Abstract
Background
Vaccination has been recommended as one of the most potent ways of controlling the mpox (formerly, monkeypox) outbreak, particularly among high-risk groups. Here, we evaluated the prevalence of mpox vaccine acceptance and uptake globally.
Methods
We searched multiple databases for peer-reviewed studies published in English from May 2022 to 25th November 2023 that evaluated mpox vaccine acceptance and/or uptake. We fit a random-effects model meta-analysis to calculate the pooled mpox vaccine acceptance and uptake rates, with their 95% confidence intervals (CI) across population outcomes. We performed subgroup analyses among the six World Health Organization (WHO) regions (Africa [AFR], Region of the Americas [AMR], South-East Asia Region [SEAR], European Region [EUR], Eastern Mediterranean Region [EMR], and the Western Pacific Region [WPR]), as well as among select population subgroups.
Results
Of the 2531 studies screened, 61 studies, with a cumulative sample size of 263,857 participants from 87 countries were eligible for inclusion. The overall vaccine acceptance and uptake rates were 59.7% and 30.9% globally. Acceptance and uptake rates among the LGBTQI+ community were 73.6% vs 39.8% globally, 60.9% vs. 37.1% in AMR, 80.9% vs. 50.0% in EUR, and 75.2% vs. 33.5% in WPR. Among PLHIV, vaccine acceptance and uptake rates were 66.4% vs. 35.7% globally, 64.0% vs. 33.9% in AMR, 65.1% vs. 27.0% in EUR, and 69.5% vs. 46.6% in WPR. Among healthcare workers, vaccination intention was 51.0% globally.
Conclusions
Tailored interventions are needed to bolster confidence in the mpox vaccine, maximize vaccine uptake, and increase vaccine access to close the gaps between acceptance and uptake especially among key populations residing in regions with low rates of acceptance and uptake.
Plain language summary
Mpox is an infection caused by the monkeypox virus and is transmitted through direct contact with infected animals or people, or indirectly through contact with contaminated materials. An unprecedented mpox outbreak spanning all continents occurred in 2022. Vaccination against the infection by high-risk groups, including the LGBTQI+ community and frontline healthcare workers has been recommended by the WHO as essential to outbreak control. To investigate the rates and factors associated with mpox vaccine acceptance and uptake across population subgroups (LGBTQI+ community, healthcare workers, people living with HIV, and the general public), we undertook this global systematic review and meta-analysis of the available evidence. Our results reveal substantial global and regional variations in the rates of mpox vaccine acceptance and uptake across population groups, with wide acceptance-uptake gaps, indicating the need for behavioral interventions to increase mpox vaccine confidence and uptake.
Similar content being viewed by others
Introduction
The World Health Organization (WHO) has recommended vaccination as one of the most effective interventions required to prevent and control the unprecedented spread of mpox (formerly monkeypox), a zoonotic viral infection that has infected about 92, 783 individuals across116 countries, with 171 deaths reported as of November 20231,2,3. Successful control of the outbreak requires optimal acceptance and uptake of the vaccination against mpox, particularly among those designated as high-risk groups, such as the lesbian, gay, bisexual, transgender, queer, and intersex+ (LGBTQI+) community4. However, this outbreak occurred at a time when the world is witnessing all-time high levels of vaccine hesitancy5,6,7,8, defined by the WHO as “a delay in the acceptance or refusal of vaccination despite the availability of vaccination services”9
Vaccination against mpox may be provided to individuals at high risk of the infection as primary preventive vaccination (PPV) prior to exposure to the mpox virus, or as post-exposure preventive vaccination (PEPV) for contacts of mpox cases4,10. In addition to the previously employed smallpox vaccine, which has been shown to be highly effective in protecting against mpox11, newer vaccines, including the MVA-BN, LC16, and the ACAM2000 have been approved in many countries for the prevention of mpox10,12. A recently published systematic review shows that these vaccines are highly effective, safe, and immunogenic, depending on the number of doses administered and that vaccines against smallpox offer cross-protection against mpox13. However, in people living with HIV (PLHIV), a population accounting for about four in ten confirmed mpox cases14,15,16,17,18, safety concerns have precluded the use of the ACAM200012. In a recently published global case series reported a severe form of mpox resembling an AIDS-defining condition, with a mortality rate that is as high as 25% among people with advanced HIV17.
Previous systematic reviews19,20,21 have attempted to identify key determinants of intention and hesitancy to vaccinate against mpox. However, these reviews were limited by having a small number of included studies and lacking representation across all six WHO regions. Also, none of these reviews reported the regional rates of intention to vaccinate against mpox among key populations designated as high-risk groups by the WHO, including the LGBTQI+ community and healthcare workers. Furthermore, the previous meta-analyses did not report vaccine uptake rates or the global vaccine acceptance rate among vulnerable groups, such as PLHIV.
In view of these literature gaps, we conduct this systematic review and meta-analysis of 61 studies involving 263,857 participants to report the global and regional prevalence of mpox vaccine acceptance and uptake among various populations, including the LGBTQI+ community, PLHIV, healthcare workers and the general public as well as the pooled rates of uptake among people who indicated their willingness to be vaccinated. Our findings reveal substantial global and regional variations in the acceptance and uptake rates of the mpox vaccine across these population groups. Moreover, we also find wide a acceptance-uptake gaps of the mpox vaccine, including across key populations like the LGBTQI+ community. These findings call for deliberate efforts to increase access to mpox vaccine especially for at-risk group in order to close the gap between intention to vaccinate and the actual vaccine uptake.
Methods
The present review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines22. The protocol for this review was registered with the International Prospective Register for Systematic Review (PROSPERO ID: CRD42022378564).
The initial literature search was conducted between 15th to 25th February 2023 in multiple electronic databases, including Medline, Embase, PubMed, Google Scholar, Web of Science, Scopus, and PsycINFO to identify studies evaluating the acceptance and/or uptake of the mpox vaccine. An updated literature search was performed on 25th November 2023. A detailed search strategy, comprising key terms, the Boolean operators, ‘AND’ and ‘OR,’ and Medical Subject Headings (MeSH), was developed for PubMed and adapted for the other databases (Supplementary Data 1). Briefly, the key terms used included “Monkeypox,” “Mpox,” “Vaccine,” “Vaccination,” “Uptake,” “Acceptance,” “Willingness,” “Intention,” “Access,” “Hesitancy,” “refusal,” “Uncertainty,” “Indecision,” “Determinants,” “Factors,” “Correlates,” and “Predictors”.
Criteria for study inclusion/exclusion
To include studies reporting the rates oof mpox vaccine acceptance, intention/willingness and uptake, we followed the guideline of the CoCoPop (condition, context, population)23 statement for review of studies reporting on prevalence/incidence. Accordingly, we included original full-text articles reporting any of this study’s outcomes of interest: prevalence rates of intention to vaccinate against mpox, prevalence of vaccine uptake, or factors associated with mpox vaccine acceptance or uptake.
We excluded studies that: (1) were available only as abstracts or preprints; (2) evaluated conditional acceptance only (e.g. willingness to pay for vaccination); (3) did not report on any of our primary outcomes of interest; (4) used only continuous variables to measure the outcome of interest without reporting the exact prevalence of acceptance, intention or uptake; and (5) involved only clinical trial of the mpox vaccine among participants without reporting the prevalence of vaccine acceptance, intention or uptake; (6) was conducted before the 2022 global outbreak.
Study selection and eligibility
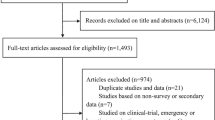
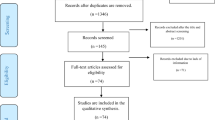
The initial literature search identified a total of 748 studies. After the removal of duplicate studies, a total of 309 articles were further screened independently using the Rayyan QCRI (Qatar Computing Research Institute)24, based on title, then abstracts, and subsequently full-text by two investigators (SKS and MSM). All discrepancies were referred to and resolved by two senior authors (FIT and ATB). Following the screening process, 142 articles were found eligible, and following the application of our exclusion criteria, a total of 39 studies were included in the first stage of our literature search. (Fig. 1). We updated our literature search to include studies published by 25th November 2023. We, in addition, searched the Regional Office for Africa Library, the African Index Medicus, and the WHO Institutional Repository for Information Sharing to identify relevant studies. We also employed direct manual search and forward and backward citation tracking to retrieve studies. These cumulative searches yielded a total of 1783 studies from which we excluded 1,658 studies for duplication and other eligibility reasons. The full texts of the remaining 125 studies were retrieved and assessed for eligibility. Among these 125 studies, we excluded 2 preprint articles, 9 review articles, 39 studies already included in our initial literature search, and 74 studies that did not meet other inclusion/exclusion criteria. Consequently, our updated search identified 22 additional studies eligible for inclusion. Thus, a total of 61 studies were included in this review.
