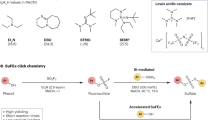
Abstract
Sulfur(VI) fluoride exchange click chemistry is a formidable tool to rapidly and effectively link chemical structures. Despite advances in the field in recent years, the installation of the sulfonyl fluoride handle still requires the use of purpose-designed, expensive and non-atom-economic reagents. The use of the SO2F2 for sulfonyl fluoride synthesis has been thwarted by the difficulties associated with the manipulation and dosage of this toxic gas, and by its apparent low reactivity with amino functionalities. Here we report a modular flow platform that can generate on demand, and efficiently dose, gaseous SO2F2. The use of flow technologies allows many lingering limitations of this transformation to be overcome, resulting in reduced reaction times, efficient reactivity and broad substrate scope. The effectiveness of the process was demonstrated by the successful synthesis of a diverse set of fluorosulfates and sulfamoyl fluorides, including those derived from biorelevant compounds, peptides and proteins.



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information.
Change history
31 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44160-024-00489-6
References
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Moorhouse, A. D., Homer, J. A. & Moses, J. E. The certainty of a few good reactions. Chem 9, 2063–2077 (2023).
Kitamura, S. et al. Sulfur(VI) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Liu, Z. et al. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018).
Zheng, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. USA 116, 18808–18814 (2019).
Jones, L. H. Emerging utility of fluorosulfate chemical probes. ACS Med. Chem. Lett. 9, 584–586 (2018).
McCann, H. M. et al. Covalent immune proximity-induction strategy using SuFEx-engineered bifunctional viral peptides. ACS Chem. Biol. 17, 1269–1281 (2022).
Gilbert, K. E. et al. Profiling sulfur(VI) fluorides as reactive functionalities for chemical biology tools and expansion of the ligandable proteome. ACS Chem. Biol. 18, 285–295 (2023).
Li, S. et al. SuFExable polymers with helical structures derived from thionyl tetrafluoride. Nat. Chem. 13, 858–867 (2021).
Dong, J., Sharpless, K. B., Kwisnek, L., Oakdale, J. S. & Fokin, V. V. SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. 53, 9466–9470 (2014).
Durie, K. et al. Multifunctional surface manipulation using orthogonal click chemistry. Langmuir. 32, 6600–6605 (2016).
Randall, J. D. et al. Modification of carbon fibre surfaces by sulfur–fluoride exchange click chemistry. ChemPhysChem. 19, 3176–3181 (2018).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Falardeau, E. R. & DesMarteau, D. D. Synthesis of pentafluorophenoxy derivatives of sulfur(IV) and -(VI) fluorides. J. Chem. Eng. Data. 21, 386–387 (1976).
Ciuffarin, E., Senatore, L. & Isola, M. Nucleophilic substitution at four-co-ordinate sulphur. Mobility of the leaving group. J. Chem. Soc., Perkin Trans. 2, 468–471 (1972).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019).
Gehringer, M. & Laufer, S. A. Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J. Med. Chem. 62, 5673–5724 (2018).
Zeng, D., Deng, W.-P. & Jiang, X. Advances in the construction of diverse SuFEx Linkers. Natl. Sci. Rev. 29, nwad123 (2023).
Schneir, A., Clark, R. F., Kene, M. & Betten, D. Systemic fluoride poisoning and death from inhalational exposure to sulfuryl fluoride. Clin. Toxicol. 46, 850–854 (2008).
Veryser, C., Demaerel, J., Bieliu̅nas, Vidmantas, Gilles, P. & De Borggraeve, W. M. Ex situ generation of sulfuryl fluoride for the synthesis of aryl fluorosulfates. Org. Lett. 19, 5244–5247 (2017).
Zhou, H. et al. Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(VI) fluorides. Org. Lett. 20, 812–815 (2018).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F−SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Kiang, T. & Zare, R. N. Stepwise bond dissociation energies for the removal of fluorine from thionyl fluoride and sulphuryl fluoride. Chem. Commun. 1228 (1980).
Plutschack, M. B., Pieber, B., Gilmore, K. & Seeberger, P. H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 117, 11796–11893 (2017).
Dallinger, D., Gutmann, B. & Kappe, C. O. The concept of chemical generators: on-site on-demand production of hazardous reagents in continuous flow. Acc. Chem. Res. 53, 1330–1341 (2020).
Capaldo, L., Wen, Z. & Noël, T. A field guide to flow chemistry for synthetic organic chemists. Chem. Sci. 14, 4230–4247 (2023).
Mallia, C. J. & Baxendale, I. R. The use of gases in flow synthesis. Org. Process Res. Dev. 20, 327–360 (2016).
Casnati, A., Gemoets, H. P., Motti, E., DellaCa’, N. & Noël, T. Homogeneous and gas–liquid Catellani‐type reaction enabled by continuous‐flow chemistry. Chem. Eur. J. 24, 14079–14083 (2018).
Straathof, N. J., Su, Y., Hessel, V. & Noël, T. Accelerated gas–liquid visible light photoredox catalysis with continuous-flow photochemical microreactors. Nat. Protoc. 11, 10–21 (2015).
Dong, Z., Wen, Z., Zhao, F., Kuhn, S. & Noël, T. Scale-up of micro- and milli-reactors: an overview of strategies, design principles and applications. Chem. Eng. Sci. X. 10, 100097 (2021).
Thirumurugan, P., Matosiuk, D. & Jozwiak, K. Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev. 113, 4905–4979 (2013).
Wang, N. et al. Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 (2018).
Boutureira, O. & Bernardes, G. J. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Acknowledgements
We acknowledge financial support from the European Union H2020 research and innovation program for an ERC CoG grant for T.N. (FlowHAT, number 101044355) and a Marie S. Curie Grant fellowship for D.M. (ELECTRORGANO, number 101022144), the Spanish Government-MCIN, the national agency of investigation-AEI/10.13039/501100011033, and the European Regional Development Fund-ERDF for project PID2020-120584RB-I00 to O.B. and and FPU Fellowship (FPU19/01969 and EST22/00303) to M.B.
Author information
Authors and Affiliations
Contributions
M.B. and D.M. conceived the project. M.B., D.M. and J. S. performed and analysed the experiments. O.B. and T.N.G. supervised the ligation of peptides and proteins. Z.Z., A.Y.V., A.F.G.G. and T.N.G. performed the analyses of the ligation of peptides and proteins. T.N. directed the project. M.B., D.M. and T.N. have written the manuscript with contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Nicholas Ball, Jiajia Dong and Christopher Hone for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–76, Tables 1–22, Discussion, NMR spectra and Schemes 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bernús, M., Mazzarella, D., Stanić, J. et al. A modular flow platform for sulfur(VI) fluoride exchange ligation of small molecules, peptides and proteins. Nat. Synth 3, 185–191 (2024). https://doi.org/10.1038/s44160-023-00441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00441-0
- Springer Nature Limited
This article is cited by
-
SO2F2 generation and reaction in flow
Nature Synthesis (2024)