which cannot readily account for coherence between the donor and acceptor states. However, without quantum effects, the rapid energy transfer cannot be satisfactorily explained either.
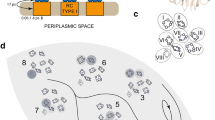
For example, the FMO molecule of bacteriochlorophyll has been the center of attention in recent years. This consists of seven trapping sites each able to hold an exciton16. When a photon is absorbed by the FMO protein, a single electron-hole pair is photoexcited in the complex. Förster theory, where the exciton hops from site to site until it finds the reaction center where it deposits its energy, fails to account for the hundreds of femtosecond timescale12,19 measured for the process. Experiments1,2,3,4,5,6,7,8 and numerical simulations22 suggest a coherent superposition of a single exciton state over the seven sites of a photosynthetic pigment. From a quantum physical perspective, the absorption of a photon, whose wavelength is 100 times larger than the length-scale of the photosynthetic aparatus (Fig. 1), projects the excited state into a quantum superposition of states spread over seven sites of different energy and with a different probability amplitude of occupation.
Light-harvesting pigment protein of the green sulphur photosynthetic bacterium Prosthecochloris aestuarii14 that lives in marine environments.
The wavelength of the incoming photon field, ~800 nm, is two orders of magnitude larger than the dimensions of the molecule.
Just before we consider the details of our generic model, let us briefly put it into context. Existing proposals23,24,25 resort to stochastic theory and introduce Markovian statistics. We adopt a nonmarkovian analysis that avoids this complexity to reveal the underlying transfer mechanism in an intuitive manner. A nonmarkovian approach was also explored in22, which resorts to additional complex features redundant in our model. Furthermore, the efficiency predicted in theory25 is lower than experimentally measured values and appears to diverge at higher temperatures from what might be expected from a system designed to shield quantum coherence. Coherent random walk proposals23,24,25 approach the problem of energy transfer from the viewpoint that the probability of transfer depends on the exciton being ejected from energy minima where it comes unstuck, through coupling to a thermal bath. The trapped excitonic states, or dark states, then rely on a coherent random walk to reach the reaction center. This contrasts with the minimalistic mechanism we present here, which dispenses with noise.
The nonmarkovian property of the system and phonon environment dynamics can formally be phrased as follows. Any Markovian dynamics have the property that the distinguishability between input states cannot increase with time and in fact, it usually decreases. If, then, we record the opposite trend in the evolution of our system, namely that the distinguishability of states increases, the immediate conclusion is that the system-to-environment coupling is nonmarkovian. The increase in distinguishability is therefore sufficient to witness nonmarkovianity. Our model employs two qubits coupled to a narrow-band phonon mode. It is nonmarkovian in that the ability to distinguish two qubit states can increase with time. This means that information flows coherently from the environment back into the system, contrary to Markovian processes. However, in our semiclassical approximation, where the phonon mode is treated as a static classical field, the ensuing evolution of the two qubits is unitary. Another way of saying this is that our vibrational modes can in fact be thought of as embedded within the system and instead of introducing noise, they promote coherence. Existing proposals assume either markovian or nonmarkovian environments. We believe instead that neither of these two statistical properties of the environment are necessary to explain the energy transfer efficiency.
A clearer understanding of the energy transfer process in complex molecules is obscured by ongoing debates surrounding the exact nature of and coupling to thermal noise in the environment. Much work has been done on modelling this in biomolecules such as FMO17,18 through atomistic simulations, but at present there is insufficient experimental data to conclusively characterise or identify the phononic or vibronic profiles believed to aid transport. In contrast, the authors of19 conclude that a non-standard phononic environment might be responsible for preserving the coherence seen in their experiments. A system that seeks to preserve coherence would, it seems reasonable, seeks to optimise or exclude coupling to a thermal bath. In the ideal and simplest case, a single-mode would be sufficient to bring into resonance energy-detuned sites.
What we do know, however, is that regardless of the exact profile of the environment, noise-assisted transport requires one or several phonon modes of appropriate frequency to bridge the energy gaps in any given system. Such a mechanism is ubiquitous in nature, as in generic double-minimum systems interacting with a quantum mechanical heat bath and in transfer kinetics in condensed hydrogen-bonded systems20,21. We underline that a realistic phononic environment is likely to present a more complicated mode structure, but our purpose here is to distil only the salient features of the transport problem to offer a fresh and intuitive understanding of how phonon-aided transport can enhance energy transfer.