From a total of 748 studies retrieved from multiple databases during the initial literature search between the 15th and 25th of February 2023, we removed 439 studies due to duplication and many other reasons. We then screened 309 studies based on their titles and abstracts and excluded 167 studies for not reporting on any of the outcomes of interest. The remaining 142 studies were sought for retrieval and assessed for full-text eligibility, Subsequently, we removed: 5 review articles, 2 studies qualified by outcome measure but conducted before 2022 (on the basis of peer review process recommendation), and 96 studies for not reporting on any of the outcomes of interest and not meeting our other inclusion criteria. Therefore, a total of 39 studies having 40 reports were included in our initial search. At the request of the editorial and peer review process, we extended our literature search to studies published by 25th November 2023, repeated the search in the select databases, manual search and employed forward and backward citation tracking, generating 1783 studies. From this (1783), we excluded 1658 studies for duplication and other eligibility reasons and sought the remaining 125 for retrieval. From this (125), we excluded: 2 studies for being preprints, 9 studies for being reviews, 39 studies for being the initially included studies, and 74 studies for not meeting the inclusion criteria thereby making the remaining 22 studies from the update to qualify for inclusion. Therefore, a total of 61 studies (39 from initial and 22 from final literature searches, respectively) were finally included. We only included studies if they: (1) are original full-text publications; (2) are not reporting a conditional acceptance; (3) reported any of our outcomes of interest (acceptance, intention, uptake, and/or associated factors), (4) did not solely use a continuous variable to measure the outcome of interest; (5) were not clinical trials only; (6) were not conducted before 2022. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
Study outcomes
The primary outcomes were: 1. Intention, defined as the unconditional willingness to receive free mpox vaccine; 2. Uptake, defined as the actual receipt of one or more of any of the vaccines approved for mpox prevention and/or treatment during the 2022 outbreak; 3. Acceptance, which comprises both the intention to receive the mpox vaccine and the actual uptake of the vaccine, in line with previous studies25. Thus, in studies that only reported intention to accept the mpox vaccine among unvaccinated people, the prevalence of acceptance was defined as the proportion of individuals intending to be vaccinated among the entire cohort. However, for studies that reported both vaccine uptake and intention to vaccinate, the prevalence of acceptance was calculated as the proportion of those already vaccinated and those willing to be vaccinated among the study cohort.
We performed stratified analyses of the intention, uptake, and acceptance rate of the mpox vaccine for the overall populations and across the various population subgroups (such as the LGBTQI+ community, PLHIV, healthcare workers, and the general public) according to WHO global regions. We also evaluated the prevalence of vaccine uptake among people who indicated acceptance. Furthermore, we assessed the prevalence of vaccine intention and acceptance among PLHIV, as well as the factors associated with mpox vaccine acceptance, intention, and/or uptake.
Data extraction
The Zotero software (version 6.0.15) was used for the retrieval of references as well as the removal of duplicate articles generated from the literature search. Thereafter, one investigator (SKS) used the Joanna Briggs Institute (JBI)26 data extraction form to extract relevant data from the retrieved articles. The data extracted were rechecked by two other investigators (MSM and BTM). The information extracted from the included articles comprised the first author’s name; publication year; study title; country/countries of study; study setting; study design sampling method; means of study administration; study period; publication status; sample size; the number of male participants; the mean/median age of the participants; the number of participants who indicated an intention to accept the mpox vaccine, actual uptake, and/or acceptance; the number of PLHIV participating in the study, along with the number of PLHIV indicating their intention to accept the vaccine; and the factors independently associated with vaccine acceptability and/or uptake. The data extracted and used for this work is available at https://doi.org/10.17605/OSF.IO/FS5QH.
Critical appraisal (quality assessment) of included studies
Two investigators (SKS and MSM) independently reviewed all articles to critically appraise their methodological rigor using an adapted version of the Newcastle Ottawa Scale (NOS)27 for cross-sectional studies, and the NOS for cohort studies28. The scale consists of seven items divided into three (3) major domains; 1) Selection, having a total of four items and a maximum score of five; 2) Comparability, having only one item and a maximum score of two; and 3) Outcome, having two items and a maximum score of three. Accordingly, a study is rated as having low (1–4), moderate (5–7), or high (8–9) quality of evidence. The scores of the two investigators were compared and reviewed by two senior authors (FIT and ATB), and where disputes occurred, a final consensus score was decided by the senior authors through revision and discussion of the articles. (Supplementary Data 2 and Supplementary Table 1).
Statistical analysis
The meta-analysis was performed using the metaprop command in Stata Version 15IC (StataCorp, College Station, Texas USA)29. Inverse variance weights were used to estimate the pooled prevalence rates of the mpox vaccine intention, uptake, and acceptance from the studies. Forest plots were used to present the results of the respective pooled proportions. The I2 measure was used to assess the percentage of total heterogeneity (variation) across the studies. Accordingly, heterogeneity was categorized as low (0–25%), moderate (26–75%), and substantial (76% to 100%)30. Because the studies were highly heterogeneous, only the random effects model was used to illustrate the pooled proportions of the mpox vaccine intention, uptake, and acceptance, as recommended31.
Stratified analyses were performed based on region and study population. To prevent the exclusion of some studies having proportions that are close to or equal to 1, the Freeman-Tukey double arcsine transformation was used32,33,34. The pooled proportions and weighted mean differences with their 95% confidence intervals (CI) were reported, and a p-value of <0.05 was considered significant.
To compute the prevalence of intention, we divided the number of those who were willing to be vaccinated by the total number of unvaccinated participants in the study and multiplied the proportion by a hundred. Furthermore, we computed the prevalence of vaccine uptake as the number of vaccinated participants divided by the total study participants multiplied by a hundred. The prevalence of acceptance was calculated as the number of participants in the acceptance group (intention + uptake) divided by the total sample size of the study multiplied by a hundred. To derive the prevalence of uptake among participants that indicated acceptance, we divided the number of vaccinated participants by the total number of participants who indicated acceptance of the vaccine and multiplied that by a hundred.
To evaluate a potential effect of each included study on the prevalence estimates, we performed a series of leave-one-out sensitivity analyses among the overall study population and across population subgroups. This analysis involved the iterative removal of a single study to report the pooled estimated prevalence rates without the excluded study. This process was repeated until all studies were individually excluded.
To evaluate potential publication bias among the included studies, we used funnel plots35, and Egger’s test36, with a P-value > 0.05 indicating no statistically significant evidence of publication bias. We also evaluated publication bias by assessing for asymmetry in the Doi plot, a plot of the normal-quantile versus effect size using the LFK index37,38. An LFK index beyond ±1 was deemed to be consistent with Doi plot asymmetry. Where Doi plot asymmetry is observed, we sequentially removed (trimmed) studies potentially causing the asymmetry until Doi plot symmetry is achieved, borrowing from Duval and Tweedie’s (2000) Trim and Fill method. We then compared the outcome prevalence before and after trimming of studies to assess the effect of the studies causing Doi plot asymmetry on the prevalence estimates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Characteristics of the studies
The present systematic review included 61 peer-reviewed studies with a cumulative sample size of 263,857. The prevalence of intention to vaccinate was not reported by nine studies39,40,41,42,43,44,45,46,47, uptake rates were reported by seventeen studies39,40,41,42,44,45,46,47,48,49,50,51,52,53,54,55,56, rates of uptake among those who indicated acceptance were reported by eight studies48,50,51,52,53,54,55,56, and mpox acceptance rate was reported by all included studies. Overall, all six WHO regions were represented, with AFR (Algeria, Ghana, Nigeria) having four studies39,57,58,59, AMR having eleven studies40,42,45,47,54,55,60,61,62,63,64, EMR (Iraq, Pakistan, Saudi Arabia) having eight studies65,66,67,68,69,70,71,72, EUR (all WHO European member countries) having seventeen studies41,44,48,51,52,56,73,74,75,76,77,78,79,80,81,82,83, SEAR (Indonesia, Bangladesh, Japan) three studies84,85,86, and the WPR (China, Malaysia, Australia) having twelve studies49,50,53,87,88,89,90,91,92,93,94,95. The acceptance rate among PLHIV was reported by fourteen studies41,47,49,50,52,53,54,63,74,78,79,87,91,96, the rate of intention to be vaccinated was reported by nine50,52,63,74,78,79,87,91,96, while uptake rate was reported by eight studies41,47,49,50,52,53,54,96. Among the LGBTQI+ community, the acceptance rate was reported by twenty-one studies42,45,47,49,50,51,52,53,55,56,62,63,74,78,79,80,87,89,91,93,95, rate of intention to vaccinate was reported by seventeen stuides50,51,52,53,55,56,62,63,74,78,79,80,87,89,91,93,95, while rate of uptake was reported by studies42,45,47,49,50,51,52,53,55,56. The rate of intention to vaccinate among healthcare workers was reported by fifteen studies57,59,61,66,69,72,73,75,82,83,84,92,94,97. Among the general public, the acceptance rate was reported by nineteen studies39,40,41,44,48,51,58,60,64,65,67,68,71,81,85,86,88,93,98, rate of intention to vaccinate was reported by fifteen stuides48,51,59,60,64,65,67,68,71,81,85,86,88,93,98, while rate of uptake was reported by six studies39,40,41,44,48,51. The rate of intention to vaccinate among university students was reported by three studies70,89,99.
Among all included studies, two were multiregional, having involved participants from more than one WHO region97,98. Apart from these two multiregional studies. All other studies were single-country studies, with US having the highest number of studies (nine)40,42,43,45,47,54,60,61,96 followed by the China (eight)49,50,51,65,67,75,89 and Saudi Arabia (four)65,66,67,69. In all, a total of 87 countries were represented in this review. All studies were conducted in 2022 and used non-probability sampling techniques for participant selection. Except for four studies that were utilized prospective cohort design41,43,44,46, all studies were cross-sectional. Also, five studies used electronic record for data collection40,41,43,46,49, while all the other studies were online surveys. The largest sample size per study was 119,34543, while the smallest was 7584. The study-level determinants of the mpox vaccine acceptance were reported in 42 out of the 60 studies included in this review (Supplementary Data 3).
