Abstract
Background
Perinatal outcomes vary for women with gestational diabetes mellitus (GDM). The precise factors beyond glycemic status that may refine GDM diagnosis remain unclear. We conducted a systematic review and meta-analysis of potential precision markers for GDM.
Methods
Systematic literature searches were performed in PubMed and EMBASE from inception to March 2022 for studies comparing perinatal outcomes among women with GDM. We searched for precision markers in the following categories: maternal anthropometrics, clinical/sociocultural factors, non-glycemic biochemical markers, genetics/genomics or other -omics, and fetal biometry. We conducted post-hoc meta-analyses of a subset of studies with data on the association of maternal body mass index (BMI, kg/m2) with offspring macrosomia or large-for-gestational age (LGA).
Results
A total of 5905 titles/abstracts were screened, 775 full-texts reviewed, and 137 studies synthesized. Maternal anthropometrics were the most frequent risk marker. Meta-analysis demonstrated that women with GDM and overweight/obesity vs. GDM with normal range BMI are at higher risk of offspring macrosomia (13 studies [n = 28,763]; odds ratio [OR] 2.65; 95% Confidence Interval [CI] 1.91, 3.68), and LGA (10 studies [n = 20,070]; OR 2.23; 95% CI 2.00, 2.49). Lipids and insulin resistance/secretion indices were the most studied non-glycemic biochemical markers, with increased triglycerides and insulin resistance generally associated with greater risk of offspring macrosomia or LGA. Studies evaluating other markers had inconsistent findings as to whether they could be used as precision markers.
Conclusions
Maternal overweight/obesity is associated with greater risk of offspring macrosomia or LGA in women with GDM. Pregnancy insulin resistance or hypertriglyceridemia may be useful in GDM risk stratification. Future studies examining non-glycemic biochemical, genetic, other -omic, or sociocultural precision markers among women with GDM are warranted.
Plain language summary
Gestational Diabetes (GDM) is high blood sugar that develops during pregnancy and may cause complications. GDM diagnosis is centered on blood sugar levels. Despite everyone receiving standard treatment, the clinical outcomes may vary from one individual to another. This indicates a need to identify factors that may help GDM diagnosis and result in improved classification of those at greatest risk for complications. Here, we systematically analyzed all published evidence for potential markers that could identify those with GDM who have greater risk of complications. We find that high maternal weight is a risk factor for offspring born larger for their gestational age. Other promising markers were identified, but further analysis is needed before they can be applied in the clinic.
Similar content being viewed by others
Introduction
Gestational diabetes (GDM) is the most common metabolic complication of pregnancy with an increasing prevalence consistent with the concomitant global increase in obesity and diabetes1. GDM traditionally refers to abnormal glucose tolerance with onset or first recognition during pregnancy, typically diagnosed between 24 and 28 weeks’ gestation2. It is associated with maternal and neonatal complications such as hypertensive disorders of pregnancy, offspring large-for-gestational-age (LGA), macrosomia, birth trauma, neonatal respiratory distress, and neonatal hypoglycemia3.
Although treating hyperglycemia lowers the risk of maternal and neonatal morbidity, some women with GDM likely would not have had perinatal complications even if left untreated4,5, while others still go on to develop complications despite adequate glycemic control6. Maternal GDM with obesity (BMI ≥ 30 kg/m2) vs. GDM without obesity is associated with a 2- to 4-fold greater risk of macrosomia7,8,9,10. Recently, differences in perinatal outcomes based on physiologic subtypes of GDM (e.g., insulin-resistant vs. insulin secretion deficient) have been described11,12,13,14. While the diagnostic criteria for GDM detect dysregulation of glucose metabolism, GDM is increasingly recognized as a heterogeneous condition, which may include sub-phenotypes6,15. As such, metabolic variations, beyond glycemic measures among women with GDM may modify its impact on maternal and fetal health16.
Several upstream determinants of metabolic health are considered risk factors for GDM and may also be markers by which to stratify risk within the population of women who develop GDM. These risk factors include genetics, higher BMI, prior medical and obstetric history, and socio-demographic factors such as race/ethnicity which may capture differences in societal and environmental factors17,18,19. Prior systematic reviews have focused on the prediction or prevention of GDM20,21,22,23, have used a normoglycemic group for comparison24,25, or have focused on glycemic markers as the sub-phenotyping variable among women with GDM26. Despite great interest in risk stratification of women with GDM, efforts to systematically review studies evaluating a spectrum of social, physiological, and biological non-glycemic factors that could identify sub-phenotypes within GDM are lacking.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine27. As part of the ADA/EASD PMDI effort to comprehensively evaluate the evidence for precision diabetes medicine and inform the 2nd International Consensus Report on Precision Diabetes Medicine28, we aimed to review the existing literature to investigate GDM sub-phenotypes and heterogeneity in association with adverse perinatal outcomes. This effort was undertaken to aid in determining whether factors other than traditional glycemic measures could refine the diagnosis of GDM. The following categories of precision markers were included: maternal anthropometrics, clinical or socio-cultural factors, diet and behaviors, non-glycemic biochemical markers, genetics/genomics or other -omics, and fetal biometry.
Our systematic review of 137 studies and 432,825 women with GDM demonstrates that perinatal outcomes vary substantially related to factors that extend beyond glycemia. Prior research has largely focused on the impact of pre-pregnancy overweight or obesity on adverse perinatal outcomes. In a meta-analysis of 10 studies of LGA and 13 studies of macrosomia, we found that the co-occurrence of pre-pregnancy overweight/obesity with GDM was associated with a 2 to 3-fold greater risk of LGA or macrosomia. Furthermore, independent of maternal BMI, those with higher triglycerides or insulin resistance, may be at higher risk of having an offspring born LGA or with macrosomia. Areas that currently require more evidence include investigations of genetics, metabolites, and other novel biomarkers, as well as integration of social, environmental, and behavioral factors. Overall, our systematic review identified critical gaps and future research areas for precision GDM diagnosis and highlighted promising biomarkers that may open the door to non-glycemic treatment targets in GDM.
Methods
A protocol for this review was registered at PROSPERO (CRD42022316260) on 11 March 2022. Nota bene, as part of the diabetes scientific community, ADA/EASD PDMI is committed to using inclusive language, especially in relation to gender. We choose to use gendered terminology throughout the article following the rationale for using female-sexed language in studies of maternal and child health29. Additionally, most of the original studies reviewed used “women” as their terminology to describe their population, as GDM per definition is a pregnancy complication, which can only occur in individuals who are assigned female sex at birth. In this review, we use the term “women” throughout, but acknowledge that not all individuals who experienced a pregnancy may self-identify as women.
Data sources and search strategy
Systematic literature searches were performed in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and EMBASE (https://www.embase.com) databases from their inception to March 2022. These databases were chosen because both can be searched using multiple retrieval approaches such as text word terms in relevant fields and standardized subject terms (controlled vocabulary). Searches were limited to databases that could return results for a combination of these two approaches, a decision informed by the mandatory requirement of the Cochrane Review quality assurance strategy. We searched for observational studies (cohort and case–control) and clinical trials that compared outcomes among women with GDM. The following categories of precision markers were included in the current search: maternal anthropometrics, clinical or socio-cultural factors (i.e., age, race/ethnicity, country of origin), diet and behaviors, non-glycemic biochemical markers (e.g., lipids, insulin, other biomarkers), genetics/genomics or other -omics (e.g., proteomics, lipidomics, metabolomics, metagenomics), and fetal biometry. The search was restricted to studies in adult humans that were published in English. The search strategy and terms are available in Supplementary Note 1. All studies were screened by at least two reviewers and conflicts were resolved by a third independent reviewer. All titles and abstracts were screened for eligibility, and those that were assessed as potentially meeting inclusion/exclusion criteria were selected for full-text evaluation. To help ensure that other important articles were not missed, during full-text evaluation, if a relevant study was referenced, we included those in full-text evaluation as well.
Inclusion criteria
Studies were considered potentially eligible for inclusion if they met the following criteria: 1) at least 100 participants and a minimum of 30 GDM cases when reporting a continuous precision marker, or 30 cases per GDM subtype, 2) reported outcome data on maternal hypertensive disorders in pregnancy, cesarean delivery, offspring anthropometry at birth (macrosomia, LGA, small-for-gestational-age [SGA]), preterm delivery, birth trauma, metabolic sequelae (e.g., hypoglycemia) or mortality, and 3) presented data in text or tables that allowed for the comparison of outcomes between subtypes of GDM or among women with GDM exclusively for continuous markers. Studies evaluating prevention, treatment, or long-term maternal and offspring prognosis were excluded as they were to be covered by the objectives of complementary systematic reviews led by other PDMI working groups30,31,32.
Exclusion criteria
As our main goal was to review studies identifying GDM sub-phenotypes beyond glycemia, we excluded studies that only reported on glycemic markers or thresholds (e.g., HbA1c, fasting glucose, oral glucose tolerance test [OGTT] glycemic values), or studies that were focused on assessing differences in outcomes based on timing of glucose measurement.
We also excluded studies that 1) measured the precision marker after GDM diagnosis (e.g., total gestational weight gain [GWG] over the whole pregnancy, or fetal biometry after 32 weeks’ gestation), 2) combined pre-existing diabetes or overt diabetes (based on non-pregnancy glycemic thresholds) with GDM, 3) included women with multi-gestations, or 4) did not contain full-length manuscripts in English.