We take as our starting point the simplest case of a donor-acceptor system coherently coupled to a phonon mode with a single optimal frequency in the limit where the coupling is weaker compared to the energy gap between the sites (J ≪ Δ). In the absence of further experimental evidence, this approach offers an intuitive insight that complements other approaches that build a case from the “ground-up” through atomistic simulation17. The single-mode approach in particular allows us to neglect dephasing and to adopt a purely quantum mechanical treatement that illustrates the salient features of the transport mechanism.
We offer a heuristic argument for our approximation of the coupling rate between individual donor and acceptor sites relative to their detuning and phonon energy (in the case of FMO). The mechanism for our transport model itself stands independently of these approximations and can be extended to other multiple minima systems.
Two-level toy system
The first mechanism we consider is a donor-acceptor system with Förster dipole-dipole exchange interaction. The problem we address is the efficiency of exciton transfer from a donor state, |d〉, to an acceptor, |a〉, given that the two are generally widely detuned in energy, as in Fig. 2(a), illustrating two energy-detuned sites 1 and 2. The detuning Δ is typically of the order of optical frequencies, while the hopping interaction strength, J, due to Förster coupling, is some two orders of magnitude smaller. In the absence of another mechanism, the excitation would have little chance transferring efficiently from the donor to the acceptor. A straightforward way of illustrating this is to focus only on the relevant subspace, by reducing the system to a two-level toy system. One level designates the donor energy and the other the acceptor energy, the two separated by the detuning Δ and driven at the rate J. This is the same as the Rabi model of a two-level system driven by an external field. The Bloch sphere representation in figure 2(b) illustrates the θ = π/2 rotation of the Bloch state-vector (indicated by the arrows). This rotation, hence the evolution of the system from the donor site |d〉 to the acceptor site |a〉, is characterised by the well- known probability of transfer:

Given that J is roughly ten to a hundred times smaller than Δ, the probability is as small as 10−2–10−4. When we consider the near unit efficiencies of energy transfer light-harvesting, this mechanism is insufficient. In practice, the donor-acceptor system is part of a complex molecule that has a multitude of vibrational degrees of freedom, in addition to the electronic component. There are usually acoustic vibrations (of order megahertz) and optical vibrations, around three orders of magnitude higher. Given that the transfer can take place at the rate of inverse J (on picosecond time scales), acoustic vibrations will be irrelevant, while optical ones play a useful role. In our system, the initially detuned donor and acceptor are shifted into resonance by a narrow band of phonons that are coherently excited.
(a) Vibrationally-assisted transition between two sites, 1 and 2, representing the exciton16 donor and acceptor states, |d〉 and |a〉, respectively. with an energy mismatch. (b) Bloch sphere representation of the transition, illustrating the detuning from resonance between the two states in the two sites. On transition between the two sites, the Bloch vector flips between the two sites which are detuned by an energy deficiency Δ. The parameters are: Δ, the coherence restitution term, where Δ = E2 − E1 and E2 − E1 is the energy difference between the excited states of sites 1 and 2. On resonance, Δ = g|α|, which is analogous to Rabi oscillations in atoms.
Typically, phonons couple to excitons in an off-resonant and dispersive fashion. Normally, we think that this naturally dephases the excitons. However, this is only really true if, generally speaking, the phonons have a broad spectrum and are subject to weak coupling to excitons. This leads to the regime given by the Born-Markov approximation26. If we have a strongly coupled narrow band of phonons instead, then the vibrational degrees of freedom act coherently and can in fact aid the transfer. This is the key mechanism we are proposing.
To see how phonons can enhance the exciton transfer, let us introduce a system of vibrations. We start with the full Hamiltonian, which includes the donor and acceptor, the phonons as well as electron-phonon coupling:

The energy difference between donor and acceptor is E2 − E1 = Δ, the exchange coupling is J, the third term is the phonon energy and the last is the electron-phonon coupling. Now we make four assumptions, which define our system:
-
1
The phonon density is narrow, so that we can approximate relevant vibrations by a single phonon mode.
-
2
The donor and acceptor are exactly out of phase in their coupling to vibrations.
-
3
The vibrational mode can be treated classically. This means that it can be treated as a coherent state (or near-coherent) of amplitude α. Thermal states are mixtures of coherent states, so they also satisfy this condition.
-
4
The frequency of phonons is much smaller than J (quantified below).