Due to a substantial overlap of participants in the study by Zucker et al. (N = 2025 participants)46 with that of Sagy et al. (N = 2054 participants)41, we used only one of the studies (Sagy et al.) for quantitative synthesis. The study by Salih et al.43 did not provided total number of uptake and n0n-uptake and was also not included for quantitative synthesis. Therefore, bringing the total number of studies eligible for meta-analysis to 59.
Risk of publication bias
Among the studies reporting overall acceptance of the mpox vaccine (n = 59), visual inspection of Begg’s funnel plot showed symmetry, and Egger’s test similarly did not demonstrate evidence of publication bias (p = 0.856) (Supplementary Fig. 1). No evidence of publication bias was demonstrated among studies (n = 51) reporting overall intention to vaccinate against mpox by both Begg’s test and Egger’s test (p = 0.993) (Supplementary Fig. 2). For studies reporting overall uptake rates of the mpox vaccine (n = 17), evidence of publication bias was demonstrated by Begg’s test and Egger’s test (p = 0.022) (Supplementary Fig. 3). However, we did not found evidence of publication bias among studies (n = 9) reporting rates of mpox vaccine uptake among people who indicated acceptance using both Begg’s test and Egger’s test (p = 0.156) (Supplementary Fig. 4).
The Begg’s test and Egger’s test (p = 0.168) performed to check for the evidence of publication bias for studies (n = 14) reporting acceptance rate in PLHIV did not show evidence suggesting publication bias (p = 0.168) (Supplementary Fig 5). Similarly, no evidence suggesting publication bias was found according to the findings of our Begg’s test and Egger’s test (p = 0.215) for studies (n = 9) that reported the rate of intention to vaccinate among PLHIV (Supplementary Fig 6). Also, our assessment did not reveal evidence to suggest publication bias for the 9 studies reporting the rates of uptake among PLHIV by both Begg’s test and Egger’s test (p = 0.165) (Supplementary Fig 7).
Assessment of publication bias using Begg’s test and Egger’s test (p = 0.060) for the 21 studies reporting rate of acceptance among the LGBTQI+ community similarly revealed no evidence (Supplementary Fig. 8). There is also an absence of publication bias evidence among studies (n = 17) reporting the rate of intention to vaccinate against the mpox among the LGBTQI+ community based on the findings of our Begg’s test and Egger’s test (p = 0.202) (Supplementary Fig. 9). Our analysis of the 10 studies reporting uptake rates of the mpox vaccine among the LGBTQI+ community using the Begg’s test and Egger’s test (p = 0.081) did not show evidence of publication bias (Supplementary Fig. 10).
Publication bias was not observed among the 15 studies reporting the rate of intention to vaccinate against the mpox among healthcare workers from Begg’s test and Egger’s test (p = 0.443) (Supplementary Fig. 11). For the studies (n = 19) reporting acceptance rates among the general public, evidence of publication bias was observed by both Begg’s test and Egger’s test (p = 0.001) (Supplementary Fig. 12). Evidence of the presence of publication bias was also observed by both Begg’s test and Egger’s test (p = 0.003) among the 15 studies reporting rates of intention to vaccinate among the general public (Supplementary Fig. 13). However, no evidence of publication bias was observed among studies (n = 6) reporting the rate of intention to vaccinate against the mpox among the general public from the Begg’s test as well as Egger’s test (p = 0.473) (Supplementary Fig. 14).
Furthermore, Doi plot asymmetry was observed in the meta-analysis of studies reporting overall global prevalence of acceptance, uptake, and intention (Supplementary Fig 15–28). However, after trimming of studies potentially causing the Doi plot asymmetry, the prevalence estimates for acceptance, uptake, and intention only changed by 0.2, 1.3, and 1.3, respectively (Supplementary Table 4). Conversely, Doi plot asymmetry was not observed in the meta-analysis of studies reporting vaccine uptake among those who indicated their willingness to receive the vaccine. The Doi plots and LFK index, as well as the sensitivity analysis, for the stratified analysis among various population subgroups are provided in Supplementary Fig 15–28 and Supplementary Table 2.
Global and regional prevalence of mpox vaccine acceptance across population groups
The overall global prevalence of mpox vaccine acceptance, pooled from fifty-nine studies (n = 142,487 participants) included in the meta-analysis was 59.7% (95% CI, 51.1–68.1%) (Supplementary Data 4). Stratified by the WHO region, the SEAR had the highest acceptance rate at 72.2% (95% CI, 60.7–82.4%), followed by the WPR at 67.3% (95% CI, 5.7–100%), then EUR at 63.8% (95% CI, 54.6–72.6%), then EMR at 52.0% (95% CI, 44.2–59.8%), then AMR at 48.9% (95% CI, 24.9–73.2%), and AFR at 41.9% (95% CI, 38.5-45.3%) (Supplementary Data 4 and Fig. 2a). The pooled global acceptance rate among multiregional studies was 60.2% (95% CI, 41.1–77.8%) (Supplementary Data 4).
a Population-wide (overall) acceptance rates of the mpox vaccine across all six WHO global regions. b Acceptance rates of the mpox vaccine among PLHIV according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). c Acceptance rates of the mpox vaccine among the LGBTQI+ community according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). d Acceptance rates of the mpox vaccine among the general public according to all six WHO global regions. Darker areas indicate higher rates, and lighter areas indicate lower rates. Maps adapted from OpenStreetMap under a Creative Commons licence CC BY-SA 2.0.
Based on data pooled from fourteen studies (n = 7593 participants), the global prevalence of mpox vaccine acceptance in PLHIV was 66.4% (95% CI, 51.4–79.9%) (Supplementary Data 4). According to the WHO region, the rate of mpox vaccine acceptance was 69.5% (95% CI, 45.3–89.1%) among PLHIV in the WPR, 66.4% (95% CI, 51.4–79.9%) among PLHIV in the AMR, and 65.3% (95% CI, 34.9–89.8%) among PLHIV in EUR (Supplementary Data 4 and Fig. 2b).
The prevalence of mpox vaccine acceptance based on data pooled from twenty-one studies conducted among 63,538 LGBTQI+ community members was 73.6% (95% CI, 67.2–79.6%) globally (Supplementary Data 4). This acceptance rate varied according to the WHO region, with the highest rate being among the LGBTQI+ community in the EUR at 80.9% (95% CI, 75.1–86.0%), followed by the WPR at 75.2% (95% CI, 60.2–87.6%), and then the AMR at 60.9% (95% CI, 35.2–83.8%) (Supplementary Data 4 and Fig. 2c).
The estimated global prevalence of mpox vaccine acceptance pooled from nineteen studies in the general public (n = 56,518 participants) was 50.9% (95% CI, 39.2–62.5%) (Supplementary Data 4). Stratified by the WHO region, the acceptance rate in the general public was 70.3% (95% CI, 68.6–72.0%) in the WPR, 56.5% (95% CI, 39.9–72.4%) in the EUR, 51.9% (95% CI, 46.0–57.8%) in the EMR, 43.3% (95% CI, 40.7–45.9%) in the AFR, 24.1% (95% CI, 7.9–45.7%) in the AMR, and 19.3% (95% CI, 18.8–19.7%) in the SEAR (Supplementary Data 4 and Fig. 2d). The pooled acceptance rate in the general public from a multiregional study was 48.9% (95% CI, 47.3-50.5%) (Supplementary Data 4).
Global and regional prevalence of intention to vaccinate against mpox across population groups
The overall global rate of intention to vaccinate against mpox, pooled from fifty-one studies (n = 127,359 participants) was 60.9% (95% CI, 52.1–69.3%) (Supplementary Data 4). There was high variation in this intention rates across the six WHO regions, with the rate of intention to vaccinate being highest in the WPR at 73.5% (95% CI, 63.0–82.9%), followed by the SEAR at 67.3% (95% CI,5.7–100%), then AMR at 59.5% (95% CI, 37.9–79.4%), then EUR at 59.3% (95% CI, 49.3–69.0%), then the EMR at 52.0% (95% CI, 44.2–59.8%) and AFR at 41.9% (95% CI, 36.6–47.4%) (Supplementary Data 4 and Fig. 3a). The pooled overall global rate of intention to vaccinate against mpox from multiregional studies was 60.2% (95% CI, 41.1–77.8%) (Supplementary Data 4).
a Population-wide (overall) rates of intention to vaccinate against mpox across all six WHO global regions. b Rates of intention to vaccinate against mpox among PLHIV according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). c Rates of intention to vaccinate against mpox among the LGBTQI+ community according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). d Rates of intention to vaccinate against mpox among healthcare workers according to all six WHO global regions. e Rates of intention to vaccinate against mpox among the general public according to all six WHO global regions. Darker areas indicate higher rates, and lighter areas indicate lower rates. Maps adapted from OpenStreetMap under a Creative Commons licence CC BY-SA 2.0.
Data pooled from nine studies in 6784 PLHIV puts the estimated the global prevalence of intention to vaccinate against mpox in this group at 75.0% (95% CI, 61.7–86.3%) (Supplementary Data 4). The rate of intention to vaccinate was 79.5% (95% CI, 50.2–97.7%) among PLHIV in the WPR, 74.8% (95% CI, 69.3–80.0%) among PLHIV in the AMR, and 71.7% (95% CI, 46.0–91.5%) among PLHIV in the EUR (Supplementary Data 4 and Fig. 3b).