Data extraction and quality assessment
Study and sample characteristics were extracted independently by two reviewers and conflicts were resolved by a third reviewer from full-text using a web-based collaboration software platform that streamlines the production of systematic literature reviews (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). The following data elements were extracted from each study when available: cohort characteristics (continent, country, study type [hospital/registry/cohort], enrollment years); participant characteristics (age, BMI, the proportion nulliparous); GDM information (sample size, diagnostic criteria or description); timing of precision marker measurement (pre-pregnancy, before or at GDM diagnosis); and perinatal outcomes (maternal, fetal/neonatal).
The risk of bias and overall quality of each study were assessed independently or in duplicate using the Joanna Briggs Institute Critical Appraisal Tool for cohort studies, which was modified specifically for the objectives of the current systematic review33 (Supplementary Note 2). We assessed the studies using a ten-question measure and considered studies with two poor quality metrics to be of low quality34.
Data synthesis and meta-analysis
For each category of precision marker, two independent reviewers jointly summarized qualitatively the findings. Given the numerous studies evaluating effect modification by maternal BMI, we performed a post-hoc meta-analysis of studies that reported data that allowed for quantitative measurement of the associations of maternal BMI with offspring LGA or macrosomia among women with GDM.
We pooled odds ratios (ORs) from individual studies to estimate the summary OR with 95% CI for each BMI category using the Dersimonian and Laird random-effects model accounting for both within- and between-study variances35. We assessed overall heterogeneity using the Cochran’s Q test and I2 statistics; I2 > 75% was considered as evidence of statistical heterogeneity36. Subgroup analyses were carried out by study enrollment period (enrollment completed prior to 2010 vs. enrollment from 2010 onwards), quality grade (≥2 poor quality metrics vs. <2 poor quality metrics), and covariate adjustment (yes vs. no). Degree of potential publication bias was evaluated using the Egger’s test and the Begg’s test37,38. Meta-analyses were conducted using R (version 4.2.3) and the ‘metafor’ R package (version 4.0-0). A two-sided p value of <0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Literature search
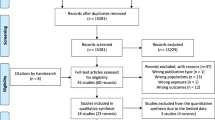
The literature search yielded 5905 non-duplicated abstracts (Fig. 1). After independent review by two investigators for each abstract, 5130 abstracts were excluded. Among the 775 full-text studies reviewed, 638 were excluded based on our study selection criteria, resulting in 137 studies that met the selection criteria. All 137 studies were observational, with no randomized clinical trials. The 137 studies were categorized into three groups depending on the precision markers examined: 1) anthropometry (maternal anthropometry/fetal biometry); 2) biochemical, genetics, -omics markers; and 3) clinical or socio-cultural factors.
Overall study characteristics
Characteristics of the 137 studies representing a total of 432,156 participants are shown in Supplementary Data 1. The median number of study participants was 587. Of these studies, 68 evaluated maternal anthropometry, 33 evaluated non-glycemic biochemical markers, and 48 evaluated clinical or socio-cultural factors (some studies reported more than one precision marker). Most studies (72%) included pregnancies from 2000 to 2020 and were from geographically diverse regions. The studies were most frequently conducted in China (20%), the US (12%), Australia (7%), and Spain (6%). The most frequent diagnostic criteria for GDM were the 2010 International Association of the Diabetes in Pregnancy Study Groups (IADPSG)39/2013 World Health Organization (WHO) criteria40.
Overall, 45% of the studies had two or more quality assessment domains categorized as low (Fig. 2). The most frequent domain ranked as low was related to confounding; ~40% of studies reported unadjusted effect size estimates. In addition, self-reported data is generally considered to be of low quality, and since many studies ascertained maternal weight and/or BMI using self-reported pre-pregnancy weight, 28% of studies had low-quality rankings on the “ascertainment of precision marker” domain. Other factors that impacted the quality rankings were mostly due to unclear reporting in the manuscripts.
The risk of bias and overall quality of each study was assessed independently or in duplicate using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for cohort studies, which was modified specifically for the objectives of the current systematic review. For each question, a reviewer could indicate “not applicable” (blank filled bars), “yes” (blue filled bars), “unclear” (orange filled bars), “no” (red filled bars). An answer of “yes” indicates less risk of bias and greater quality, and answer of “no” indicates a higher risk of bias and lower quality.
Studies of anthropometry as a precision marker
Study characteristics—a total of 68 studies of women with GDM described associations of pre-pregnancy overweight and obesity (defined by maternal BMI based on WHO classifications41 or region-specific cut-offs) with adverse pregnancy and perinatal outcomes7,8,9,10,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105. A small number of studies described the relationship of early gestational weight gain (early GWG) prior to GDM diagnosis (n = 4)49,106,107,108, or fetal biometry ultra-sound measures (biparietal, head, abdominal circumference or femur length) before 32 weeks’ gestation (n = 9)42,55,64,87,98,109,110,111,112 with adverse perinatal outcomes. The characteristics of these studies are summarized in Supplementary Data 2. The median number of GDM cases was 594.
Maternal anthropometry—studies evaluating the relationship between maternal BMI and adverse pregnancy outcomes tended to be retrospective hospital record cohort or case-control studies relying on self-reported pre-pregnancy weight. All but nine studies44,59,65,80,88,94,95,96,103,113,114,115 reported that maternal overweight and obesity were associated with greater risk of at least one adverse perinatal outcome, most commonly offspring LGA or macrosomia.
Thirty-eightstudies reported data that could be meta-analyzed to offer a quantitative assessment of maternal BMI as a precision marker for risk of macrosomia or LGA7,8,9,10,42,45,46,47,48,49,54,55,56,58,61,66,67,69,70,71,72,73,77,79,81,82,84,88,89,90,92,93,94,98,100,101,105,113. Among women with GDM, the association of maternal BMI and macrosomia was reported in 23 studies (n = 34,016)7,8,9,10,45,47,48,56,61,70,71,72,73,77,81,84,90,92,93,94,98,100,113 and the association of maternal BMI and LGA was reported in 26 studies (n = 31,287)8,10,42,46,49,54,55,58,66,67,69,70,71,72,77,79,81,82,84,88,89,90,93,94,101,105. Across the 38 studies, there were differences in how the BMI categories were constructed and which category was used as a reference. Pooled estimates from the 13 studies that reported maternal overweight/obesity categories (versus BMI in normal range) and macrosomia (Fig. 3; n = 28,763)7,9,10,45,47,51,61,70,73,81,90,93,94 and the ten studies that reported maternal overweight/obesity categories (versus BMI in normal range) and LGA (Fig. 4; n = 20,070)10,49,70,79,81,90,93,94,101 are reported below. Meta-analysis of other categories of BMI and reference groups (e.g., obese vs. non-obese) can be found in Supplementary Fig. 1 and Supplementary Fig. 2.
Square represents the odds ratio on the log scale; confidence interval (CI). a It shows the odds ratio (95% CI) for the association of maternal BMI categorized in overweight/obesity vs. normal range and offspring macrosomia among 13 studies that included a total 28,763 participants. b Odds ratio for the association of continuous maternal BMI (per kg/m2) and offspring macrosomia among three studies that included 2611 participants. Abbreviations: LGA large-for-gestational age.
Square represents the odds ratio on the log scale; confidence interval (CI). a Odds ratio (95% CI) for the association of maternal BMI categorized in overweight/obesity vs. normal range and offspring LGA among 10 studies that included a total 20,070 participants. b Odds ratio for the association of continuous maternal BMI (per kg/m2) and offspring LGA among 10 studies that included 6113 participants. Abbreviations: LGA: large-for-gestational age.
Macrosomia—the pooled OR from 13 studies of macrosomia (n = 28,763)7,9,10,45,47,51,61,70,73,81,90,93,94 was 2.65 (95% CI: 1.91–3.68) for overweight/obesity compared to normal BMI with heterogeneity noted across the pooled studies (I2 > 75%) (Fig. 3). Study-specific ORs based on country of recruitment are presented in each figure. The association between overweight/obesity and macrosomia among women with GDM in subgroup meta-analyses is shown in Supplementary Table 1. Subgroup analysis of studies with low quality (n = 3) yielded an OR of 6.44 (95% CI: 0.84-49.42), and among studies of high quality (n = 10) the I2 was 0% indicating study quality as a source of heterogeneity in the full analysis. Subgroup analyses focusing on studies with enrollment during or after 2010 (all of which used IADPSG diagnosis criteria), studies of high quality, or studies with covariate adjustment did not substantially change the results. No indication of publication bias was suggested based on either the Egger’s test (P = 0.32) or the Begg’s test (P = 0.51). Three studies examined maternal BMI as a continuous variable associated with macrosomia in women with GDM (n = 2611)8,98,113. A one-unit increment of maternal BMI (kg/m2) was significantly associated with an increased risk of macrosomia (OR: 1.12, 95% CI: 1.05–1.19) (Fig. 3).
LGA—the pooled OR from 10 studies of LGA (n = 20,070)10,49,70,79,81,90,93,94,101 was 2.23 (95% CI: 2.00–2.49) for overweight/obesity vs. normal BMI (Fig. 4). Study-specific ORs based on country of recruitment are presented in each figure. Subgroup analysis, where we stratified studies by enrollment during or after 2010 (all of which used IADPSG diagnostic criteria), or study quality rating, or restricted to studies with covariate adjustment did not produce substantially different results (Supplementary Table 2). No indication of publication bias was suggested based on either the Egger’s test (P = 0.69) or the Begg’s test (P = 0.29). Ten studies reported associations of continuous BMI with offspring LGA (n = 6113)8,42,49,55,58,66,67,69,88,89. A one unit increment of maternal BMI (kg/m2) was associated with increased odds of LGA (summary OR: 1.09, 95% CI: 1.06–1.12) (Fig. 4).
