Abstract
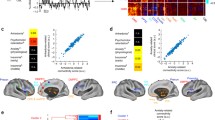
Over 20 years of neuroimaging experiments into aberrant task-based brain activity in unipolar depression have failed to reliably delineate a convergent set of anatomical regions. Here we examined whether study-derived coordinates might delineate a dysfunctional brain network in unipolar depression rather than isolated neuroanatomical foci, utilizing data from 57 studies with 99 individual neuroimaging task-based experiments, testing either emotional or cognitive processing (n = 1,058). We further assessed clinical relevance by computing optimal network-based personalized targets in 26 individuals who previously received transcranial magnetic stimulation for unipolar depression. Although coordinates were neuroanatomically heterogeneous, they localized to highly robust distributed brain networks. Importantly, these networks closely recapitulated clinically meaningful and independently derived models of depression circuitry, quantified by spatial correlation (P < 0.00002). Therapeutic outcome of transcranial magnetic stimulation was dependent on how effectively this circuit was targeted (P = 0.018). These findings indicate that neuroimaging findings in depression, which previously appeared irreconcilable, localize to highly robust and clinically meaningful distributed brain networks.






Similar content being viewed by others
Data availability
The meta-analysis derived coordinate data have been published previously1. HCP datasets are available for download to anyone agreeing to the open access data use terms (https://db.humanconnectome.org/). The clinical data remain subject to privacy and ethical restrictions. The depression cohort dataset was collected separately from the present study (ACTRN12610001071011) and remains subject to privacy and ethical restrictions.
Code availability
Preprocessing software and code is available at https://fmriprep.org/en/stable/. Custom analysis scripts were developed and implemented in MATLAB R2017a, and are available on reasonable request to the corresponding author (R.F.H.C.), although not for commercial applications.
References
Müller, V. I. et al. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry 74, 47–55 (2017).
Barch, D. M. & Pagliaccio, D. Consistency, replication, and meta-analyses of altered brain activity in unipolar depression. JAMA Psychiatry 74, 56–57 (2017).
Ioannidis, J. P., Fanelli, D., Dunne, D. D. & Goodman, S. N. Meta-research: evaluation and improvement of research methods and practices. PLoS Biol. 13, e1002264 (2015).
Darby, R. R., Joutsa, J. & Fox, M. D. Network localization of heterogeneous neuroimaging findings. Brain 142, 70–79 (2019).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017).
Carp, J. On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front. Neurosci. 6, 149 (2012).
Marquand, A. F. et al. Conceptualizing mental disorders as deviations from normative functioning. Mol. Psychiatry 24, 1415–1424 (2019).
Molenberghs, P., Sale, M. V. & Mattingley, J. B. Is there a critical lesion site for unilateral spatial neglect? A meta-analysis using activation likelihood estimation. Front. Hum. Neurosci. 6, 78 (2012).
Fornito, A., Zalesky, A. & Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 16, 159–172 (2015).
Downar, J. & Daskalakis, Z. J. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 6, 231–240 (2013).
Boes, A. D. et al. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075 (2015).
Siegel, J. S. et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc. Natl Acad. Sci. USA 113, E4367–E4376 (2016).
Padmanabhan, J. L. et al. A human depression circuit derived from focal brain lesions. Biol. Psychiatry 86, 749–758 (2019).
Horn, A. The impact of modern-day neuroimaging on the field of deep brain stimulation. Curr. Opin. Neurol. 32, 511–520 (2019).
Siddiqi, S. H. et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat. Hum. Behav. 5, 1707–1716 (2021).
Baldermann, J. C. et al. Connectivity profile predictive of ffective deep brain stimulation in obsessive-compulsive disorder. Biol. Psychiatry 85, 735–743 (2019).
Eickhoff, S. B. et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85 (2016).
Cash, R. F., Cocchi, L., Lv, J., Fitzgerald, P. B. & Zalesky, A. Functional magnetic resonance imaging–guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry 78, 337–339 (2021).
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D. & Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603 (2012).
Weigand, A. et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 84, 28–37 (2018).
Cash, R. F. et al. Personalized connectivity‐guided DLPFC‐TMS for depression: advancing computational feasibility, precision and reproducibility. Hum. Brain Mapping 42, 4155–4172 (2021).
Cash, R. F. et al. Personalized brain stimulation of memory networks. Brain Stimul. 15, 1300–1304 (2022).
Fox, M. D., Liu, H. & Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66, 151–160 (2013).
Tik, M. et al. Acute TMS/fMRI response explains offline TMS network effects—an interleaved TMS-fMRI study. NeuroImage 267, 119833 (2023).
Kong, G., Wei, L., Wang, J., Zhu, C. & Tang, Y. The therapeutic potential of personalized connectivity-guided transcranial magnetic stimulation target over group-average target for depression. Brain Stimul. 15, 1063–1064 (2022).
Stöhrmann, P. et al. Effects of bilateral sequential theta-burst stimulation on functional connectivity in treatment-resistant depression: first results. Preprint at medRxiv https://doi.org/10.1101/2022.02.16.22271078 (2022).
Cash, R. F. H. et al. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol. Psychiatry 86, e5–e7 (2019).
Hamani, C. et al. The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry 69, 301–308 (2011).
Groenewold, N. A., Opmeer, E. M., de Jonge, P., Aleman, A. & Costafreda, S. G. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 37, 152–163 (2013).
Heilbronner, S. R., Safadi, Z. & Haber, S. N. in Neuromodulation in Psychiatry (eds Hamami, C. et al.) Ch. 3 (Wiley, 2016).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93 (2011).