Among the LGBTQI+ community, the estimated global prevalence of intention to vaccinate against mpox (pooled from seventeen studies in 61,118 participants) was 77.1% (95% CI, 72.3–81.5%) (Supplementary Data 4). According to the WHO region, the prevalence rate of intention to vaccinate was highest among the LGBTQI+ community in the AMR at 82.4% (95% CI, 76.0–88.1%), followed by the WPR at 78.0% (95% CI, 66.5–87.7%), and then the EUR at 74.2% (95% CI, 67.5–80.3%) (Supplementary Data 4 and Fig. 3c).
The global prevalence of intention to vaccinate against mpox pooled using data from fifteen studies in 9,064 healthcare workers was 51.0% (95% CI, 39.7–62.3 (Supplementary Data 4). Wide variations existed in the rates of healthcare worker intention to vaccinate against mpox across all six WHO region, with the highest rate of intention to vaccinate being 81.9% (95 CI, 80.0–83.7%) among healthcare workers in the WPR, then 77.3% (95% CI, 667–853.5%) in the SEAR, 49.7% (95% CI, 42.8–56.7%) in the AMR, 46.5% (95% CI, 28.9–64.6%) in the EMR, 40.8% (95% CI, 19.6–63.9%) in the EUR, and 39.0% (95% CI, 34.7–43.3%) in the AFR (Supplementary Data 4 and Fig. 3d). The pooled rate of intention to vaccinate among healthcare workers from a multiregional study was 54.5% (95% CI, 52.9–56.1%) (Supplementary Data 4).
Based in data available form fifteen studies (n = 43,810 participants), the estimated global prevalence of intention to vaccinate against mpox in the general public was 52. 3% (95% CI, 38.1–66.4%) (Supplementary Data 4). However, the results showed high variation according to the WHO region, with a highest rate the rate of 70.3% (95% CI, 68.6–72.0%) in the WPR, 51.9% (95% CI, 46.0–57.8%) in the EMR, 50.0% (95% CI, 25.3–74.8%) in EUR, 46.1% (95% CI, 42.2–50.1%) in the AFR, 33.9% (95% CI, 31.4–36.4%) in the AMR, and 19.3% (95% CI, 18.8–19.7%) in the SEAR (Supplementary Data 4 and Fig. 3e). The pooled rate of intention to vaccinate from a multiregional study was 48.9% (95% CI, 47.3–50.5%) (Supplementary Data 4). Furthermore, the global prevalence of intention to vaccinate against mpox among university students, pooled from three studies (n = 13,094 participants), was 59.4% (95% CI, 41.8–75.9%) (Supplementary Data 4).
Global and regional prevalence of mpox uptake across population groups
Overall, the global prevalence of mpox vaccine uptake, pooled from seventeen studies (n = 26,186 participants), was 30.9% (95% CI, 21.0–41.7%) (Supplementary Data 4). Uptake rates varied by WHO region: 36.9% (95% CI, 15.4–61.6%) in EUR, 33.5% (95% CI, 21.9–46.3%) in the WPR, 28.3% (95% CI, 15.9–42.7%) in AMR, and 5.0% (95% CI:3.7–6.7%) in AFR (Supplementary Data 4 and Fig. 4a).
a Population-wide (overall) rates of uptake of the mpox vaccine across all six WHO global regions. b Rates of uptake of the mpox vaccine among the accepting group according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). c Rates of uptake of the mpox vaccine among PLHIV according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). d Rates of uptake of the mpox vaccine among the LGBTQI+ community according to WHO global regions (using data available for only three regions: AMR, EUR, and WPR). e Rates of uptake of the mpox vaccine among the general public according to WHO global regions (using data available for only three regions: AFR, AMR, and EUR). Darker areas indicate higher rates, and lighter areas indicate lower rates. Maps adapted from OpenStreetMap under a Creative Commons licence CC BY-SA 2.0.
The pooled global prevalence of mpox vaccine uptake among those who indicated their intention to receive the vaccine (uptake rate among the accepting group) based on data from nine studies involving 11,058 participants was 36.1% (95% CI, 19.9-54.1%) (Supplementary Data 4). Based on the WHO region, the uptake rate among the accepting group was 46.8% (95% CI, 42.1–51.5%) in the AMR, 33.7% (95% CI, 9.7–63.4%) in EUR, and 30.3% (95% CI, 28.4–32.1%) in the WPR (Supplementary Data 4 and Fig. 4b).
Globally, the prevalence of mpox vaccine uptake pooled from eight studies involving 1,933 PLHIV was 35.7% (95% CI, 27.3–44.6%) (Supplementary Data 4). PLHIV living in the WHO WPR region had the highest uptake rate at 46.6% (95% CI, 41.0–52.2%), followed by the PLHIV in the AMR at 33.9% (95% CI, 17.1–53.0%), and then EUR at 27.0% (95% CI, 24.7–29.4%) (Supplementary Data 4 and Fig. 4c).
The global prevalence of mpox vaccine uptake in the LGBTQI+ community pooled from ten studies (N = 8803 participants) was 39.8% (95% CI, 30.7–49.3%) (Supplementary Data 4). Stratified by the WHO region, the rate of uptake was highest in the EUR at 80.50% (95% CI, 24.4–75.6%), followed by the AMR at 37.1% (95% CI, 22.6–53.0%), and then the WPR at 33.5% (95% CI, 21.9–46.3%) (Supplementary Data 4 and Fig. 4d).
Among the general public, the estimated global prevalence of mpox vaccine uptake pooled from six studies involving 17,110 participants was 20.2% (95% CI, 6.9–38.3%) (Supplementary Data 4). The rate of uptake was 27.6% (95% CI, 3.1–64.0%) in the EUR, 12.8% (95% CI, 12.2–13.5%) in the AMR, and 5.0% (95% CI, 3.7–6.7%) in the AFR (Supplementary Data 4 and Fig. 4e).
The results of all the meta-analysis performed for the prevalence of mpox vaccine acceptance, intention, and uptake across all the population groups have been provided in the Supplementary Information (Supplementary Fig. 29–57).
Sensitivity analysis
The overall population-wide pooled prevalence of mpox vaccine acceptance was not influenced by a single study according to our leave-one-out sensitivity analysis with the pooled estimates varying between 59.0% (95% CI, 53.0–65.0%; p = 0.000) and 61.0% (95% CI: 54.0–67.0%; p = 0.000) (Supplementary Fig. 58). Similarly, we found no evidence of an overriding influence of a single study on the pooled acceptance rates in our leave-one-out sensitivity analysis among PLHIV (64.0% (95% CI, 50.0–77.0%; p = 0.000) to 70.0% (95% CI, 58.0–81.0%; p = 0.000)) (Supplementary Fig. 59), LGBTQI+ community (72.0% [95% CI, 62.0–81.0%; p = 0.000] to 76.0% (95% CI, 67.0–84.0%; p = 0.000)) (Supplementary Fig. 60), and the general public (48.0% (95% CI, 38.0–57.0%; p = 0.000) to 53.0% (95% CI, 42.0–64.0%)) (Supplementary Fig. 61).
For the intention to vaccinate outcome, our leave-one-out sensitivity analysis showed that no single study had an overriding influence on the overall pooled prevalence of intention to vaccinate, with the pooled estimates varying between 60.0% (95% CI, 54.0–66.0%; p = 0.000) and 62.0% (95% CI: 55.0–68.0%; p = 0.000) (Supplementary Fig. 62). Similarly, we found no evidence of an overriding influence of a single study on the pooled intention rates in our leave-one-out sensitivity analysis for PLHIV (72.0% (95% CI, 59.0–84.0%; p = 0.000) to 78.0% (95% CI, 66.0–88.0%; p = 0.000)) (Supplementary Fig. 63), LGBTQI+ community (75.0% (95% CI, 68.0–82.0%; p = 0.000) to 79.0% (95% CI, 72.0–84.0%; p = 0.000)) (Supplementary Fig. 64), healthcare workers (48.0% (95% CI, 38.0–58.0%; p = 0.000) to 55.0% (95% CI, 45.0–64.0%; p = 0.000)) (Supplementary Fig. 65), and the general public (48.0% (95% CI, 39.0–58.0%; p = 0.000) to 55.0% (95% CI, 43.0–66.0%; p = 0.000)) (Supplementary Fig. 66). However, the leave-one-out analysis found evidence of an overriding influence of the multiregional study by Abd Elhafeez et al.99 and the Malasian study by Lin et al.90 (50.0% (95% CI, 118.0–83.0%; p = 0.000) vs. 72.0% (95% CI, 64.0–80.0%; p = 0.000)) on the pooled rate of intention to vaccinate (59.4; 95% CI, 41.8–75.9%) among university students (Supplementary Fig. 67).
The leave-one-out sensitivity analysis for our population-wide mpox vaccine uptake outcome revealed that estimated pooled prevalence of uptake was not influenced by omission of any of the included studies, with the pooled estimates varying between 28.0% (95% CI, 17.0–40.0%; p = 0.000) and 34.0% (95% CI: 22.0–46.0%; p = 0.000) (Supplementary Fig. 68). Similarly, we found no evidence of an overriding influence of any study on the pooled mpox vaccine uptake rates in our leave-one-out sensitivity analysis among PLHIV (34.0% (95% CI, 25.0–43.0%; p = 0.000) to 39.0% (95% CI, 29.0–49.0%; p = 0.000)) (Supplementary Fig. 69), and the LGBTQI+ community (35.0% (95% CI, 28.0–42.0%; p = 0.000) to 42.0% (95% CI, 30.0–54.0%; p = 0.000)) (Supplementary Fig. 70). However, the leave-one-out sensitivity analysis revealed evidence of an overriding influence of the study by Ewijk et al.44 and the study by Gallè et al.48 (11.0% (95% CI, 1.0–3.0%; p = 0.000) vs. 26.0% (95% CI, 4.0–59.0%) on the pooled prevalence of uptake (20.2%; 95% CI, 6.9–38.3%) among the general public (Supplementary Fig. 71). Similarly, we found evidence of an overriding influence of the study by Palich et al.56 and the study by Gallè et al.48 (29.0% (95% CI, 15.0–46.0%; p = 0.000) vs. 42.0% (95% CI, 24.0–62.0%; p = 0.000)) on the pooled prevalence of vaccine uptake (36.1%; 95% CI, 19.9–54.1) among the accepting group (Supplementary Fig. 72).