Numerous studies among women with GDM also found associations between maternal overweight/obesity and greater risk of cesarean delivery44,51,54,64,70,72,73,75,78,92,95,105 or preeclampsia and other hypertensive disorders of pregnancy46,47,53,54,70,72,92,99,100,102,104. Four studies reported an association with neonatal hypoglycemia56,58,61,62, two reported an association with a composite outcome of neonatal morbidity and/or admission to NICU47,96, and one reported an increased risk of major congenital malformations63.
Early gestational weight gain (GWG)—three of four49,106,107,108 studies of early GWG (prior to GDM diagnosis) in women with GDM found positive associations with LGA49,106,108, one of which reported that trimester-specific weight gain above the Institute of Medicine Guidelines116 was additionally associated with increased risk of preeclampsia and macrosomia106.
Fetal biometry—among the nine studies with a fetal biometry ultra-sound measure near the time of GDM diagnosis, six found that larger fetal abdominal42,55,87,110,112 or biparietal circumference98 was positively associated with greater neonatal size (birthweight, LGA, macrosomia).
Studies of biochemical, genetics, or -omics as precision markers
Study characteristics—of the studies that reported biochemical, genetic, or -omics (e.g., metabolomic, lipidomic) markers, 14 described associations of lipid classes (triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol) with adverse pregnancy and perinatal outcomes7,65,66,74,82,88,89,97,98,99,113,117,118,119. There were 12 studies that described associations of insulin sensitivity/resistance profiles12,114,115,120,121,122,123,124 or insulin secretion indices109,117,125,126 with perinatal outcomes. A small number of studies subtyped GDM based on adipokines (n = 2)109,127, metabolomics (n = 1)128, non-coding RNA (n = 2)129,130, and variants in candidate genes (n = 2)131,132. The characteristics of these studies are summarized in Supplementary Data 3, which also includes studies with measurement of other biochemical markers (e.g., proteinuria, platelet count). The median (range) number of GDM cases was 242 (64-2647).
Lipid subclasses—among the 14 studies measuring triglycerides prior to or at the time of GDM diagnosis7,65,66,74,82,88,89,97,98,99,113,117,118,119, half reported that higher triglycerides positively correlated with increased birthweight or risk of LGA or macrosomia after adjusting for maternal BMI7,82,88,89,97,98,113, whereas six reported no association with neonatal size65,66,74,117,118,119, and one reported no association with preeclampsia99. Of the studies measuring total, LDL, or HDL cholesterol (n = 12)65,66,74,88,89,97,98,99,113,115,118,119, one found a positive association of LDL with LGA89, and three reported lower mean HDL levels were associated with LGA66,74,119.
Insulin profiles and indices—a variety of methods of calculating insulin resistance/sensitivity and insulin secretory response using timed insulin and glucose during the OGTT for GDM subtyping were described. The homeostatic model assessment of insulin resistance (HOMA-IR or HOMA2-IR) calculated at the time of GDM diagnosis (http://www.dtu.ox.ac.uk/homacalculator/)133 was most commonly used. The Matsuda index134, modeled using glucose and insulin values across the OGTT, was the most frequent measure of insulin sensitivity. HOMA-B/HOMA-2B (http://www.dtu.ox.ac.uk; homeostatic model assessment of beta-cell function; fasting insulin and fasting glucose model), and the Stumvoll first phase insulin estimate (modeled using timed insulin and glucose values from OGTT)135,136 were the most utilized indices defining insulin secretory response. Other indices such as the insulinogenic index and disposition index were utilized rarely120,122.
All four studies examining HOMA-IR found that women with GDM and high HOMA-IR (highest quartile or >2.0) had a significantly increased risk of LGA or macrosomia compared to those with GDM and lower HOMA-IR115,121,124,126, although in one study the statistical comparison was to normal glucose tolerant women126. In two studies, insulin-related measures such as a defect in insulin sensitivity, insulin secretion, or a combination of both were not associated with differences in perinatal outcomes12,123. Three studies reported on insulin profiles among participants with and without GDM114,120,122. In two of these, participants with GDM who were insulin resistant had higher rates of LGA and macrosomia (where women with GDM without greater than usual insulin resistance had similar rates as women without GDM); however, no statistical tests for significant differences in the outcome rates among the subtypes of GDM were reported114,122. A study of insulin secretion peaks during an OGTT found that a delayed insulin secretion peak was associated with increased risk of preeclampsia, LGA, and neonatal hypoglycemia125, whereas both a study of insulin levels following a 50 g glucose load and a study of fasting plasma insulin found no association with adverse perinatal outcomes109,117.
Adipokines—two studies measured adiponectin, leptin109,127, and one additionally measured visfatin127. Neither study found that adiponectin or leptin was associated with perinatal outcomes among women with GDM; however, higher visfatin levels were associated with lower risk of LGA127.
Metabolomics—a single study utilizing mass spectrometry examined the association of plasma levels of carnitine and 30 acylcarnitines with adverse perinatal complications in women with GDM128. Carnitine and acylcarnitine levels together with clinical factors were used to construct a nomogram to predict macrosomia within women with GDM, which resulted in an area under the receiver operating characteristic (AUROC) curve of 0.78.
Non-coding RNAs—two studies examined the association of different classes of non-coding RNAs with various adverse pregnancy outcomes129,130. One study of circulating long non-coding RNAs (lncRNAs) measured in 63 women with GDM found that including XLOC_014172 and RP11-230G5.2 in a prediction model for macrosomia resulted in an AUROC curve of 0.962129. In a study of high or low plasma levels of circular RNA circATR2, high circATR2 was associated with higher rates of prematurity, miscarriage, intrauterine death, fetal malformations, intrauterine infection and hypertension but not macrosomia or fetal distress130.
Genetic studies—we did not identify any genome-wide-association studies reporting on GDM subtypes. Two studies used a candidate gene approach to subtype women with GDM based on their genotype and examine associations with pregnancy outcomes131,132. One study of a variant in the patatin-like phospholipase-3 (PNPLA3)/adiponutrin gene (rs738409 C.G) found that carrying the G allele (n = 96) compared with being a CC homozygote (n = 104) was associated with lower fasting insulin, insulin resistance, and LGA132. In a study of SNP 45TG in exon 2 of the adiponectin gene, the G allele and GG + TG genotypes were associated with GDM, lower adiponectin levels, and among the women with GDM, greater incidence of macrosomia and neonatal hypoglycemia compared to the TT group131.
Studies of clinical and sociocultural factors as precision markers
Study characteristics—of the studies reporting associations of clinical, sociocultural factors or composites of multiple risk factors among women with GDM55,59,60,65,69,78,80,85,86,96,97,103,104,113,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171, 14 compared differences in adverse perinatal outcomes between different races, ethnicities, or countries of origin69,96,147,148,149,150,152,154,155,156,157,158,159,160, and six included multiple risk factors as a composite variable78,86,141,144,162,163. Four studies investigated psychological factors, and five reported on concomitant presence of pre-eclampsia or hypertensive disorders of pregnancy in women with GDM. The characteristics of these studies are summarized in Supplementary Data 4. Half of the studies included pregnancies from 1990-2009, with four studies from the 1980s. A third of studies diagnosed GDM using 2010 IADPSG criteria, and 20% did not report diagnostic criteria. The median (range) number of GDM cases in these 48 studies was 950 (100–170,572).
Race/ethnicity—studies have reported various findings comparing outcomes in women with GDM from different races or ethnicities69,96,147,148,149,150,152,154,155,156,157,158,159,160. In the US, women with GDM who identified as Black or African American were at higher risk of perinatal complications, including fetal death148,154. Findings were inconsistent regarding risk of reported complications in women with GDM who identified as Hispanic (versus non-Hispanic): two studies did not find major differences in adverse outcomes149,158, while one large study reported a higher rate of preterm birth148. In Hawaii, White women with GDM were at greater risk of macrosomia compared to other race/ethnicity groups (Hawaiian/Pacific Islander, Filipina, or other Asian women)152. Several studies in Australia, US, and Canada comparing women with GDM from different race/ethnicity groups found that women who identified as Asian were less likely to have LGA offspring (compared to White-identified women)147,150,154,155,159,161. In two Canadian studies, women with GDM from First Nations or Indigenous groups were at higher risk of perinatal complications150,157.
Clinical, and co-existing medical factors and conditions—many studies that met our inclusion criteria also reported the association of other factors such as prior history of GDM, macrosomia, polycystic ovary syndrome (PCOS), or family history of diabetes with risk of perinatal adverse outcomes among women with GDM. Given that these risk factors were not pre-specified in our search, our summary of these studies should be considered as a scoping (rather than systematic) review.
Of the studies examining multiple clinical or sociocultural factors (e.g., BMI, maternal age, prior GDM pregnancy), four found that the presence of one or more risk factors was associated with greater neonatal size (birthweight percentile, LGA, macrosomia), compared to women with GDM and no risk factors78,86,141,162. Two of these studies reported a higher risk of cesarean delivery78,86. One study reported that GDM with one or more risk factors was associated with cesarean delivery and not neonatal size144, and another found no difference in perinatal outcomes among women with or without risk factors163.