Wang, C. et al. Disrupted functional connectivity patterns of the insula subregions in drug-free major depressive disorder. J. Affect. Disord. 234, 297–304 (2018).
Sheline, Y. I., Price, J. L., Yan, Z. & Mintun, M. A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl Acad. Sci. USA 107, 11020–11025 (2010).
Downar, J. Orbitofrontal cortex: a ‘non-rewarding’new treatment target in depression? Curr. Biol. 29, R59–R62 (2019).
Bakker, N. et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 8, 208–215 (2015).
Mayberg, H. S. Limbic–cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9, 471–481 (1997).
Papez, J. W. A proposed mechanism of emotion. Arch. NeurPsych. 38, 725–743 (1937).
Binder, D. K. & Iskandar, B. J. Modern neurosurgery for psychiatric disorders. Neurosurgery 47, 9–23 (2000).
Stockmeier, C. A. & Rajkowska, G. Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues Clin. Neurosci. 6, 185 (2004).
Cash, R. F. H. et al. Using brain imaging to improve spatial targeting of TMS for depression. Biol. Psychiatry 90, 689–700 (2020).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
Conradi, H., Ormel, J. & De Jonge, P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol. Med. 41, 1165–1174 (2011).
Figee, M. & Mayberg, H. The future of personalized brain stimulation. Nat. Med. 27, 196–197 (2021).
Li, B. J. et al. A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci. Ther. 24, 1004–1019 (2018).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Gray, J. P., Müller, V. I., Eickhoff, S. B. & Fox, P. T. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am. J. Psychiatry 177, 422–434 (2020).
Wang, Q. et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. Neuroimage 224, 117307 (2021).
Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 (2014).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007).
Vincent, J. L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
Smith, S. M. et al. Resting-state fMRI in the Human Connectome Project. Neuroimage 80, 144–168 (2013).
Tian, Y., Margulies, D. S., Breakspear, M. & Zalesky, A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 23, 1421–1432 (2020).
Birn, R. M. et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 83, 550–558 (2013).
Noble, S. et al. Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cerebral Cortex 27, 5415–5429 (2017).
Choe, A. S. et al. Reproducibility and temporal structure in weekly resting-state fMRI over a period of 3.5 years. PLoS ONE 10, e0140134 (2015).
Gordon, E. M. et al. Precision functional mapping of individual human brains. Neuron 95, 791–807.e7 (2017).
Cui, Z. et al. Individual variation in functional topography of association networks in youth. Neuron 106, 340–353.e8 (2020).
Coalson, T. S., Van Essen, D. C. & Glasser, M. F. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc. Natl Acad. Sci. USA 115, E6356–E6365 (2018).
Fox, M. D. et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl Acad. Sci. USA 111, E4367–E4375 (2014).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: identifying differences in brain networks. Neuroimage 53, 1197–1207 (2010).
Fitzgerald, P. B., Hoy, K. E., Anderson, R. J. & Daskalakis, Z. J. A study of the pattern of response to rTMS treatment in depression. Depress Anxiety 33, 746–753 (2016).
Abbott, L. F. & Nelson, S. B. Synaptic plasticity: taming the beast. Nat. Neurosci. 3, 1178–1183 (2000).
Fox, M. D., Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243 (2012).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Xia, M., Wang, J. & He, Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE 8, e68910 (2013).
Beam, W., Borckardt, J. J., Reeves, S. T. & George, M. S. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2, 50–54 (2009).
Acknowledgements
We thank S. Siddiqi for providing the convergent and lesion circuits. We thank all participants, nurses and staff involved in the collection of clinical data. We thank all who contributed to the original experiments from which the present coordinates are derived and those involved in the Human Connectome Project. We thank all the funding bodies below, and note that the funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. R.F.H.C. was funded by the Australian Research Council (DE200101708) and Brain and Behavior Research Foundation. A.Z. was supported by the Australian National Health and Medical Research Council Senior Research Fellowship B (ID: 1136649). P.B.F. has received equipment for research from Cervel Neurotech, Medtronic, MagVenture A/S and Brainsway. S.B.E. acknowledges funding by the European Union’s Horizon 2020 Research and Innovation Program (grant agreements 945539 (HBP SGA3) and 826421 (VBC), the Deutsche Forschungsgemeinschaft (DFG), Project-ID 431549029 – SFB 1451) and the National Institute of Health (NIH, 2R01-MH074457).
Author information
Authors and Affiliations
Contributions
Conception and study design: R.F.H.C., A.Z., S.B.E. and V.I.M. Preprocessing and data analysis: R.F.H.C. and A.Z. Clinical data was contributed by P.B.F. Interpretation R.F.H.C., A.Z., S.B.E. and V.I.M. Paper writing: R.F.H.C. and A.Z. with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Martin Tik, Ruiyang Ge, Deborah Klooster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cash, R.F.H., Müller, V.I., Fitzgerald, P.B. et al. Altered brain activity in unipolar depression unveiled using connectomics. Nat. Mental Health 1, 174–185 (2023). https://doi.org/10.1038/s44220-023-00038-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00038-8
- Springer Nature America, Inc.
This article is cited by
-
Divergent suicidal symptomatic activations converge on somato-cognitive action network in depression
Molecular Psychiatry (2024)
-
The future of brain circuit-targeted therapeutics
Neuropsychopharmacology (2024)
-
Elevating the field for applying neuroimaging to individual patients in psychiatry
Translational Psychiatry (2024)
-
Non-invasively targeting, probing and modulating a deep brain circuit for depression alleviation
Nature Mental Health (2023)
-
Embracing the heterogeneity in depression neuroimaging
Nature Mental Health (2023)