Factors associated with mpox vaccine acceptance and uptake
The most commonly reported correlates of the intention to accept the mpox vaccine and uptake include:
Age
Older age has been reported to be associated with higher intention to accept51,89,97, lower intention to accept65,93,94,98, and higher hesitancy95. Younger age was associated with higher uptake47,53,96
Sex
Being male is associated with a higher intention to accept58,60,97, and returning for a second dose43.
Level of education
Attaining a university-level degree has been reported to be associated with both lower65,87,93,95 and a higher92,95 likelihood of intention to accept. Similarly, having a below university-level degree has been reported to be associated with a lower likelihood of intention to accept among Chinese MSM91 and a higher likelihood of intention to accept according to a study among Chinese Healthcare workers92. Higher education level has also being reported to be associated with uptake of more than one dose45.
Income
Having a higher income has been reported to be associated with both higher80,97 and lower93 likelihood of intention to accept. Low income has been reported to be associated with lower uptake46.
Mpox-related concern
Concern about the mpox51,65,73,74,75,77,78,91,93,97,98, including being more worried about the mpox than COVID-1965,75,93,98, has been reported to be associated with a higher likelihood of intention to accept, while the belief that mpox is being overemphasized is associated with a lower likelihood of intention to accept51,60.
Perceived mpox susceptibility
The perception of being highly susceptible or at risk of mpox has been associated with a higher likelihood of intention to accept the mpox vaccine45,47,50,51,60,61,74,87,89,91,98.
Mpox-related knowledge
Having an above-average level of knowledge about mpox is associated with a higher likelihood of intention to accept60,75,87,88,92,93,98, and lower hesitancy95.
Mpox-related information
Receiving information about mpox from health authorities is associated with a higher likelihood of intention to accept vaccination65,75,93,97.
Mpox vaccine trust
Trusting the mpox vaccine to be safe is associated with higher acceptance51,60,63,72,91,94.
Vaccination history
Having a good vaccination history has been reported to be associated with higher intention to accept72 and actual uptake46. Previous vaccination against COVID-1957,60,73,86 and seasonal influenza82 were associated with a higher likelihood of intention to accept while the refusal of COVID-19 vaccination was associated with lower acceptance58.
Mpox vaccine mandate
Holding the opinion that mpox vaccination be made compulsory for high-risk groups is associated with high intention to accept78,94.
Sexual behavior
Having multiple sexual partners is associated with higher acceptance50,77,78,85,91, higher uptake49, and lower hesitancy79, while being bisexual is associated with lower acceptance50,80. On the other hand, engaging in chemsex74 and using condoms87 were all associated with a higher likelihood of intention to accept. Being MSM was associated with a higher likelihood of intention to accept80,86 and a higher uptake49.
HIV PrEP
Being on HIV PrEP is associated with a higher intention51,74 to accept as well as actual uptake41,49,53 of the mpox vaccine.
STI history
A recent diagnosis of STI within the previous two years has been reported to be associated with higher intention to accept the mpox vaccine74, and the actual uptake49,52,53.
HIV co-infection
Being HIV-infected has being reported to be associated with higher intention to accept among MSM residing in the EUR74, while a study among men attending a clinic in Israel41,46 reported HIV infection to be associated with a lower uptake.
Comorbidity
Having a chronic disease has been reported to be associated with a higher likelihood of intention to accept89,98, but with a lower likelihood of uptake41 of the mpox vaccine.
Discussion
This systematic review and meta-analysis evaluated the prevalence of mpox vaccine acceptance and uptake globally, regionally, and across key population subgroups. We also identified the factors associated with vaccine acceptance/uptake. Our findings revealed a suboptimal pooled overall global rate of the mpox vaccine acceptance rate (59.7%). This study also demonstrated substantial global and regional variations in the rates of mpox vaccine acceptance and uptake, overall and across key population groups (PLHIV, LGBTQI+ community, and healthcare workers), with the highest acceptance rate (73.6%) observed among the LGBTQI+ community. Our study also identified several modifiable behavioral factors associated with a higher likelihood of mpox vaccine acceptance, including being concerned about getting infected, the perception of being highly at risk, and knowledge about mpox, as well as receiving information about the disease from healthcare authorities
This systematic review and meta-analysis are, to the best of our knowledge, the largest study reporting the global prevalence of acceptance and uptake of the mpox vaccine, with representation from all WHO regions and almost all countries reporting a confirmed case of the disease. Also, to the best of our knowledge, this is the first meta-analysis to report the global prevalence of mpox vaccine uptake. The pooled overall global rate of the mpox vaccine acceptance rate from all the included studies was 59.7%, The overall pooled global rate of intention to vaccinate against mpox in this study (60.9%) falls within the range of 56.0% (11 studies)19 and 61.05% (29 studies)21 reported by previous meta-analyses. The overall global pooled uptake rate was 30.9%, with the LGBTQI+ community having a substantially higher uptake rate (39.8%) than the general public (20.2%). Among PLHIV, the pooled global acceptance and uptake rates were 66.4% (with substantial variations across the WHO regions) and 35.7%.
The findings of our meta-analysis revealed a remarkable gap between the overall global rate of intention to vaccinate against mpox (60.9%) and the overall global mpox vaccine uptake rate (30.9%). Moreover, further analyses of our data showed that only 36.6% of those who indicated their intention to receive the mpox vaccine actually received the vaccine. These findings indicate the existence of a major lag in vaccine uptake among those intending to vaccinate. Several factors, including variations in the timing of the studies and the availability of the vaccine across individual study settings, may have contributed to these observed gaps. Nonetheless, aggressive vaccination policies and strategies may be needed to bridge these gaps and ultimately meet the demands of the unvaccinated people who intend to vaccinate. The need for these policies is particularly crucial because unvaccinated individuals who intend to vaccinate remain at high risk of switching back to being uncertain or refusing to vaccinate100. Also, it is worth noting that the observed global overall prevalence of intention to vaccinate against mpox (60.9%) is substantially lower than the rate (95.3%)101 reported for the malaria vaccine, although it is relatively similar to the rates of intention to vaccinate against COVID-19 widely reported in previous studies (range: 60% − 65%)102,103,104,105. Similarly, the observed rates of the overall mpox vaccine uptake (30.9%) were relatively lower than the overall rates of the COVID-19 vaccine uptake (42.3%) reported in a previous large–scale meta-analysis25. The variations in rates of uptake and intention to vaccinate across these diseases may be explained by the differences in the relative prevalence, severity, and case fatality associated with the diseases.
Furthermore, our meta-analysis demonstrated that the global prevalence of mpox vaccine acceptance among PLHIV (66.4%) is comparable to that of the COVID-19 vaccine (67.0%)106. We also found that the global intention to vaccinate against mpox among PLHIV (75.0%) and the actual vaccine uptake (35.7%) were substantially higher than the observed pooled global overall rates of the mpox vaccine intention (60.9%) and uptake (30.9%). Furthermore, given that the prevalence of HIV coinfection among patients with mpox has been reported to be as high as 40%14,15,17,18, a high rate of intention to be vaccinated against mpox among PLHIV may substantially lower the overall prevalence of mpox if appropriate measures are put in place to improve vaccine uptake among PLHIV, particularly those who are already intending to be vaccinated.
The results of our meta-analysis also revealed considerable variations in the overall and population-specific rates of acceptance, intention, and uptake of the mpox vaccine across WHO regions. The overall acceptance rate for the WPR was 72.2%, the SEAR had 67.3%, EUR had 63.8%, the EMR had 52.0%, AMR had 48.9%, and AFR had 41.9%, while the rate of uptake was 46.8% among the accepting group for the AMR, 33.7% among those in EUR, and 30.3% among those in the WPR. The overall intention to vaccinate and the actual vaccine uptake were 73.5% and 33.5% for the WPR, 59,3% and 36.9% for EUR, 44.8% and 28.3% for AMR, and 41.9% and 5.0% in AFR. These findings have important implications. First, compared to the WPR and EUR, the WHO AFR had the lowest overall rate of intention and uptake despite being home to mpox-endemic countries, like Nigeria, which has been the source of most mpox outbreaks, including the 2022 outbreak that started in the UK107. Therefore, strong public health policies specific to mpox awareness and prevention are needed particularly in the WHO African region to prevent future outbreaks. Second, a relatively weaker health system in the WHO AFR may explain the endemicity of the disease and outbreaks in countries within the region. Therefore, as part of strengthening the global health system, building capacity for disease surveillance, emergency preparedness and response in the WHO AFR has been suggested as a potent means of substantially rolling back the spectrum of the mpox endemicity in the region108,109. Furthermore, vaccinating animals in settings with the confirmed animal-to-human transmission may be employed to successfully eradicate the disease108.