In general, there was no consistent association of maternal age, parity, prior GDM, or family history of diabetes with risk of adverse perinatal outcomes in women with GDM. Studies that identified co-existing medical conditions (e.g., PCOS, preeclampsia, hypertensive disorders of pregnancy, infertility treatment) showed that PCOS was a marker for higher risk of preeclampsia140,145. Co-existing preeclampsia or hypertensive disorder of pregnancy in women with GDM was associated with smaller size at birth compared to GDM pregnancies without preeclampsia or hypertensive disorder of pregnancy57,80.
Four studies reporting on psychological factors found that worse depression, anxiety, or diabetes distress scores146,151,153,164 were markers of greater risk of various adverse perinatal outcomes, or were related to differences in neonatal size146,151,153. There was a lack of studies evaluating diet or physical activity as risk markers among women with GDM. Three studies included other risk factors (e.g., fetal sex, seasonality of conception, epicardial fat)167,170,171 with findings that will need replication before conclusions can be drawn.
Discussion
Our systematic review of 137 studies and 432,825 women with GDM demonstrates that perinatal outcomes vary substantially related to factors that extend beyond glycemia. Prior research has largely focused on the impact of pre-pregnancy overweight or obesity on adverse perinatal outcomes. In a meta-analysis of 10 studies of LGA (n = 20,070) and 13 studies of macrosomia (n = 28,763), we found that the co-occurrence of pre-pregnancy overweight/obesity with GDM was associated with a 2 to 3-fold greater risk of LGA or macrosomia. Notably, even across the spectrum of maternal size, a one-unit increase in BMI is associated with a 9% greater risk of LGA and a 12% greater risk of macrosomia. Furthermore, independent of maternal BMI, those with higher triglycerides or insulin resistance, may be at higher risk of having an offspring born LGA or with macrosomia. Studies reporting on genetics and ‘omics were scarce, and we could not draw conclusions on these potential precision markers. There was inconsistent evidence that individual maternal clinical and sociocultural factors were associated with greater risk of perinatal complications.
Anthropometry as a precision marker
Findings from our meta-analysis provide strong evidence that women with GDM and overweight/obesity compared to women with GDM and BMI in the normal range are at greater risk of fetal overgrowth. Although assessment of the relative contribution of maternal glycemia versus obesity to adverse pregnancy and perinatal outcomes was beyond the scope of this review, the risks associated with obesity and GDM are likely to be additive54. Current evidence suggests metabolic alterations that accompany obesity increase the risk of adverse perinatal outcomes172. This underscores the need to better refine the phenotyping of women with GDM based on lipids, insulin resistance, and other metabolic alterations that may contribute to fetal overgrowth.
Fetal biometry is not a novel precision marker of overgrowth risk and arguably reflects the consequences of GDM. Nevertheless, few research studies have evaluated a combination of early ultrasound fetal growth biometry with other metabolic data, in association with, or prediction of, adverse perinatal outcomes. These studies may help identify early metabolic biomarker profiles (and, therefore, targets) of birth size.
Biochemical, genetics, or -omics as precision markers
Most studies examining lipids in association with adverse perinatal outcomes have measured a standard lipid panel that includes three measures of cholesterol levels (total, LDL and HDL cholesterol) and triglycerides. Half of the studies reported higher triglycerides, independent of BMI, were associated with macrosomia or LGA, with fewer studies finding that higher LDL or lower HDL was associated with neonatal size. Data on the association of triglycerides with neonatal size in women with GDM align with a recent meta-analysis of studies in the general pregnant population that higher triglycerides were associated with increased birthweight and higher risk of LGA and macrosomia173. However, not all studies included in our review reported positive associations, and many factors, such as differences in timing of blood collection and variability in the distribution of characteristics across studies, could explain inconsistencies. Few studies examined the joint effects of multiple lipid subclasses, and future studies should include other and more detailed lipid measures to further clarify the mechanisms leading to fetal overgrowth (which lipids or lipid fractions, placental transfer, etc.) so novel therapeutic approaches can be developed and tested.
Many studies reported data on insulin profiles among women with GDM but made statistical comparisons to normal glucose-tolerant women. In general, it appears that women with GDM who have greater insulin resistance are at increased risk of fetal overgrowth and LGA. Subtyping by the presence or absence of insulin resistance and deficiency was described by Powe et al. in 2016, but this paper was not included in our review because of small GDM sub-group sample sizes11. Many of the subsequent studies included here used a similar insulin resistance/insulin deficiency classification rubric and referred to Powe et al. as part of the rationale or methodology. There are inadequate data to determine whether a predominant defect in insulin secretion without excess insulin resistance is related to adverse perinatal outcomes. Insulin sensitivity or resistance in pregnancy can be estimated using insulin or C-peptide and glucose values at multiple time-points during the standard OGTT (e.g., using the Matsuda formula which has been validated in pregnancy); however, the studies that calculated insulin resistance using HOMA-IR, which simply requires fasting insulin and glucose values, found that this index was a marker for higher rates of LGA or macrosomia in women with GDM, making it an appealing simple biochemical marker115,121,124,126. If GDM subtyping based on insulin physiology is to be translated clinically, there is a need for laboratory standardization of insulin (or C-peptide) assays to support establishing clinical thresholds.
Given the role of adiponectin as an insulin sensitizer174 and leptin as modulator of food intake and energy expenditure175, as well as the robust body of data tying maternal adiposity to pregnancy outcomes in GDM, it is surprising that our review only identified two studies that reported associations between adipokines and adverse perinatal outcomes among women with GDM. It is difficult to assess if this reflects a publication bias where null findings have been excluded or a true lack of research in this area. Future studies assessing adipose-derived peptides as precision markers among women with GDM should also consider additional effect modification by insulin sensitivity or maternal adiposity. This latter point may be particularly relevant as previous studies of adipokines in general pregnancy have reported effect modification by maternal BMI176,177.
No studies that met our inclusion criteria included measures of branched-chain amino acids, which have been implicated in diabetes risk and complications both within and outside of pregnancy178,179. Although we recognize that pregnancy cohorts not restricted to GDM have found associations of amino acids with glucose metabolism and perinatal outcomes180,181,182,183,184; whether amino acid subclasses or indeed hormonal profiles might be used as potential precision markers among women with GDM that identify increased risk of adverse perinatal outcomes has not been adequately studied and future research in this area is needed.
Studies performed to date attempting to identify genetic markers that predict adverse outcomes among women with GDM are not only limited in number but have major methodologic limitations. First, the identified studies have had small sample sizes and were performed in homogenous populations, without replication in independent cohorts. Moreover, reviewed genetic studies used a targeted approach examining either a single or limited number of variants/molecules. Future studies among larger diverse populations are needed for genome-wide association studies. None of the studies included in our review focused on metagenomics.
Clinical and sociocultural factors as precision markers
Certain racial/ethnic groups (such as Asian, First Nations/Indigenous, Hispanic) have been observed to have an increased risk of GDM. In the current review, being part of a minoritized race/ethnicity group was associated with adverse outcomes only in some instances. In studies where differences were observed, they mirrored patterns of health disparities reflecting different perinatal complication rates in the general population185. We note that these racial and ethnic categories and their relationship with outcomes are highly dependent on the overall social context (countries or regions) and may reflect experiences of racism, some aspects of culture, socioeconomic status, and many other factors that influence health outcomes such as diet and environmental pollutants or toxicants17,18,19. Studies that met our inclusion criteria did not directly investigate some of the correlates of race and ethnicity (e.g., diet, environmental exposures). We encourage future studies to carefully consider and collect data on the sociocultural influences and other correlates of race and ethnicity, and separately investigate genetic similarity, ancestry, or the inequities related to racism.
Limitations
It is important to highlight that in our review all the studies that met the inclusion/exclusion criteria were observational in design, and thus inadequate to provide the level of evidence required to change clinical practice currently. Different criteria for diagnosing GDM have been adopted at different time periods and across different geographic regions, which ultimately has led to women with differing degrees of hyperglycemia and maternal/fetal risks being diagnosed with GDM186,187. In our summary of the literature, IADPSG was the most common reported diagnostic criteria in the studies that were included in our systematic review. We included all studies meeting pre-specified inclusion/exclusion criteria regardless of differences in diagnostic approach. We evaluated non-glycemic precision markers that could reliably distinguish at-risk sub-phenotypes irrespective of the timing, threshold value, or number of above-threshold values. In the current review, the meta-analysis estimates of maternal BMI as a precision marker of macrosomia or LGA risk were similar regardless of diagnostic criteria or time period of study enrollment. We note that not all articles provided details on the definition of macrosomia or LGA, and therefore, this may have introduced some error in the meta-analysis estimates.
In our review, there was an inclusion criterion that manuscripts be published in English, which somewhat limits the global scope of included studies and representation of some regions of the world (e.g., no studies from India were included). Given the increasing prevalence of GDM and risk of diabetes, studies of the utility of precision markers for diagnosing GDM from different regions are needed. We excluded studies with less than 100 participants (and <30 GDM cases), as it would be difficult to draw sound conclusions from studies with fewer participants. However, this undoubtedly limited the inclusion of pilot studies or with smaller sample size, which could have limited the diversity of populations included. Lastly, the GDM diagnosis PMDI working group is a partnership that was initiated by the ADA and EASD which invited GDM experts from across the globe. Although we are an international working group of clinical and research scientists, our expertise may not completely capture the vast global spectrum of scientific and clinical work related to GDM. We hope this initiative will spark additional collaborations and multinational efforts that will continue to contribute to precision initiatives to help identify successes and opportunities in tailored GDM diagnostics.
