Abstract
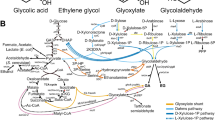
Gas-fermenting acetogens can upgrade one-carbon (C1) compounds (such as CO2 and CO) to the two-carbon (C2) metabolite acetyl coenzyme A (CoA) and convert sugar feedstocks to acetyl-CoA with minimal CO2 emissions. Fulfilling the biosynthetic potential of these microbes requires overcoming challenges in pathway engineering. Here we design a synthetic acetyl-CoA bi-cycle—in addition to the natural carbon-fixing pathways—for C2 metabolite synthesis. This pathway produces an acetyl-CoA by fixation of two CO2 equivalents via three functional modules acting in sequence: carbon fixation, gluconeogenesis and non-oxidative glycolysis. The pathway was examined by in silico thermodynamic and kinetic analyses. The prototypic pathway was implemented in a syngas-fermenting organism, Clostridium ljungdahlii DSM 13528, by expressing a heterologous phosphoketolase that can work with other native enzymes in the host acetogen. The carbon conversion pathway is possible under various growth conditions and is independent of the Wood–Ljungdahl pathway for the valorization of H2 and CO2. This study reports the improvement of carbon conversion using a reductive acetyl-CoA bi-cycle and the potential impact of redox homoeostasis in the acetogenic host for industrial applications of gas fermentation.









Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within this paper and the Supplementary Information.
Code availability
The scripts and models used in this paper are available at https://doi.org/10.5281/zenodo.4548907. The code for thermodynamics, enzyme protein cost and robustness analysis can be found at https://github.com/Chaowu88/PathParser.
References
Sun, X., Atiyeh, H. K., Huhnke, R. L. & Tanner, R. S. Syngas fermentation process development for production of biofuels and chemicals: a review. Bioresource Technol. Rep. 7, 100279 (2019).
Liew, F. et al. Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 7, 694 (2016).
Jones, S. W. et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat. Commun. 7, 12800 (2016).
Maru B. T., Munasinghe P. C., Gilary H., Jones S. W. & Tracy B. P. Fixation of CO2 and CO on a diverse range of carbohydrates using anaerobic, non-photosynthetic mixotrophy. FEMS Microbiol. Lett. 365, 29462309 (2018).
Kopke, M. & Simpson, S. D. Pollution to products: recycling of ‘above ground’ carbon by gas fermentation. Curr. Opin. Biotechnol. 65, 180–189 (2020).
Debabov, V. G. Acetogens: biochemistry, bioenergetics, genetics, and biotechnological potential. Microbiology 90, 273–297 (2021).
Takors, R. et al. Using gas mixtures of CO, CO2 and H2 as microbial substrates: the do’s and don’ts of successful technology transfer from laboratory to production scale. Microb. Biotechnol. 11, 606–625 (2018).
Schuchmann, K. & Müller, V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014).
Sanchez-Andrea, I. et al. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 11, 5090 (2020).
Cohen, S. E. et al. Negative-stain electron microscopy reveals dramatic structural rearrangements in Ni–Fe–S-dependent carbon monoxide dehydrogenase/acetyl-CoA synthase. Structure 29, 43–49 e43 (2021).
Can, M., Armstrong, F. A. & Ragsdale, S. W. Structure, function, and mechanism of the nickel metalloenzymes. Chem. Rev. 114, 4149–4174 (2014).
Roberts, J. R., Lu, W. P. & Ragsdale, S. W. Acetyl-coenzyme A synthesis from methyltetrahydrofolate, CO, and coenzyme A by enzymes purified from Clostridium thermoaceticum: attainment of in vivo rates and identification of rate-limiting steps. J. Bacteriol. 174, 4667–4676 (1992).
Straub, M., Demler, M., Weuster-Botz, D. & Durre, P. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii. J. Biotechnol. 178, 67–72 (2014).
Song, Y. et al. Functional cooperation of the glycine synthase-reductase and Wood–Ljungdahl pathways for autotrophic growth of Clostridium drakei. Proc. Natl Acad. Sci. USA 117, 7516–7523 (2020).
Bogorad, I. W., Lin, T.-S. & Liao, J. C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 502, 693–697 (2013).
Köpke, M. et al. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl Acad. Sci. USA 107, 13087–13092 (2010).
Xiong, W. et al. CO2-fixing one-carbon metabolism in a cellulose-degrading bacterium Clostridium thermocellum. Proc. Natl Acad. Sci. USA 113, 13180–13185 (2016).
Furdui, C. & Ragsdale, S. W. The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood–Ljungdahl pathway. J. Biol. Chem. 275, 28494–28499 (2000).
Shin, J., Song, Y., Jeong, Y. & Cho, B. K. Analysis of the core genome and pan-genome of autotrophic acetogenic bacteria. Front Microbiol 7, 1531 (2016).
Tremblay, P.-L., Zhang, T., Dar, S. A., Leang, C. & Lovley, D. R. The Rnf Complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4, e00406–e00412 (2013).
Liew, F. et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 40, 104–114 (2017).
Liu, J. K. et al. Predicting proteome allocation, overflow metabolism, and metal requirements in a model acetogen. PLoS Comput. Biol. 15, e1006848 (2019).
Flamholz, A., Noor, E., Bar-Even, A., Liebermeister, W. & Milo, R. Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc. Natl Acad. Sci. USA 110, 10039–10044 (2013).