The higher rates of acceptance and uptake observed among the LGBTQI+ community (73.6% and 39.8%, respectively – relative to the population-wide average of 49% acceptance and 11% uptake rate – may indicate the group’s higher risk perception and better awareness compared to the general public. Among healthcare workers, who are also at a high risk of contracting mpox, the prevalence of intention to vaccinate against mpox (51.9%) is comparable to the acceptance rate of the COVID-19 vaccine (55.9%-65.7)104,105,110. However, the prevalence of intention to vaccinate among the general public, considered to be at a lower risk of mpox is substantially lower than the rate reported for the COVID-19 vaccine (52.3% for mpox vs. 61.0%-81.65% for COVID-19)104,105,110. These findings further illustrate the potential role of risk perception on vaccine acceptance. Importantly, despite the WHO’s recommendation of vaccination against mpox by the high-risk LGBTQI+ community111, only 36.1% of those intending to receive the vaccine had taken one or more doses of the vaccine against mpox either as PPV or PEPV. Although the proportion of uptake among those intending to be vaccinated is substantially higher for the LGBTQI+ community (39.8%) compared to the population-wide average of 30.9%, the high burden of mpox among the LGBTQI+ implies that vaccine uptake among this vulnerable group is still suboptimal. Therefore, further research is needed to develop strategies to improve vaccine uptake to meet the vaccination demands of unvaccinated LGBTQI+ community members who intend to get vaccinated.
Furthermore, our narrative synthesis highlighted important correlates of the acceptance of the mpox vaccine. Age, sex, level of education, and level of income are among the most reported sociodemographic determinants of mpox vaccine acceptance. Therefore, public health intervention programs aimed at enhancing positive community attitudes toward the mpox vaccination program need to consider these sociodemographic characteristics in order to maximize acceptance and uptake of the vaccine. Our review has shown that having a few sexual partners and being bisexual are associated with lower vaccine acceptance rate; therefore, it is vital that intervention programs take into account the sexual behaviors of the target population. Furthermore, our results showed that MSM who are not on HIV PrEP have lower acceptance of the mpox vaccination. This finding indicates the need for deliberate health education and awareness efforts aimed at addressing vaccination hesitancy in this high-risk group. Moreover, MSM who are not on HIV PrEP may be targeted for HIV testing during mpox vaccination and appropriately counseled for commencement of PrEP if found not positive for HIV, as recommended112.
Finally, this review also found some important modifiable behavioral factors associated with mpox vaccine acceptance, including concerns about the disease, the perception of being highly at risk, and knowledge about mpox, as well as the source of information about the disease. These findings indicate the critical need for behavioral interventions to increase knowledge and clear misperceptions related to mpox in the community, especially among high-risk groups such as the LGBTQI+ community. Accordingly, these interventions need to incorporate measures that favor public receipt of mpox-related information from reliable sources like health institutions and/or professionals. This consideration is particularly important given that the current 2022 mpox outbreak occurred during the COVID-19 pandemic era, a period that has been characterized by unprecedented levels of vaccine hesitancy, mostly fueled by a phenomenon termed “infodemic”, defined by the WHO as “too much information, including false or misleading information in digital and physical environments during a disease outbreak”113 As evidence has strongly linked infodemic to vaccine hesitancy100,114,115, efforts to maximize the mpox vaccine acceptance also need to include measures to combat misinformation-induced hesitancy, especially the misinformation spread via online media. Active engagement of public health professionals and institutions in online media campaigns has been recommended as one of the potent ways of addressing this growing problem116. Also, since an individual’s level of trust in the mpox vaccine and previous vaccination against COVID-19 and influenza history are also strong correlates of mpox vaccine acceptance, efforts are needed to accelerate public trust in vaccines, and emphasis should be given to those individuals with poor vaccination history. Also, because trust in vaccines is a highly delicate topic, caution must be undertaken before enacting mandatory vaccine policies, as these policies may fester hesitancy, further spawn the growing anti-vaccine activism, and potentially expel some individuals who were previously intent on getting vaccinated. Of note, we did not find a single study conducted in the WHO AFR that reported the specific prevalence of vaccine intention, acceptance, or uptake among the LGBTQI + , even though this population has been numerously identified as a high-risk group and mpox is known to be endemic in many countries in the region. Therefore, future studies from this region should focus on evaluating the prevalence of and factors associated with mpox vaccine intention, uptake, and acceptance among the LGBTQI+ communities.
Among the key strengths of this review is a literature search involving multiple databases, which provided a high number of included studies (sixty-one) having a cumulative sample size of 263,857 from 87 countries across all six WHO regions. Second, our critical appraisal showed that the majority of the included studies have high methodological rigor. Third, this study is the first meta-analysis to evaluate the gap between acceptance and uptake, by evaluating the rate of vaccine uptake among those who indicated vaccine acceptance. The limitations of this review are mostly related to the included studies, including the utilization of online surveys by a vast majority of the included studies, which may introduce participant recruitment bias, and exclude people with no/or limited access to the internet. Second, there is high degree of statistical heterogeneity in the overall analysis, which remained present when we disaggregated the data for each of the population groups and performed a subgroup analysis by region. However, we have performed and reported the results of our leave-one-out sensitivity analysis for each of the three major outcomes across all population groups. Third, nearly all of the studies employed a non-probability sampling technique, which may be associated with selection bias. Fourth, due to limited availability of funds for cross-language translations, only studies published in the English language were considered, thereby potentially limiting the generalizability of our pooled estimates. Fifth, some population regions, like the SEAR have only three studies, further limiting the generalizability of the sub-group analyses by WHO regions.
Conclusion
This review demonstrated the existence of substantial regional variations in the rates of mpox vaccine acceptance and uptake, as well as the presence of a wide gap between the rate of vaccine acceptance and vaccine uptake. Among the LGBTQI+ community, a group designated as a high-risk group for mpox, only about one-third of those who indicated vaccine acceptance actually received at least a single dose, and an even wider acceptance-uptake gap was reported for the general population. Targeted intervention programs to maximize mpox vaccine uptake, which account for the socio-demographic and other behavioral predictors of low mpox vaccine acceptance, particularly among high-risk groups are needed to reduce the overall global burden of mpox.
Data availability
All the source data supporting the results presented in this work is accessible at https://doi.org/10.17605/OSF.IO/FS5QH.
Code availability
All the code for the data analyzed in this work is openly available at Open Science Framework (via https://doi.org/10.17605/OSF.IO/FS5QH)117.
References
Andre, F. E. et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 86, 140–146 (2008).
Greenwood, B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. B: Biol. Sci. 369, 20130433 (2014).
Mpox (monkeypox) November 2023 (Accessed 26 December 2023). https://www.who.int/news-room/fact-sheets/detail/monkeypox.
Mpox (Monkeypox). https://www.ecdc.europa.eu/en/mpox-monkeypox (Accessed 19 Feb 2023).
Bergen, N. et al. Global state of education-related inequality in COVID-19 vaccine coverage, structural barriers, vaccine hesitancy, and vaccine refusal: findings from the Global COVID-19 Trends and Impact Survey. Lancet Glob Health https://doi.org/10.1016/S2214-109X(22)00520-4 (2022).
Wiegand, M. et al. Global declines in vaccine confidence from 2015 to 2022: a large-scale retrospective analysis. https://doi.org/10.2139/ssrn.4438003.
Eagan, R. L., Larson, H. J. & de Figueiredo, A. Recent trends in vaccine coverage and confidence: a cause for concern. Hum. Vaccines Immunother. 19, 2237374 (2023).
de Figueiredo, A., Temfack, E., Tajudeen, R. & Larson, H. J. Declining trends in vaccine confidence across sub-Saharan Africa: a large-scale cross-sectional modeling study. Hum. Vaccines Immunother. 19, 2213117 (2023).
MacDonald, N. E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 33, 4161–4164 (2015).
Vaccines and immunization for monkeypox: Interim guidance, 16 November 2022 (Accessed 18 Feb 2023). https://www.who.int/publications-detail-redirect/WHO-MPX-Immunization.
Monkeypox-WHO Fact Sheet (Accessed 18 Feb 2023). https://www.who.int/news-room/fact-sheets/detail/monkeypox.
CDC. Mpox and HIV. Centers for Disease Control and Prevention. (Accessed 18 Feb 2023). https://www.cdc.gov/poxvirus/monkeypox/prevention/hiv.html.
Ghazy, R. M. et al. Systematic review on the efficacy, effectiveness, safety, and immunogenicity of Monkeypox Vaccine. Vaccines 11, 1708 (2023).
Curran K. G. HIV and sexually transmitted infections among persons with Monkeypox—Eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 71 https://doi.org/10.15585/mmwr.mm7136a1 (2022).
Ortiz-Saavedra, B. et al. Epidemiologic situation of HIV and Monkeypox coinfection: a systematic review. Vaccines 11, 246 (2023).
Thornhill, J. P. et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 387, 679–691 (2022).
Mitjà, O. et al. Mpox in people with advanced HIV infection: a global case series. Lancet https://doi.org/10.1016/S0140-6736(23)00273-8 (2023).
Suñer, C. et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect. Dis. 23, 445–453 (2023).
Ulloque-Badaracco, J. R. et al. Acceptance towards Monkeypox vaccination: a systematic review and meta-analysis. Pathogens 11, 1248 (2022).
Lounis, M. & Riad, A. Monkeypox (MPOX)-related knowledge and vaccination hesitancy in non-endemic countries: concise literature review. Vaccines 11, 229 (2023).
León-Figueroa, D. A., Barboza, J. J., Valladares-Garrido, M. J., Sah, R. & Rodriguez-Morales, A. J. Prevalence of intentions to receive monkeypox vaccine. A systematic review and meta-analysis. https://www.researchsquare.com/article/rs-3387241/latest (Accessed 18 Nov 2023).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269 (2009).