Future directions
Our systematic review has identified several major areas for further research. There remains a need for mechanistic studies to provide an understanding of why the identified precision biomarkers are associated with an increased risk of adverse pregnancy outcomes. Replication studies of any potential biomarkers are needed in large and diverse populations across the world, and these should be accompanied by work to standardize the laboratory analysis of biomarkers used to diagnose subtypes of GDM at certain thresholds. Additionally, it will be worthwhile to leverage the information provided herein to investigate whether the identified precision markers for identifying at-risk GDM subtypes are dependent on the criteria used for GDM diagnosis. Multinational studies measuring environmental and behavioral factors such as dietary intake, physical activity, differences in socioeconomic opportunity, and neighborhood characteristics are needed, given their potential impact on perinatal outcomes among women with GDM. Moreover, large studies including participants from many regions across the world with measurements of genetic variants and multi-omics that integrate clinical and sociocultural data are needed and could provide insight into the determinants and causal pathways of heterogeneity within GDM and its outcomes. This may require applying approaches often used in systems biology, machine learning, or in the aggregation and analysis of large datasets from different sources using methods such as multilayer networks and clustering188.
For future clinical implications, some of the next questions are: if precision markers such as insulin resistance or higher triglycerides are part of causal pathways that lead to adverse outcomes, can we directly target them safely in pregnancy? Although somewhat controversial, Metformin targets insulin resistance and is accepted in some contexts for use during pregnancy; however, well-designed clinical trials for insulin-resistant GDM women are needed to accurately estimate the benefit-to-risk ratio. There is a need to test whether dietary approaches, supplements, or other therapeutics can effectively and safely reduce triglycerides among women with GDM. Thus, scientific identification of the physiological and mechanist attributes that lead to pregnancy-induced excessive insulin resistance and triglycerides, with linkage to prognostic differences, will improve the development of novel therapeutic options that are targeted to the causal pathways in pregnancy.
Conclusions
We conducted a systematic review to identify potential precision markers that would refine the diagnosis of GDM and identify women with GDM at higher risk of perinatal complications. The results of our meta-analyses that included over 20,000 women demonstrated that a higher maternal BMI in women with GDM is a marker for risk of offspring LGA or macrosomia. Other promising precision markers include maternal triglycerides and insulin resistance indices (e.g., HOMA-IR); however, these biomarkers require additional replication and development of standardized clinical laboratory assays before implementation in clinical practice. Areas that currently require substantially more evidence include investigations of genetics, metabolites, and other novel biomarkers, as well as integration of social, environmental, and behavioral factors. Advances in computing and the promotion of cross-disciplinary team science may be one approach for addressing these gaps and future directions. Overall, our systematic review identified critical gaps and future research areas for precision GDM diagnosis and highlighted promising biomarkers that may open the door to non-glycemic treatment targets in GDM.
Data availability
Complete lists of the publications where data were extracted for this study are provided in Supplementary Data 1. Source data for Fig. 2 are available in Supplementary Data 5. Additional information is available via contact with the corresponding author. Data for meta-analyses are available via contact with Dr. Jiaxi Yang.
References
Zhu, Y. Y. & Zhang, C. L. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr. Diabetes Rep. 16, https://doi.org/10.1007/s11892-015-0699-x (2016).
World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res. Clin. Pract. 103, 341–363 (2014).
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 5, 47 (2019).
Landon, M. B. et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 361, 1339–1348 (2009).
Crowther, C. A. et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352, 2477–2486 (2005).
Powe, C. E., Hivert, M. F. & Udler, M. S. Defining heterogeneity among women with gestational diabetes mellitus. Diabetes 69, 2064–2074 (2020).
Hashemipour, S. et al. Level of maternal triglycerides is a predictor of fetal macrosomia in non-obese pregnant women with gestational diabetes mellitus. Pediatr. Neonatol. 59, 567–572 (2018).
Leng, J. et al. GDM women’s pre-pregnancy overweight/obesity and gestational weight gain on offspring overweight status. PLoS One 10, e0129536 (2015).
Ouzounian, J. G. et al. Pre-pregnancy weight and excess weight gain are risk factors for macrosomia in women with gestational diabetes. J. Perinatol. 31, 717–721 (2011).
Scifres, C., Feghali, M., Althouse, A. D., Caritis, S. & Catov, J. Adverse outcomes and potential targets for intervention in gestational diabetes and obesity. Obstet. Gynecol. 126, 316–325 (2015).
Powe, C. E. et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39, 1052–1055 (2016).
Benhalima, K. et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 62, 2118–2128 (2019).
Immanuel, J. et al. Metabolic phenotypes of early gestational diabetes mellitus and their association with adverse pregnancy outcomes. Diabet. Med., https://doi.org/10.1111/dme.14413 (2020).
Feghali, M. N. et al. 82: Subtypes of gestational diabetes mellitus based on mechanisms of hyperglycemia. Am. J. Obstetr. Gynecol. 220, S66 (2019).
Francis, E. C., Kechris, K., Jansson, T., Dabelea, D. & Perng, W. Novel metabolic subtypes in pregnant women and risk of early childhood obesity in offspring. JAMA Netw. Open 6, e237030 (2023).
Nolan, C. J. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care 18, https://doi.org/10.2337/diacare.18.12.1550 (1995).
Fang Zhang, F. et al. Trends and disparities in diet quality among us adults by supplemental nutrition assistance program participation status. JAMA Netw. Open 1, e180237 (2018).
Panza, G. A. et al. Links between discrimination and cardiovascular health among socially stigmatized groups: a systematic review. PLoS One 14, e0217623 (2019).
Ruiz, D., Becerra, M., Jagai, J. S., Ard, K. & Sargis, R. M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 41, 193–205 (2018).
Chu, S. Y. et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30, 2070–2076 (2007).
Yao, D. et al. Relationship between maternal central obesity and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. J. Diabetes Res. 2020, 6303820 (2020).
Powe, C. E. Early pregnancy biochemical predictors of gestational diabetes mellitus. Curr. Diabetes Rep. 17, 12 (2017).
Griffith, R. J. et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane reviews. Cochrane Database Syst. Rev., https://doi.org/10.1002/14651858.CD012394.pub3 (2020).
Farhat, S., Hemmatabadi, M., Ejtahed, H. S., Shirzad, N. & Larijani, B. Microbiome alterations in women with gestational diabetes mellitus and their offspring: a systematic review. Front. Endocrinol. 13, 1060488 (2022).
Sriboonvorakul, N., Hu, J., Boriboonhirunsarn, D., Ng, L. L. & Tan, B. K. Proteomics studies in gestational diabetes mellitus: a systematic review and meta-analysis. J. Clin. Med. 11, https://doi.org/10.3390/jcm11102737 (2022).
Mou, S. S. et al. Association between HbA1c levels and fetal macrosomia and large for gestational age babies in women with gestational diabetes mellitus: a systematic review and meta-analysis of 17,711 Women. J. Clin. Med. 12, https://doi.org/10.3390/jcm12113852 (2023).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45, 261–266 (2022).
Tobias, D. K. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 29, 2438–2457 (2023).
Gribble, K. D. et al. Effective communication about pregnancy, birth, lactation, breastfeeding and newborn care: the importance of sexed language. Front. Glob. Womens Health 3, 818856 (2022).
Benham, J. L. et al. Precision gestational diabetes treatment: a systematic review and meta-analyses. Commun. Med. 3, 135 (2023).
Lim, S. et al. Participant characteristics in the prevention of gestational diabetes as evidence for precision medicine: a systematic review and meta-analysis. Commun. Med. 3, 137 (2023).
Semnani-Azad, Z. et al. Predictors and risk factors of short-term and long-term outcomes among women with gestational diabetes mellitus (GDM) and their offspring: Moving toward precision prognosis? medRxiv, 2023.2004.2014.23288199, https://doi.org/10.1101/2023.04.14.23288199 (2023).
McArthur, A., Klugárová, J., Yan, H. & Florescu, S. Innovations in the systematic review of text and opinion. Int. J. Evid. Based Healthc. 13, 188–195 (2015).
Zeraatkar, D. et al. Effect of lower versus higher red meat intake on cardiometabolic and cancer outcomes: a systematic review of randomized trials. Ann. Intern. Med. 171, 721–731, (2019).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986).
Cochran, W. G. Some methods for strengthening the common χ2 tests. Biometrics 10, 417–451 (1954).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Metzger, B. E. et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010).
World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy (World Health Organization, Geneva, 2013).
Lim, J. U. et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 12, 2465–2475 (2017).
Antoniou, M. C. et al. Main fetal predictors of adverse neonatal outcomes in pregnancies with gestational diabetes mellitus. J. Clin. Med. 9, 1–12 (2020).
Much, D. et al. Risk stratification in women with gestational diabetes according to and beyond current WHO criteria. Hormone Metab. Res. 48, 16–19 (2015).
de Paula Bertoli, J. P. et al. Obesity in patients with gestational diabetes: Impact on newborn outcomes. Obes. Med. 20, https://doi.org/10.1016/j.obmed.2020.100296 (2020).
Sun, D., Li, F., Zhang, Y. & Xu, X. Associations of the pre-pregnancy BMI and gestational BMI gain with pregnancy outcomes in Chinese women with gestational diabetes mellitus. Int. J. Clin. Exp. Med. 7, 5784–5789 (2014).
Weschenfelder, F., Hein, F., Lehmann, T., Schleußner, E. & Groten, T. Contributing factors to perinatal outcome in pregnancies with gestational diabetes—what matters most? A retrospective analysis. J. Clin. Med. 10, 1–12 (2021).
Nobumoto, E. et al. Effect of the new diagnostic criteria for gestational diabetes mellitus among Japanese women. Diabetol. Int. 6, 226–231 (2015).