Noor, E. et al. Pathway thermodynamics highlights kinetic obstacles in central metabolism. PLoS Comput. Biol. 10, e1003483 (2014).
Wu, C. et al. A generalized computational framework to streamline thermodynamics and kinetics analysis of metabolic pathways. Metab. Eng. 57, 140–150 (2020).
Tran, L. M., Rizk, M. L. & Liao, J. C. Ensemble modeling of metabolic networks. Biophys. J. 95, 5606–5617 (2008).
Tan, Y., Rivera, J. G., Contador, C. A., Asenjo, J. A. & Liao, J. C. Reducing the allowable kinetic space by constructing ensemble of dynamic models with the same steady-state flux. Metab. Eng. 13, 60–75 (2011).
Lee, Y., Lafontaine Rivera, J. G. & Liao, J. C. Ensemble modeling for robustness analysis in engineering non-native metabolic pathways. Metab. Eng. 25, 63–71 (2014).
Orth, J. D., Thiele, I. & Palsson, B. Ø. What is flux balance analysis? Nat. Biotechnol. 28, 245–248 (2010).
Ebrahim, A., Lerman, J. A., Palsson, B. O. & Hyduke, D. R. COBRApy: constraints-based reconstruction and analysis for Python. BMC Syst. Biol. 7, 74 (2013).
Perez, J. M., Richter, H., Loftus, S. E. & Angenent, L. T. Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 110, 1066–1077 (2013).
Whitham, J. M. et al. Characterization of Clostridium ljungdahlii OTA1: a non-autotrophic hyper ethanol-producing strain. Appl. Microbiol. Biotechnol. 101, 1615–1630 (2017).
Emerson, D. F. et al. Enhancing hydrogen-dependent growth of and carbon dioxide fixation by Clostridium ljungdahlii through nitrate supplementation. Biotechnol. Bioeng. 116, 294–306 (2019).
Klask, C. M., Kliem-Kuster, N., Molitor, B. & Angenent, L. T. Nitrate feed improves growth and ethanol production of Clostridium ljungdahlii with CO2 and H2, but results in stochastic inhibition events. Front Microbiol 11, 724 (2020).
Könneke, M. et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl Acad. Sci. USA 111, 8239–8244 (2014).
Bengelsdorf, F. R. et al. Bacterial anaerobic synthesis gas (syngas) and CO2 + H2 fermentation. Adv. Appl. Microbiol. 103, 143–221 (2018).
Jeong, Y., Song, Y., Shin, H. S. & Cho, B. K. Draft genome sequence of acid-tolerant Clostridium drakei SL1T, a potential chemical producer through syngas fermentation. Genome Announc. 2, e00387–e00414 (2014).
Kusel, K., Dorsch, T., Acker, G., Stackebrandt, E. & Drake, H. L. Clostridium scatologenes strain SL1 isolated as an acetogenic bacterium from acidic sediments. Int. J. Syst. Evol. Microbiol. 50, 537–546 (2000).
Nagarajan, H. et al. Characterizing acetogenic metabolism using a genome-scale metabolic reconstruction of Clostridium ljungdahlii. Microb Cell Fact 12, 118 (2013).
Acknowledgements
We acknowledge A.M. Grunden at North Carolina State University for providing an ACS-deficient C. ljungdahlii OTA1. This work was authored in part by the National Renewable Energy Laboratory, operated by the Alliance for Sustainable Energy for the US Department of Energy (DOE) under contract no. DE-AC36-08GO28308. This work is supported by a Laboratory Directed Research and Development (LDRD) project at the National Renewable Energy Laboratory to W.X., C.W., J.L., X.G. and K.J.C., partially by Shell International Exploration and Production to W.X., P.M. and J.L., a DOE Bioenergy Technologies Office (BETO) Co-Optimization of Fuels and Engines (Co-Optima) project to W.X., C.W., B.Y., J.H., C.U., D.P. and N.T., as well as the Start-up Fund of Miami University to X.W. and S.S. The views expressed in the article do not necessarily represent the views of the DOE or the US Government. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US Government purposes.
Author information
Authors and Affiliations
Contributions
W.X. projects conception. C.W., W.X. designed and performed all computational biology analysis. J.L. designed and performed genetic engineering on C. ljungdahlii. J.L., C.U., X.G., B.Y., J.H. and W.X. performed experiments including cell culture, enzyme assay, gas fermentation and product measurement. C.W., C.U., J.H., X.G. and W.X. designed and performed 13C-labelling experiments and data analysis. S.S., X.W., C.W., and W.X. generated and analysed proteomics data. C.W. and W.X. wrote the manuscript with input from all co-authors and revisions from K.J.C., P.M., N.T. and D.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Stephen Ragsdale, Michael Köpke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Information, Methods, Tables 1–5 and Figs. 1–11.
Source data
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 8
Phylogenetic analysis Source Data.
Source Data Fig. 8
Phylogenetic analysis Source Data.
Rights and permissions
About this article
Cite this article
Wu, C., Lo, J., Urban, C. et al. Acetyl-CoA synthesis through a bicyclic carbon-fixing pathway in gas-fermenting bacteria. Nat. Synth 1, 615–625 (2022). https://doi.org/10.1038/s44160-022-00095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00095-4
- Springer Nature Limited
This article is cited by
-
Light-driven biosynthesis of volatile, unstable and photosensitive chemicals from CO2
Nature Synthesis (2023)
-
Divide and conquer towards synthetic autotrophy
Nature Catalysis (2023)
-
Redesigning CO2 fixation
Nature Synthesis (2022)