Munn, Z., Stern, C., Aromataris, E., Lockwood, C. & Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 18, 5 (2018).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 210 (2016).
Wang, Q. et al. Mapping global acceptance and uptake of COVID-19 vaccination: a systematic review and meta-analysis. Commun. Med. 2, 1–10 (2022).
Munn, Z., Tufanaru, C. & Aromataris, E. JBI’s systematic reviews: data extraction and synthesis. AJN Am. J. Nurs. 114, 49–54 (2014).
Herzog, R. et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health 13, 154 (2013).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://api.semanticscholar.org/CorpusID:79550924 (2000).
Nyaga, V. N., Arbyn, M. & Aerts, M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health 72, 39 (2014).
Higgins, J. P. et al. Cochrane Handbook for Systematic Reviews of Interventions (John Wiley & Sons, 2019).
9.5.3 Strategies for addressing heterogeneity. https://handbook-5-1.cochrane.org/chapter_9/9_5_3_strategies_for_addressing_heterogeneity.htm (Accessed 17 Oct 2022).
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E. & Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 67, 974–978 (2013).
Lin, L. & Xu, C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Sci. Rep. 3, e178 (2020).
Freeman, M. F. & Tukey, J. W. Transformations related to the angular and the square root. Ann. Math. Stat. 21, 607–611 (1950).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Furuya-Kanamori, L., Barendregt, J. J. & Doi, S. A. R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc 16, 195–2023 (2018).
Shamim, M. A. Real-life implications of prevalence meta-analyses? Doi plots and prediction intervals are the answer. Lancet Microbe 4, e490 (2023).
Al-Mustapha, A. I. et al. A cross-sectional survey of public knowledge of the monkeypox disease in Nigeria. BMC Public Health 23, 591 (2023).
Payne, A. B. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons — 43 U.S. Jurisdictions, July 31–October 1, 2022. MMWR Morb. Mortal Wkly. Rep. 71. https://doi.org/10.15585/mmwr.mm7149a5 (2022).
Sagy Y. W. et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med. https://doi.org/10.1038/s41591-023-02229-3 (2023).
Owens, C. & Hubach R. D. Rural-urban differences in monkeypox behaviors and attitudes among men who have sex with men in the United States. J. Rural Health 39, 508–515.
Salih, T. Demographic disparities in mpox vaccination series completion, by route of vaccine administration—California, August 9, 2022–March 31, 2023. MMWR Morb. Mortal Wkly. Rep. 72 https://doi.org/10.15585/mmwr.mm7230a4 (2023).
van Ewijk, C. E. et al. Acceptance and timeliness of post-exposure vaccination against mpox in high-risk contacts, Amsterdam, the Netherlands, May–July 2022. Vaccine 41, 6952–6959 (2023).
Curtis, M. G. et al. Predictors of Mpox vaccine uptake among sexual and gender minority young adults living in Illinois: Unvaccinated vs. double vs. single dose vaccine recipients. Vaccine 41, 4002–4008 (2023).
Zucker R. et al. Examining the patterns of mpox vaccine uptake in a vulnerable population. Sex Transm. Dis. 50, 680–684.
Abara, W. E. et al. Characteristics of mpox vaccine recipients among a sample of men who have sex with men with presumed exposure to mpox. Sex Transm, Dis, 50, 458–461 (2023).
Gallè, F. et al. “Monkeypox: what do you know about that?” italian adults’ awareness of a new epidemic. Pathogens 11, 1285 (2022).
Chow E. P. F., Chen M. Y., Bradshaw C. S., Towns J. M., Fairley C. K. Accessing first doses of mpox vaccine made available in Victoria, Australia. Lancet Reg Health – West Pac. 31. https://doi.org/10.1016/j.lanwpc.2023.100712 (2023).
MacGibbon, J. et al. Mpox (monkeypox) knowledge, concern, willingness to change behaviour, and seek vaccination: results of a national cross-sectional survey. Sex Health 20, 403–410 (2023).
Smith, L. E. et al. Did mpox knowledge, attitudes and beliefs affect intended behaviour in the general population and men who are gay, bisexual and who have sex with men? An online cross-sectional survey in the UK. BMJ Open 13, e070882 (2023).
Svartstein, A. S. W. et al. Mpox incidence and vaccine uptake in men who have sex with men and are living with HIV in Denmark. Vaccines 11, 1167 (2023).
Chow E. P. F. et al. Mpox knowledge, vaccination and intention to reduce sexual risk practices among men who have sex with men and transgender people in response to the 2022 mpox outbreak: a cross-sectional study in Victoria, Australia. Sex Health. https://doi.org/10.1071/SH23075 (2023).
Filardo, T. D. et al. Mpox vaccine acceptability among people experiencing homelessness in San Francisco — October–November 2022. Vaccine 41, 5673–5677 (2023).
Gilbert, M. et al. Uptake of Mpox vaccination among transgender people and gay, bisexual and other men who have sex with men among sexually-transmitted infection clinic clients in Vancouver, British Columbia. Vaccine 41, 2485–2494 (2023).
Palich, R. et al. High uptake of vaccination against mpox in men who have sex with men (MSM) on HIV pre-exposure prophylaxis (PrEP) in Paris, France. Sex Transm Infect. https://doi.org/10.1136/sextrans-2023-055885 (2023).
Lounis, M., Bencherit, D. & Abdelhadi, S. Knowledge and awareness of Algerian healthcare workers about human monkeypox and their attitude toward its vaccination: An online cross-sectional survey. Vacunas https://doi.org/10.1016/j.vacun.2022.11.003 (2023).
Ghazy, R. M. et al. Monkeypox vaccine acceptance among Ghanaians: a call for action. Vaccines 11, 240 (2023).
Ghazy, R. M. et al. Psychological antecedents of healthcare workers towards monkeypox vaccination in Nigeria. Vaccines 10, 2151 (2022).
Winters, M., Malik, A. A. & Omer, S. B. Attitudes towards Monkeypox vaccination and predictors of vaccination intentions among the US general public. PLoS ONE 17, e0278622 (2022).
Bates, B. R. & Grijalva M. J. Knowledge, attitudes, and practices towards monkeypox during the 2022 outbreak: An online cross-sectional survey among clinicians in Ohio, USA. J Infect Public Health https://doi.org/10.1016/j.jiph.2022.11.004 (2022).
Torres, T. S. et al. Evaluation of Mpox knowledge, stigma, and willingness to vaccinate for mpox: cross-sectional web-based survey among sexual and gender minorities. JMIR Public Health Surveill 9, e46489 (2023).
Araoz-Salinas, J. M. et al. Perceptions and Intention to Get Vaccinated against Mpox among the LGBTIQ+ community during the 2022 outbreak: a cross-sectional study in Peru. Vaccines 11, 1008 (2023).
Caycho-Rodríguez, T. et al. Relationship between fear of monkeypox and intention to be vaccinated against Monkeypox in a Peruvian sample. The mediating role of conspiracy beliefs about Monkeypox. Eval. Health Prof. https://doi.org/10.1177/01632787231180195 (2023).
Temsah, M. H. et al. Monkeypox caused less worry than COVID-19 among the general population during the first month of the WHO Monkeypox alert: experience from Saudi Arabia. Travel Med. Infect. Dis. 49, 102426 (2022).
Alhasan, K. et al. Mpox perceptions and vaccine advocacy among the healthcare workers of solid organ transplant centers: a multicenter, cross-sectional survey in Saudi Arabia. Healthcare 11, 603 (2023).
Meo, S. A., Al-Khlaiwi, T., Aljofan, Z. F., Alanazi, A. I. & Meo, A. S. Public perceptions of the emerging human monkeypox disease and vaccination in riyadh, saudi arabia: a cross-sectional study. Vaccines 10, 1534 (2022).
Ahmed, S. K. et al. Knowledge, attitude and worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) Outbreak in 2022: an online cross-sectional study. Vaccines 11, 610 (2023).
Alarifi, A. M., Alshahrani, N. Z. & Sah, R. Are Saudi healthcare workers willing to receive the monkeypox virus Vaccine? Evidence from a descriptive-baseline survey. Trop. Med. Infect. Dis. 8, 396 (2023).
Kumar, N. et al. Monkeypox cross-sectional survey of knowledge, attitudes, practices, and willingness to vaccinate among university students in Pakistan. Vaccines 11, 97 (2023).
Jamaleddine, Y. et al. Knowledge and attitude towards monkeypox among the Lebanese population and their attitude towards vaccination. J. Prev. Med. Hyg. 64, E13–E26 (2023).
Mahameed, H. et al. Previous vaccination history and psychological factors as significant predictors of willingness to receive mpox vaccination and a favorable attitude towards compulsory vaccination. Vaccines 11, 897 (2023).
Gagneux-Brunon, A., Dauby, N., Launay, O. & Botelho-Nevers, E. Attitudes towards monkeypox vaccination among healthcare workers in France and Belgium: an element of complacency? J Hosp Infect 130, 144–145 (2022).
Reyes-Urueña, J. et al. High monkeypox vaccine acceptance among male users of smartphone-based online gay-dating apps in Europe, 30 July to 12 August 2022. Eurosurveillance 27, 2200757 (2022).
Sahin, T. K. et al. Knowledge and attitudes of Turkish physicians towards human Monkeypox disease and related vaccination: a cross-sectional study. Vaccines 11, 19 (2023).
Riad, A. et al. Monkeypox knowledge and vaccine hesitancy of Czech healthcare workers: a health belief model (HBM)-based study. Vaccines 10, 2022 (2022).