Alfadhli, E. M. Maternal obesity influences Birth Weight more than gestational Diabetes author. BMC Preg. Childbirth 21, 111 (2021).
Barnes, R. A. et al. Predictors of large and small for gestational age birthweight in offspring of women with gestational diabetes mellitus. Diabet. Med. 30, 1040–1046 (2013).
Barquiel, B. et al. HbA1c and gestational weight gain are factors that influence neonatal outcome in mothers with gestational diabetes. J. Womens Health 25, 579–585 (2016).
Barquiel, B. et al. Optimal gestational weight gain for women with gestational diabetes and morbid obesity. Matern. Child. Health J. 22, 1297–1305 (2018).
Ben-Haroush, A., Hadar, E., Chen, R., Hod, M. & Yogev, Y. Maternal obesity is a major risk factor for large-for-gestational-infants in pregnancies complicated by gestational diabetes. Arch. Gynecol. Obstet. 279, 539–543 (2009).
Blickstein, I. et al. The effect of gestational diabetes, pre-gravid maternal obesity, and their combination (‘diabesity’) on outcomes of singleton gestations. J. Matern. Fetal Neonatal Med. 31, 640–643 (2018).
Catalano, P. M. et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35, 780–786 (2012).
Chee, C., Hibbert, E. J., Lam, P., Nanan, R. & Liu, A. Sonographic and other nonglycemic factors can predict large-for-gestational-age infants in diet-managed gestational diabetes mellitus: a retrospective cohort study. J. Diabetes 12, 562–572 (2020).
Collins, K., Oehmen, R. & Mehta, S. Effect of obesity on neonatal hypoglycaemia in mothers with gestational diabetes: a comparative study. Aust. N.Z. J. Obstet. Gynaecol. 58, 291–297 (2018).
Cosson, E. et al. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: a retrospective observational study. Diabetes Metab. 42, 38–46 (2016).
Cremona, A. et al. Maternal obesity and degree of glucose intolerance on neonatal hypoglycaemia and birth weight: a retrospective observational cohort study in women with gestational diabetes mellitus. Eur. J. Pediatr. 179, 653–660 (2020).
Ducarme, G. et al. Efficacy of maternal and biological parameters at the time of diagnosis of gestational diabetes mellitus in predicting neonatal morbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 221, 113–118 (2018).
Filardi, T. et al. Impact of risk factors for gestational diabetes (GDM) on pregnancy outcomes in women with GDM. J. Endocrinol. Investig. 41, 671–676 (2018).
Fuka, F. et al. Factors associated with macrosomia, hypoglycaemia and low Apgar score among Fijian women with gestational diabetes mellitus. BMC Preg. Childbirth 20, 133 (2020).
García-Patterson, A. et al. Maternal body mass index is a predictor of neonatal hypoglycemia in gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 97, 1623–1628 (2012).
García-Patterson, A. et al. In human gestational diabetes mellitus congenital malformations are related to pre-pregnancy body mass index and to severity of diabetes. Diabetologia 47, 509–514 (2004).
Gascho, C. L., Leandro, D. M., Ribeiro, E. S. T. & Silva, J. C. Predictors of cesarean delivery in pregnant women with gestational diabetes mellitus. Rev. Bras. Ginecol. Obstet. 39, 60–65 (2017).
Gorban de Lapertosa, S., Alvariñas, J., Elgart, J. F., Salzberg, S. & Gagliardino, J. J. The triad macrosomia, obesity, and hypertriglyceridemia in gestational diabetes. Diabetes Metab. Res. Rev. 36, e3302 (2020).
Grotenfelt, N. E. et al. Neonatal outcomes among offspring of obese women diagnosed with gestational diabetes mellitus in early versus late pregnancy. J Public Health 41, 535–542 (2019).
Hagiwara, Y. et al. Should the IADPSG criteria be applied when diagnosing early-onset gestational diabetes? Diabetes Res. Clin. Pract. 140, 154–161 (2018).
Hardy, D. S. A multiethnic study of the predictors of macrosomia. Diabetes Educ. 25, 925–933 (1999).
Hernandez-Rivas, E. et al. Gestational diabetes in a multiethnic population of Spain: clinical characteristics and perinatal outcomes. Diabetes Res. Clin. Pract. 100, 215–221 (2013).
Hildén, K., Hanson, U., Persson, M. & Fadl, H. Overweight and obesity: a remaining problem in women treated for severe gestational diabetes. Diabet. Med. 33, 1045–1051 (2016).
Hod, M. et al. Perinatal complications following gestational diabetes mellitus how ‘sweet’ is ill? Acta Obstet. Gynecol. Scand. 75, 809–815 (1996).
Huet, J., Beucher, G., Rod, A., Morello, R. & Dreyfus, M. Joint impact of gestational diabetes and obesity on perinatal outcomes. J. Gynecol. Obstet. Hum. Reprod. 47, 469–476 (2018).
Ijäs, H. et al. Independent and concomitant associations of gestational diabetes and maternal obesity to perinatal outcome: a register-based study. PLoS One 14, e0221549 (2019).
Krstevska, B. et al. Association between foetal growth and different maternal metabolic characteristics in women with gestational diabetes mellitus. Prilozi 30, 103–114 (2009).
Langer, O. Obesity or diabetes: which is more hazardous to the health of the offspring? J. Matern. Fetal Neonatal Med. 29, 186–190 (2016).
Li, G. et al. Differential effect of pre-pregnancy low BMI on fetal macrosomia: a population-based cohort study. BMC Med. 19, 175 (2021).
Martin, K. E., Grivell, R. M., Yelland, L. N. & Dodd, J. M. The influence of maternal BMI and gestational diabetes on pregnancy outcome. Diabetes Res. Clin. Pract. 108, 508–513 (2015).
Matta-Coelho, C., Monteiro, A. M., Fernandes, V., Pereira, M. L. & Souto, S. B. Universal vs. risk-factor-based screening for gestational diabetes-an analysis from a 5-Year Portuguese cohort. Endocrine 63, 507–512 (2019).
Mustaniemi, S. et al. Normal gestational weight gain protects from large-for-gestational-age birth among women with obesity and gestational diabetes. Front. Public Health 9, 550860 (2021).
Nunes, J. S. et al. The influence of preeclampsia, advanced maternal age and maternal obesity in neonatal outcomes among women with gestational diabetes. Rev. Bras. Ginecol. Obstet. 42, 607–613 (2020).
Olmos, P. R. et al. Gestational diabetes and pre-pregnancy overweight: possible factors involved in newborn macrosomia. J. Obstetr. Gynaecol. Res. 38, 208–214 (2012).
Olmos, P. R. et al. Maternal hypertriglyceridemia: a link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity 22, 2156–2163 (2014).
Pezzarossa, A. et al. Effects of maternal weight variations and gestational diabetes mellitus on neonatal birth weight. J. Diabetes Complic. 10, 78–83 (1996).
Philipson, E. H., Kalhan, S. C., Edelberg, S. C. & Williams, T. G. Maternal obesity as a risk factor in gestational diabetes. Am. J. Perinatol. 2, 268–270 (1985).
Pintaudi, B. et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol. 55, 1261–1273 (2018).
Quaresima, P. et al. Appropriate timing of gestational diabetes mellitus diagnosis in medium- and low-risk women: effectiveness of the Italian NHS recommendations in preventing fetal macrosomia. J. Diabetes Res. 2020, 5393952 (2020).
Schaefer-Graf, U. M. et al. How many sonograms are needed to reliably predict the absence of fetal overgrowth in gestational diabetes mellitus pregnancies? Diabetes Care 34, 39–43 (2011).
Simeonova-Krstevska, S. et al. Effect of lipid parameters on foetal growth in gestational diabetes mellitus pregnancies.Pril (Makedon Akad. Nauk. Umet. Odd Med. Nauki) 35, 131–136 (2014).
Son, G. H., Kwon, J. Y., Kim, Y. H. & Park, Y. W. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 89, 700–704 (2010).
Tavares, M., Lopes, É. S., Barros, R., Azulay, R. S. S. & Faria, M. D. S. Profile of pregnant women with gestational diabetes mellitus at increased risk for large for gestational age newborns. Rev. Bras. Ginecol. Obstet. 41, 298–305 (2019).
Usami, T. et al. Comparison of pregnancy outcomes between women with early-onset and late-onset gestational diabetes in a retrospective multi-institutional study in Japan. J. Diabetes Investig. 11, 216–222 (2020).
Wahabi, H. A., Fayed, A. A., Alzeidan, R. A. & Mandil, A. A. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr. Disord. 14, 47 (2014).
Wang, L. F. et al. Influence of pre-pregnancy obesity on the development of macrosomia and large for gestational age in women with or without gestational diabetes mellitus in Chinese population. J. Perinatol. 35, 985–990 (2015).
Wang, N., Ding, Y. & Wu, J. Effects of pre-pregnancy body mass index and gestational weight gain on neonatal birth weight in women with gestational diabetes mellitus. Early Hum. Dev. 124, 17–21 (2018).
Yogev, Y. & Langer, O. Pregnancy outcome in obese and morbidly obese gestational diabetic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 137, 21–26 (2008).
Yuen, L., Wong, V. W., Wolmarans, L. & Simmons, D. Comparison of pregnancy outcomes using different gestational diabetes diagnostic criteria and treatment thresholds in multiethnic communities between two tertiary centres in Australian and New Zealand: do they make a difference? Int. J. Environ. Res. Public Health 18, https://doi.org/10.3390/ijerph18094588 (2021).