The Hamiltonian representing the donor-acceptor qubit coupled to a single vibrational mode (assumption 1) is given by:

Here ω is the phonon frequency and gd and ga are the exciton-phonon coupling strengths for the donor and acceptor respectively. Assumption 2 states that the coupling is of opposite phase, which means that, gd = −ga = g. This is of central importance to our mechanism since this means the energy shift acts in such a way as to bring the donor and acceptor into resonance, provided that the product of the phonon coupling strength and its amplitude of oscillation is equal to the detuning. Implementing assumption 3 means rewriting the last term as  , where α is the real part of the amplitude of the phononic coherent state. Because we assume that phonons evolve slowly compared to relevant time scales (assumption 4), the amplitude is practically time-independent. The resulting Hamiltonian now represents effectively a DC Stark shift in excitons, induced by phonons:
, where α is the real part of the amplitude of the phononic coherent state. Because we assume that phonons evolve slowly compared to relevant time scales (assumption 4), the amplitude is practically time-independent. The resulting Hamiltonian now represents effectively a DC Stark shift in excitons, induced by phonons:

If Δ = +gα, then the donor-acceptor system is DC Stark-shifted into full resonance. This could be the modulation postulated in19, namely that the protein could be modulating the electron-phonon coupling. Now that the transfer due to the ensuing Rabi oscillations becomes resonant, it follows that the probability of transfer reaches unity. In practise, that probability will of course be imperfect. So let us consider how accurately each of the assumptions must hold to maintain a high fidelity of excitation transfer.
There are four main types of errors in our system, all stemming from the approximations introduced in the vibrational degrees of freedom. One is that the phonons will most certainly not be single-mode, but will instead have some spread, which we label δω. Each of these modes will couple with a different strength to excitons, say gi, where i = 1, 2, …, 7 is the FMO site index. The second error could come from the fact that the donor and acceptor are not exactly out of phase in the phonon mode. The third type of error comes from the spread in phonon number in each mode. Our mechanism works ideally with phonon states that are near number-squeezed, in the sense that the dispersion in phonon numbers does not exceed the root of the mean number of phonons. This is why we use coherent states in our calculations, although phase-coherence is by no means prerequisite and the corresponding number-state mixture would also suffice to achieve the same evolution. This is why thermal states can also achieve a high efficiency. Finally, the fourth type of error derives from the assumption that the phonon modes are static, i.e. that the oscillation frequency is much smaller than J. In effect, this means taking cosωt to be equal to unity. To second-order approximation, this error equals ω2t2, which contributes gαω2/J2 to the detuning between excitons. We can illustrate the robustness of the system with the following general argument.
It can readily be seen from equation (1), that for a transfer efficiency of 75% (i.e. an efficiency drop of 25%), the error on ω including all possible sources needs to be as high as 50% of the oscillation frequency, ω, since J ~ 1013 Hz and p ~ 1 − (δω/J)2, where δω is the error on ω. It follows that the mechanism provides ample overhead on errors, since it tolerates inaccuracies of up to 50% in J, while maintaining the on-resonant conditions that lead to the very high efficiencies of energy transfer. Importantly, we note that the highest classical limit for quantum state transfer is  27, so even with a 50% error, our mechanism shows that quantum coherence still contributes amply to the efficiency of energy transfer.
27, so even with a 50% error, our mechanism shows that quantum coherence still contributes amply to the efficiency of energy transfer.
The intuitive formulation of our system makes it accessible to experimental tests. For example, the assumption that the FMO protein maintains a narrow phononic band can be verified in tests aiming to reveal a high degree of temperature insensitivity of the phononic mode. This stands to reason since our mechanism is based on processes that occur at frequencies in the optical and near optical regime, whose energy is well above kT at room temperature and below. Although the latest experimental results do not seek to ascertain the profile of the phonon modes as a function of temperature, they do confirm that coherence persist even at ambient temperature4. This supports the speculation view that the FMO protein could have evolved a specialised mechanism to modulate the electron-phonon interaction19.
A seven-site system
We illustrate the generality of our mechanism by applying it to the seven-site system in the light-harvesting complex of Prosthecochloris aestuarii, a green sulphur photosynthetic bacterium (Figure 1) living in marine environments. The Hamiltonians of many FMO complexes and of Pr. aestuarii in particular have been extensively studied and their energies catalogued. The Hamiltonian we use here is represented by a seven-dimensional matrix24,28.
Although the exciton energies, Ei, at sites i = 1, 2, …, 7 and the hopping rates Jij between sites i and j are well characterised, we use a range of relevant phonon frequencies and their exciton coupling strengths. As before, we assume a narrow-band of vibrational modes to bring the seven sites into resonance with each other. Even though our mechanism remains minimalistic, it is difficult to treat analytically.