Wang, H., Paulo, K. J. I., d’Abreu de, Gültzow, T., Zimmermann, H. M. L. & Jonas, K. J. Monkeypox self-diagnosis abilities, determinants of vaccination and self-isolation intention after diagnosis among MSM, the Netherlands, July 2022. Eurosurveillance 27, 2200603 (2022).
Zucman, D., Fourn, E., Touche, P., Majerholc, C. & Vallée, A. Monkeypox vaccine hesitancy in french men having sex with men with PrEP or living with HIV in France. Vaccines 10, 1629 (2022).
Dukers-Muijrers, N. H. T. M. et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey). Front. Public Health 10, 1058807 (2023).
Paparini, S. et al. Public understanding, awareness, and response to monkeypox virus outbreak: a cross-sectional survey of the most affected communities in the United Kingdom during the 2022 public health emergency. https://doi.org/10.1101/2022.08.25.22279207 (2022).
Peptan, C., Băleanu, V. D. & Mărcău, F. C. Study on the vaccination of the population of Romania against Monkeypox in terms of medical security. Vaccines 10, 1834 (2022).
Riccò, M. et al. When a neglected tropical disease goes global: knowledge, attitudes and practices of Italian physicians towards Monkeypox, preliminary results. Trop. Med. Infect. Dis. 7, 135 (2022).
Riad, A. et al. Belarusian healthcare professionals’ views on Monkeypox and vaccine hesitancy. Vaccines 11, 1368 (2023).
Salim, N. A., Septadina, I. S., Permata, M. & Hudari, H. Knowledge, attitude, and perception of anticipating 2022 global human monkeypox infection among internal medicine residents at Palemberg indonesia: an online survey. J. Kedokt. Dan Kesehat. Publ. Ilm Fak. Kedokt. Univ. Sriwij. 9, 253–262 (2022).
Islam, M. R. et al. Assessment of vaccine perception and vaccination intention of Mpox infection among the adult males in Bangladesh: a cross-sectional study findings. PLoS ONE 18, e0286322 (2023).
Hori, D., Kaneda, Y., Ozaki, A. & Tabuchi, T. Sexual orientation was associated with intention to be vaccinated with a smallpox vaccine against mpox: a cross-sectional preliminary survey in Japan. Vaccine 41, 3954–3959 (2023).
Zheng, M. et al. Knowledge and vaccination acceptance toward the human monkeypox among men who have sex with men in Chin. Front. Public Health. 10 https://europepmc.org/articles/PMC9640956 (2022).
Dong, C., Yu, Z., Zhao, Y. & Ma, X. Knowledge and vaccination intention of monkeypox in China’s general population: a cross-sectional online survey. Travel Med. Infect. Dis. https://doi.org/10.1016/j.tmaid.2022.102533 (2022).
Chen, Y. et al. Knowledge of Human Mpox (Monkeypox) and Attitude towards Mpox Vaccination among Male Sex Workers in China: A Cross-Sectional Study. Vaccines 11, 285 (2023).
Lin, G. S. S., Tan, W. W., Chan, D. Z. K., Ooi, K. S. & Hashim, H. Monkeypox awareness, knowledge, and attitude among undergraduate preclinical and clinical students at a Malaysian dental school: An emerging outbreak during the COVID-19 era. Asian Pac J Trop Med 15, 461 (2022).
Fu, L. et al. Perception of and vaccine readiness towards Mpox among men who have sex with men living with HIV in China: a cross-sectional study. Vaccines 11, 528 (2023).
Peng, X. et al. Perceptions and worries about monkeypox, and attitudes towards monkeypox vaccination among medical workers in China: a cross-sectional survey. J. Infect. Public Health 16, 346–353 (2023).
Wang, B. et al. Perceptions, precautions, and vaccine acceptance related to monkeypox in the public in China: a cross-sectional survey. J. Infect. Public Health 16, 163–170 (2023).
Hong, J. et al. The willingness of Chinese healthcare workers to receive monkeypox vaccine and its independent predictors: a cross-sectional survey. J. Med. Virol. e28294. https://doi.org/10.1002/jmv.28294 (2022).
Zheng, M. et al. Mpox Vaccination Hesitancy and Its Associated Factors among Men Who Have Sex with Men in China: A National Observational Study. Vaccines 11, 1432 (2023).
Castel, A. D. et al. Mpox awareness, risk reduction, and vaccine acceptance among Pwh in Washington, DC. Top. Antivir. Med. 401–402 (2023).
Swed, S. et al. A multinational cross-sectional study on the awareness and concerns of healthcare providers toward monkeypox and the promotion of the monkeypox vaccination. Front. Public Health https://doi.org/10.3389/fpubh.2023.1153136 (2023).
Swed, S. et al. Monkeypox post-COVID-19: knowledge, worrying, and vaccine adoption in the Arabic general population. Vaccines 11, 759 (2023).
Abd ElHafeez, S. et al. Assessing disparities in medical students’ knowledge and attitude about monkeypox: a cross-sectional study of 27 countries across three continents. Front. Public Health 11, 1192542 (2023).
Loomba, S., de Figueiredo, A., Piatek, S. J., de Graaf, K. & Larson, H. J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 5, 337–348 (2021).
Sulaiman, S. K. et al. A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLoS ONE 17, e0278224 (2022).
Robinson, E., Jones, A., Lesser, I. & Daly, M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 39, 2024–2034 (2021).
Kazeminia, M., Afshar, Z. M., Rajati, M., Saeedi, A. & Rajati, F. Evaluation of the acceptance rate of covid-19 vaccine and its associated factors: a systematic review and meta-analysis. J Prev 43, 421–467 (2022).
Alimohamadi, Y., Hosamirudsari, H., Hesari, E. & Sepandi, M. Global COVID-19 vaccine acceptance rate: a systematic review and meta-analysis. J. Public Health. https://doi.org/10.1007/s10389-022-01757-5 (2022).
Norhayati, M. N., Che Yusof, R. & Azman, Y. M. Systematic review and meta-analysis of COVID-19 vaccination acceptance. Front. Med. 8, 783982 (2022).
Sulaiman, S. K., Musa, M. S., Tsiga-Ahmed, F. I., Sulaiman, A. K. & Bako, A. T. A systematic review and meta-analysis of the global prevalence and determinants of COVID-19 vaccine acceptance and uptake in people living with HIV. Nat. Hum. Behav. 1–15. https://doi.org/10.1038/s41562-023-01733-3 (2023).
Monkeypox —United Kingdom of Great Britain and Northern Ireland. (Accessed 17 Apr 2023) https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON381.
Burki, T. The end of the mpox pandemic? Lancet Infect. Dis. 23, 159–160 (2023).
Americas TLRH. The cycle of neglect: the mpox emergency in the Americas is far from ending. Lancet Reg Health—Am. https://doi.org/10.1016/j.lana.2023.100429 (2023).
Wang, Q., Yang, L., Jin, H. & Lin, L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev. Med. 150, 106694 (2021).
Public health advice for gay, bisexual and other men who have sex with men on the recent outbreak of monkeypox (Accessed 19 March 2023). https://www.who.int/publications/m/item/monkeypox-public-health-advice-for-men-who-have-sex-with-men.
Mussini, C., Guaraldi, G. & Orkin, C. Monkeypox vaccination—an opportunity for HIV prevention. Lancet HIV 9, e741–e742 (2022).
Infodemic. (Accessed 3 Sep 2022). https://www.who.int/health-topics/infodemic.
Pierri, F. et al. Online misinformation is linked to early COVID-19 vaccination hesitancy and refusal. Sci. Rep. 12, 5966 (2022).
Kabir Sulaiman, S. et al. Prevalence, determinants, and reasons for malaria vaccine hesitancy among caregivers of under-five children in Nigeria: results from a nationwide cross-sectional survey. Vaccine 41, 1503–1512 (2023).
Teng, S., Jiang, N. & Khong, K. W. Using big data to understand the online ecology of COVID-19 vaccination hesitancy. Humanit. Soc. Sci. Commun. 9, 1–15 (2022).
Sulaiman, S. K. Mapping global prevalence and determinants of mpox vaccine acceptance and uptake in key populations: a systematic review and meta-analysis. https://doi.org/10.17605/OSF.IO/FS5QH (2023).
Acknowledgements
The authors solely supported this work. No external funding was received for the work.
Author information
Authors and Affiliations
Contributions
S.K.S. conceived the study. S.K.S., M.S.M., B.T.M., F.I.T., A.K.S., and A.T.B. all contributed to the study methodology. S.K.S., M.S.M., and A.T.B. performed all statistical analysis. S.K.S., M.S.M., and A.T.B. were responsible for data curation. S.K.S. prepared the original manuscript draft; A.T.B., M.S.M., and S.K.S. led the critical review and editing of subsequent manuscript drafts along with contributions from F.I.T., A.K.S., and B.T.M.; A.K.S. led the study supervision; All authors contributed resources. All authors contributed to study validation. A.T.B. and S.K.S. were responsible for study visualization. S.K.S. was responsible for the project administration.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Ramy Mohamed Ghazy, Esteban Alarcon-Braga, and Mohamed Lounis for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sulaiman, S.K., Isma’il Tsiga-Ahmed, F., Musa, M.S. et al. Global prevalence and correlates of mpox vaccine acceptance and uptake: a systematic review and meta-analysis. Commun Med 4, 136 (2024). https://doi.org/10.1038/s43856-024-00564-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00564-1
- Springer Nature Limited