Zawiejska, A., Wender-Ozegowska, E., Brazert, J. & Sodowski, K. Components of metabolic syndrome and their impact on fetal growth in women with gestational diabetes mellitus. J. Physiol. Pharmacol. 59, 5–18 (2008).
Zou, Y. et al. Establishment of a nomogram model to predict macrosomia in pregnant women with gestational diabetes mellitus. BMC Preg. Childbirth 21, 581 (2021).
Barden, A. et al. Factors predisposing to pre-eclampsia in women with gestational diabetes. J. Hypertens. 22, 2371–2378 (2004).
Barquiel, B. et al. Body weight, weight gain and hyperglycaemia are associated with hypertensive disorders of pregnancy in women with gestational diabetes. Diabetes Metab. 40, 204–210 (2014).
Fonseca, L. et al. Third trimester HbA1c and the association with large-for-gestational-age neonates in women with gestational diabetes. Arch. Endocrinol. Metab. 65, 328–335 (2021).
Phaloprakarn, C. & Tangjitgamol, S. Risk assessment for preeclampsia in women with gestational diabetes mellitus. J. Perinat. Med. 37, 617–621 (2009).
Thevarajah, A. & Simmons, D. Risk factors and outcomes for neonatal hypoglycaemia and neonatal hyperbilirubinaemia in pregnancies complicated by gestational diabetes mellitus: a single centre retrospective 3-year review. Diabet. Med. 36, 1109–1117 (2019).
Yogev, Y., Langer, O., Brustman, L. & Rosenn, B. Pre-eclampsia and gestational diabetes mellitus: does a correlation exist early in pregnancy? J. Matern. Fetal Neonatal Med. 15, 39–43 (2004).
Yue, S. et al. Clinical consequences of gestational diabetes mellitus and maternal obesity as defined by Asian BMI thresholds in Viet Nam: a prospective, hospital-based, cohort study. BMC Preg. Childbirth 22, 195 (2022).
Shi, P., Liu, A. & Yin, X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Preg. Childbirth 21, 508 (2021).
Aiken, C. E. M., Hone, L., Murphy, H. R. & Meek, C. L. Improving outcomes in gestational diabetes: does gestational weight gain matter? Diabet. Med. 36, 167–176 (2019).
Horosz, E., Bomba-Opon, D. A., Szymanska, M. & Wielgos, M. Maternal weight gain in women with gestational diabetes mellitus. J. Perinat. Med. 41, 523–528 (2013).
Bomba-Opon, D. A., Horosz, E., Szymanska, M. & Wielgos, M. Maternal plasma adipokines and insulin concentrations in relation to fetal biometry in the gestational diabetes. Neuro Endocrinol. Lett. 31, 568–572 (2010).
Lee, B. H., Park, T. C. & Lee, H. J. Association between fetal abdominal circumference and birthweight in maternal hyperglycemia. Acta Obstet. Gynecol. Scand. 93, 786–793 (2014).
Leung, W. C., Lam, H., Lee, C. P. & Lao, T. T. Doppler study of the umbilical and fetal middle cerebral arteries in women with gestational diabetes mellitus. Ultrasound Obstetr. Gynecol. 24, 534–537 (2004).
Simpson, K. J., Pavicic, M. & Lee, G. T. What is the accuracy of an early third trimester sonogram for identifying LGA infants born to GDM patients diagnosed with the one-step approach? J. Matern. Fetal Neonatal Med. 31, 2628–2633 (2018).
Rao, C. & Ping, F. Second-trimester maternal lipid profiles rather than glucose levels predict the occurrence of neonatal macrosomia regardless of glucose tolerance status: a matched cohort study in Beijing. J. Diabetes Complic. 35, 107948 (2021).
Gibbons, K. S. et al. Prediction of large-for-gestational age infants in relation to hyperglycemia in pregnancy—a comparison of statistical models. Diabetes Res. Clin Pract. 178, 108975 (2021).
Sun, Y. Y. et al. Increasing insulin resistance predicts adverse pregnancy outcomes in women with gestational diabetes mellitus. J. Diabetes 12, 438–446 (2020).
Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines (Washington, DC, 2009).
Knopp, R. H., Magee, M. S., Walden, C. E., Bonet, B. & Benedetti, T. J. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 15, 1605–1613 (1992).
Herrera Martínez, A. et al. Hyperlipidemia during gestational diabetes and its relation with maternal and offspring complications. Nutr. Hosp. 35, 698–706 (2018).
Xiao, Y. & Zhang, X. Association between maternal glucose/lipid metabolism parameters and abnormal newborn birth weight in gestational diabetes complicated by preeclampsia: a retrospective analysis of 248 cases. Diabetes Ther. 11, 905–914 (2020).
Liu, Y. et al. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: a prospective cohort study of perinatal outcomes. J. Transl. Med. 16, 289 (2018).
Li, J. et al. Roles of insulin resistance and beta cell dysfunction in macrosomia among Chinese women with gestational diabetes mellitus. Prim. Care Diabetes 12, 565–573 (2018).
Wang, N. et al. Contribution of gestational diabetes mellitus heterogeneity and prepregnancy body mass index to large-for-gestational-age infants—a retrospective case-control study. J. Diabetes 13, 307–317 (2021).
Madsen, L. R. et al. Do variations in insulin sensitivity and insulin secretion in pregnancy predict differences in obstetric and neonatal outcomes? Diabetologia 64, 304–312 (2021).
Lin, J., Jin, H. & Chen, L. Associations between insulin resistance and adverse pregnancy outcomes in women with gestational diabetes mellitus: a retrospective study. BMC Preg. Childbirth 21, 526 (2021).
Zhang, N. J., Tao, M. F., Li, H. P., Zhao, F. & Wang, F. H. The relationship between patterns of insulin secretion and risks of gestational diabetes mellitus. Int. Fed. Gynaecol. Obstetr. 150, 318–323 (2020).
Immanuel, J. et al. Metabolic phenotypes of early gestational diabetes mellitus and their association with adverse pregnancy outcomes. Diabet. Med. 38, e14413 (2021).
Park, S. et al. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 67, 196–201 (2013).
Sun, M. et al. The alteration of carnitine metabolism in second trimester in GDM and a nomogram for predicting macrosomia. J. Diabetes Res. 2020, 4085757 (2020).
Lu, J., Wu, J., Zhao, Z., Wang, J. & Chen, Z. Circulating LncRNA serve as fingerprint for gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol. Biochem. 48, 1012–1018 (2018).
Zhu, C., Liu, Y. & Wu, H. Overexpression of circactr2 in gestational diabetes mellitus predicts intrauterine death, fetal malformation, and intrauterine infection. Diabetes, Metab. Syndrome Obes. Targets Ther. 14, 4655–4660 (2021).
Han, Y. et al. Association of adiponectin gene polymorphism 45TG with gestational diabetes mellitus diagnosed on the new IADPSG criteria, plasma adiponectin levels and adverse pregnancy outcomes. Clin. Exp. Med. 15, 47–53 (2015).
Bo, S. et al. Isoleucine-to-methionine substitution at residue 148 variant of PNPLA3 gene and metabolic outcomes in gestational diabetes. Am. J. Clin. Nutr. 101, 310–318 (2015).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487 (2004).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Stumvoll, M., Van Haeften, T., Fritsche, A. & Gerich, J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 24, 796–797 (2001).
Powe, C. E., Locascio, J. J., Gordesky, L. H., Florez, J. C. & Catalano, P. M. Oral glucose tolerance test-based measures of insulin secretory response in pregnancy. J. Clin. Endocrinol. Metab. 107, e1871–e1878 (2022).
Ding, T. T., Xiang, J., Luo, B. R. & Hu, J. Relationship between the IADPSG-criteria-defined abnormal glucose values and adverse pregnancy outcomes among women having gestational diabetes mellitus: a retrospective cohort study. Medicine 97, e12920 (2018).
Anyaegbunam, A. M., Scarpelli, S. & Mikhail, M. S. Chronic hypertension in gestational diabetes: influence on pregnancy outcome. Gynecol. Obstet. Investig. 39, 167–170 (1995).
Wang, X., Zhang, X., Zhou, M., Juan, J. & Wang, X. Association of gestational diabetes mellitus with adverse pregnancy outcomes and its interaction with maternal age in Chinese urban women. J. Diabetes Res. 2021, 5516937 (2021).
Manoharan, V. & Wong, V. W. Impact of comorbid polycystic ovarian syndrome and gestational diabetes mellitus on pregnancy outcomes: a retrospective cohort study. BMC Preg. Childbirth 20, 484 (2020).
Cosson, E. et al. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 36, 598–603 (2013).
Scime, N. V. et al. Pregnancy complications and risk of preterm birth according to maternal age: a population-based study of delivery hospitalizations in Alberta. Acta Obstet. Gynecol. Scand. 99, 459–468 (2020).
Lamminpää, R., Vehviläinen-Julkunen, K., Gissler, M., Selander, T. & Heinonen, S. Pregnancy outcomes in women aged 35 years or older with gestational diabetes—a registry-based study in Finland. J. Matern. Fetal Neonatal Med. 29, 55–59 (2016).
Benhalima, K. et al. Risk factor screening for gestational diabetes mellitus based on the 2013 WHO criteria. Eur. J. Endocrinol. 180, 353–363 (2019).
Alshammari, A., Hanley, A., Ni, A., Tomlinson, G. & Feig, D. S. Does the presence of polycystic ovary syndrome increase the risk of obstetrical complications in women with gestational diabetes? J. Matern. Fetal Neonatal. Med. 23, 545–549 (2010).