The time-evolution of the probability amplitudes of a quantum superposition of an exciton in seven sites in the Pr. aestuarii light harvesting protein was simulated numerically. Figure 3(a) shows the evolution resulting in the capture of the exciton by site 3 within ~200 fs with 99.99% probability. Once the exciton is confined to site 3, its energy is deposited in the reaction center. We find, however, that when the excitonic wavefunction is superposed equally over all seven sites, figure 3(b), the probability of confining the exciton in site 3 fails to reach unity, even at multiple periods of the evolution on the timescales of relevant biological processes. A similar outcome is shown in figure 3(c), where the system is initialized with an exciton localised in a single site, here chosen arbitrarily at site 2, since our simulations showed that this outcome was site-independent. Our results further indicate that for the Pr. aestuarii FMO complex, the excitonic wavefunction favours site 6, which has a higher occupation probability, at ~20%. This is to be expected considering that the exciton energies are different and therefore couple with different strengths to sunlight. Current experimental data cannot for now confirm with greater certainty what the initial state in the FMO complex is, but our analysis suggests that it would be surprising to find all sites excited with the same quantum mechanical amplitude. Although the energetic distribution of seven sites appears to be random and asymmetric, we can reasonably speculate that the FMO protein has evolved in such a way as to capture photons with an energy likely to generate an exciton in an optimal superposition, that is, one leading to efficient energy transfer.
Simulated time-evolution of the probability distribution of a quantum superposition of an exciton's wavefunction across sites 1 to 7 of the FMO complex in Prosthecochloris aestuarii.
(a) shows an ideal quantum superposition leading to excitonic confinement in site 3 (designated reaction centre) after ~220 fs. (b) shows the evolution for an initial state in an equal superposition of the exciton in all seven sites and (c), for an arbitrarily chosen initial pure state in site 2. The curves were obtained by plotting the relevant diagonal elements extracted from the evolution of the full state, including all the off-diagonal elements.
Discussion and conclusion
The question as to how the exciton is trapped in the reaction center remains open. This is a separate but related problem, since without trapping, the excitation will forever oscillate between sites under the action of the unitary evolution. Our mechanism could, with minor modification, tackle the problem of reversibility. When the exciton is captured by the site at the reaction center, a mechanism inspired by the physics of quantum dots can be used, whereby an electron flopping between two potential traps can be confined in one when a rapidly oscillating field of large amplitude is applied at the potential boundary29. Whether this mechanism, which is analogous to conventional dephasing, also occurs in FMO complexes is open to further investigation, but it is forseeable that the requirement on the timing of dephasing need not be stringent.
The appeal of the mechanism presented here is that it offers an intuitive system that predicts the effects observed in experiments on LHC complexes and achieves this without resorting to Markovian dynamics or quantum entanglement30,31,32. It also seems plausible that such a mechanism could be engineered into biomimetic artificial media such as low-dimensional heterostructures with applications in light-harvesting and efficient energy transport.
References
Fleming, G. R. & Scholes, G. D. Quantum mechanics for plants. Nature 431, 256–257 (2004).
Brixner, T. et al. Two-dimensional spectroscopy of electronic couplings in photosynthesis. Nature 434, 625–628 (2005).
Lee, H., Cheng, Y.-C. & Fleming, G. R. Coherence dynamics in photosynthesis: protein protection of excitonic coherence. Science 316, 1462–1465 (2007).
Collini, E. et al. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 463, 644–647 (2010).
Panitchayangkoona, G. et al. Long-lived quantum coherence in photosynthetic complexes at physiological temperature. PNAS 107, 12766–12770 (2010).
Panitchayangkoon, G. et al. Direct evidence of quantum transport in photosynthetic light-harvesting complexes. PNAS 108, 20908–20912 (2011).
Hayes, D., Griffin, G. B. & Engel, G. S. Engineering Coherence Among Excited States in Synthetic Heterodimer Systems. Science 340, 1431–1434 (2013).
Hildner, R., Brinks, D., Nieder, J. B., Cogdell, R. J. & van Hulst, N. F. Quantum Coherent Energy Transfer over Varying Pathways in Single Light-Harvesting Complexes. Science 340, 1448–1451 (2013).
Schrödinger, E. What is life. Cambridge University Press, (1944).
Buckley, P. & Peat, F. D. Glimpsing Reality: Ideas in Physics and the Link to Biology. University of Toronto Press, rev. ed. (1996).
Ball, P. Physics of life: The dawn of quantum biology. Nature 474, 272–274 (2011).
Arndt, M., Juffmann, T. & Vedral, V. Quantum physics meets biology. HFSP J. 3, 386–400 (2009).
Legett, A. J. et al. Dynamics of the dissipative two-state system. Rev. Mod. Phys. 59, 1–85 (1987).
Tronrud, D. E., Wen, J., Gay, L. & Blankenship, R. E. Photosynth. Res. 100, 79–87 (2009).
Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Physik 437, 55 (1948).
Scholes, G. D. & Rumbles, G. Excitons in nanoscale systems. Nature Materials 5, 683–696 (2006).
Shim, S., Rebentrost, P., Valleau, S. & Aspuru-Guzik, A. Atomistic Study of the Long-Lived Quantum Coherences in the Fenna-Matthews-Olson Complex. Biophysical J. 102, 649660 (2012).
Berkelbach, T. C., Markland, T. E. & Reichman, D. R. Reduced density matrix hybrid approach: Application to electronic energy transfer. J. Chem. Phys. 136, 084104 (2012).
Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782–786 (2007).
Meyer, R. & Ernst, R. R. Transitions induced in a double minimum system by interaction with a quantum mechanical heat bath. J. Chem. Phys. 93, 5518 (1990).
Heuer, A. & Haeberlen, U. The dynamics of hydrogens in double well potentials: The transition of the jump rate from the low temperature quantummechanical to the high temperature activated regime. J. Chem. Phys. 95, 4201 (1991).
Ishizaki, A. & Fleming, G. R. Theoretical examination of quantum coherence in a photosynthetic system at physiological temperature. PNAS 106, 17255–17260 (2009).
Olaya-Castro, A., Lee, C. F., Olsen, F. F. & Johnson, N. F. Efficiency of energy transfer in a light-harvesting system under quantum coherence. Phys. Rev. B 78, 085115 (2008).
Caruso, F., Chin, A. W., Datta, A., Huelga, S. F. & Plenio, M. B. Highly efficient energy excitation transfer in light-harvesting complexes: The fundamental role of noise-assisted transport. J. Chem. Phys. 131, 105106 (2009).
Rebentrost, P., Mohseni, M., Kassal, I., Lloyd, S. & Aspuru-Guzik, A. Environment-assisted quantum transport. New J. Phys. 11, 033003(2009).
Gardiner, C. W. & Zoller, P. Quantum Noise: A Handbook of Markovian and Non-Markovian Quantum Stochastic Methods with Applications to Quantum Optics. Springer (2010).
Massar, S. & Popescu, S. Optimal extraction of information from finite quantum ensembles. Phys. Rev. Lett. 74, 1259 (1995).
Adolphs, J. & Renger, T. How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys. J. 91, 2778–2797 (2006).
Nazir, A. Correlation-dependent coherent to incoherent transitions in resonant energy transfer dynamics. Phys. Rev. Lett. 103, 146404 (2009).
Sarovar, M., Ishizaki, A., Fleming, G. R. & Whaley, K. B. Quantum entanglement in photosynthetic light harvesting complexes. Nature Phys. 6, 462 (2009).
Vedral, V. Quantum Correlations in Biomolecules, 22nd Solvay Conference on Chemistry. Procedia Chemistry 3, 172175 (2011).
Caruso, F., Chin, A. W., Datta, A., Huelga, S. F. & Plenio, M. B. Entanglement and entangling power of the dynamics in light-harvesting complexes. Phys. Rev. A 81, 062346 (2010).
Acknowledgements
The authors thank Dr Wonmin Son and Dr Kavan Modi for insightful comments and gratefully acknowledge financial support from the Oxford Martin Programme on Bio-Inspired Quantum Technologies, the Singapore Ministry of Education and National Research Foundation, the UK EPSRC, the Royal Society and the Wolfson Trust. V.V. is Fellow of Wolfson College, Oxford and T.F. is James Martin Fellow of the Oxford Martin School.
Author information
Authors and Affiliations
Contributions
T.F. and V.V. contributed equally to this work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Farrow, T., Vedral, V. Scale-estimation of quantum coherent energy transport in multiple-minima systems. Sci Rep 4, 5520 (2014). https://doi.org/10.1038/srep05520
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05520
- Springer Nature Limited
This article is cited by
-
Simulating molecules on a cloud-based 5-qubit IBM-Q universal quantum computer
Communications Physics (2021)