Lee, K. W. et al. Neonatal outcomes and its association among gestational diabetes mellitus with and without depression, anxiety and stress symptoms in Malaysia: a cross-sectional study. Midwifery 81, 102586 (2020).
Wan, C. S. et al. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: a comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J. Diabetes 11, 809–817 (2019).
Esakoff, T. F., Caughey, A. B., Block-Kurbisch, I., Inturrisi, M. & Cheng, Y. W. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J. Matern. Fetal Neonatal Med. 24, 422–426 (2011).
Berggren, E. K. et al. Perinatal outcomes in Hispanic and non-Hispanic white women with mild gestational diabetes. Obstet. Gynecol. 120, 1099–1104 (2012).
Chen, L. et al. Diabetes in pregnancy in associations with perinatal and postneonatal mortality in First Nations and non-Indigenous populations in Quebec, Canada: population-based linked birth cohort study. BMJ Open 9, e025084 (2019).
Cosson, E. et al. Psychosocial deprivation in women with gestational diabetes mellitus is associated with poor fetomaternal prognoses: an observational study. BMJ Open 5, e007120 (2015).
Tsai, P. J., Roberson, E. & Dye, T. Gestational diabetes and macrosomia by race/ethnicity in Hawaii. BMC Res. Notes 6, 395 (2013).
Schmidt, C. B. et al. Diabetes distress is associated with adverse pregnancy outcomes in women with gestational diabetes: a prospective cohort study. BMC Preg. Childbirth 19, 223 (2019).
Mocarski, M. & Savitz, D. A. Ethnic differences in the association between gestational diabetes and pregnancy outcome. Matern. Child Health J. 16, 364–373 (2012).
Makgoba, M., Savvidou, M. D. & Steer, P. J. The effect of maternal characteristics and gestational diabetes on birthweight. BJOG 119, 1091–1097 (2012).
Fadl, H. E., Ostlund, I. K. & Hanson, U. S. Outcomes of gestational diabetes in Sweden depending on country of birth. Acta Obstet. Gynecol. Scand. 91, 1326–1330 (2012).
Dyck, R. F., Karunanayake, C., Pahwa, P., Stang, M. & Osgood, N. D. Epidemiology of diabetes in pregnancy among first nations and non-first nations women in Saskatchewan, 1980‒2013. Part 2: predictors and early complications; results from the DIP: ORRIIGENSS project. Can. J. Diabetes 44, 605–614 (2020).
Contreras, K. R., Kominiarek, M. A. & Zollinger, T. W. The impact of tobacco smoking on perinatal outcome among patients with gestational diabetes. J. Perinatol. 30, 319–323 (2010).
Kwong, W. et al. Perinatal outcomes among different asian groups with gestational diabetes mellitus in Ontario: a cohort study. Can. J. Diabetes 43, 606–612 (2019).
Fraser, D. et al. Gestational diabetes among Bedouins in southern Israel: comparison of prevalence and neonatal outcomes with the Jewish population. Acta Diabetol. 31, 78–81 (1994).
Ajala, O. & Chik, C. Ethnic differences in antepartum glucose values that predict postpartum dysglycemia and neonatal macrosomia. Diabetes Res. Clin. Pract. 140, 81–87 (2018).
Hammoud, N. M., de Valk, H. W., Biesma, D. H. & Visser, G. H. Gestational diabetes mellitus diagnosed by screening or symptoms: does it matter? J. Matern. Fetal Neonatal Med. 26, 103–105 (2013).
Weeks, J. W., Major, C. A., de Veciana, M. & Morgan, M. A. Gestational diabetes: does the presence of risk factors influence perinatal outcome? Am. J. Obstet. Gynecol. 171, 1003–1007 (1994).
Packer, C. H., Pilliod, R. A., Chatroux, L. R., Caughey, A. B. & Valent, A. M. Increased rates of adverse perinatal outcomes in women with gestational diabetes and depression. J. Matern. Fetal Neonatal Med. 34, 3862–3866 (2021).
Li, G., Fan, L., Zhang, L., Zhang, W. & Huang, X. Metabolic parameters and perinatal outcomes of gestational diabetes mellitus in women with polycystic ovary syndrome. J. Perinat. Med. 38, 141–146 (2010).
Szymanska, M., Horosz, E., Szymusik, I., Bomba-Opon, D. & Wielgos, M. Gestational diabetes in IVF and spontaneous pregnancies. Neuro Endocrinol. Lett. 32, 885–888 (2011).
Tundidor, D. et al. Perinatal maternal and neonatal outcomes in women with gestational diabetes mellitus according to fetal sex. Gend. Med. 9, 411–417 (2012).
Zhang, S. et al. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J. Hum. Hypertens. 31, 731–736 (2017).
Kouhkan, A. et al. Obstetric and perinatal outcomes of singleton pregnancies conceived via assisted reproductive technology complicated by gestational diabetes mellitus: a prospective cohort study. BMC Preg. Childbirth 18, 495 (2018).
Meek, C. L. et al. Seasonal variations in incidence and maternal-fetal outcomes of gestational diabetes. Diabet. Med. 37, 674–680 (2020).
Liu, J., Song, G., Meng, T. & Zhao, G. Epicardial adipose tissue thickness as a potential predictor of gestational diabetes mellitus: a prospective cohort study. BMC Cardiovasc. Disord. 20, 184 (2020).
Catalano, P. M. & Shankar, K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1 (2017).
Wang, J. et al. Gestational dyslipidaemia and adverse birthweight outcomes: a systematic review and meta-analysis. Obes. Rev. 19, 1256–1268 (2018).
Iwabu, M., Okada-Iwabu, M., Yamauchi, T. & Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 1, 15013 (2015).
Pan, W. W. & Myers, M. G. Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 19, 95–105 (2018).
Hinkle, S. N. et al. Maternal adipokines longitudinally measured across pregnancy and their associations with neonatal size, length, and adiposity. Int. J. Obes., https://doi.org/10.1038/s41366-018-0255-2 (2018).
Francis, E. C. et al. Maternal proinflammatory adipokines throughout pregnancy and neonatal size and body composition: a prospective study. Curr. Dev. Nutr. 5, nzab113 (2021).
Guasch-Ferre, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
White, S. L. et al. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 60, 1903–1912 (2017).
Zhao, L. et al. Association of circulating branched-chain amino acids with gestational diabetes mellitus: a meta-analysis. Int. J. Endocrinol. Metab. 17, e85413–e85413 (2019).
Scholtens, D. M. et al. Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 37, 158–166 (2014).
Liu, T. et al. Comprehensive analysis of serum metabolites in gestational diabetes mellitus by UPLC/Q-TOF-MS. Anal. Bioanal. Chem. 408, 1125–1135 (2016).
Hajduk, J. et al. A combined metabolomic and proteomic analysis of gestational diabetes mellitus. Int. J. Mol. Sci. 16, 30034–30045 (2015).
Dudzik, D. et al. Metabolic fingerprint of gestational diabetes mellitus. J. Proteom. 103, 57–71 (2014).
Howell, E. A. Reducing disparities in severe maternal morbidity and mortality. Clin. Obstet. Gynecol. 61, 387–399 (2018).
White, S. L., Ayman, G., Bakhai, C., Hillier, T. A. & Magee, L. A. Screening and diagnosis of gestational diabetes. BMJ 381, e071920 (2023).
Sweeting, A., Wong, J., Murphy, H. R. & Ross, G. P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 43, 763–793 (2022).
Sánchez-Valle, J. & Valencia, A. Molecular bases of comorbidities: present and future perspectives. Trends Genet. https://doi.org/10.1016/j.tig.2023.06.003.
Acknowledgements
A.S.: Grant support from the NHMRC and ADS; C.P.: Grant support from NIH/NIDDK and Robert Wood Johnson Foundation; D.S.: Grant support from NIH/NIDDK; E.C.F.: Grant support from NIH/NICHD and NIH/NHLBI; M.F.H.: Grant support from ADA, and NIH/NICHD; S.H.K.: Grant support from NHGRI; S.L.W.: Grant support from the MRC; W.L.: Grant support from NIH/NIDDK; Y.Z.: Grant support from NIH/NIDDK and NIH/NHLBI. The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL).
Author information
Authors and Affiliations
Consortia
Contributions
E.C.F.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote the first draft of the manuscript, and contributed to revisions. C.E.P.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript. W.L.L.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript. S.L.W.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript. D.M.S.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript. J.Y.: Conducted meta-analyses and wrote results. Y.Z.: screened abstracts/full text, interpreted results, wrote/revised manuscript. C.Z.: screened abstracts/full text, interpreted results, wrote/revised manuscript. M.F.H.: coordinated group, screened abstracts/full text, interpreted results, wrote/revised manuscript. S.H.K.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript. A.S.: contributed to the conceptualization of the research question, screened abstracts/full text, interpreted results, wrote/revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: C.E.P. is an Associate Editor of Diabetes Care, receives payments from Wolters Kluwer for UpToDate chapters on diabetes in pregnancy, and has received payments for consulting and speaking from Mediflix Inc. All other authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Elpida Vounzoulaki, A Seval Ozgu-Erdinc, Sarah Seaton, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Francis, E.C., Powe, C.E., Lowe, W.L. et al. Refining the diagnosis of gestational diabetes mellitus: a systematic review and meta-analysis. Commun Med 3, 185 (2023). https://doi.org/10.1038/s43856-023-00393-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-023-00393-8
- Springer Nature Limited