Abstract
Microbial anaerobic oxidation of methane coupled to the reduction of Mn(IV)-oxides, typically MnO2, derived from continental weathering and riverine transport has been proposed as a globally important sink of methane. However, the potential role of hydrothermal Mn(IV) oxide-rich sediments as a methane sink, and the mechanisms of underpinning methane oxidation at high-temperature hydrothermal fields remain poorly understood. Here, we report the occurrence of almost pure rhodochrosite with extremely negative δ13CPDB values (as low as –76.4‰) in direct association with hausmannite formed through hydrothermal activity in the Late Triassic Heqing Mn deposit in Southern China. Based on detailed petrography and geochemistry, this rhodochrosite is interpreted as the result of the thermochemical oxidation of methane by hausmannite during early diagenesis. Given high hydrothermal Mn2+ and CH4 release coupled to low sulfate concentration observed in the Archean oceans, we propose that hydrothermal Mn(IV) oxides may have been effective methane sinks in Earth’s ancient oceans.
Similar content being viewed by others
Introduction
Methane, as an important greenhouse gas, is of great significance to the global carbon cycle and climate change1. Microbial anaerobic oxidation of methane (AOM) using dissolved sulfate as the electron accepter is considered the main methane-consuming mechanism in low-temperature modern marine sediments, effectively preventing methane emission into the upper oceans and atmosphere2,3,4. The occurrence of the process involves an anaerobic methanotrophic archaea and sulfate-reducing bacteria interacting in a syntrophic fashion within anoxic seawater and/or sediments3,4,5,6. At temperatures above 90 °C – the known limits of anaerobic methane-oxidizing microbes7,8,9 – the reduction of sulfate can be coupled to the thermochemical oxidation of methane10. Authigenic carbonate with extremely depleted 13C isotopic values, as low as −30‰, reflect the mixing ratio between ocean dissolved inorganic carbon (DIC) (at 0–2‰), highly negative DIC produced by the various microbial metabolic pathways that preferentially oxidize 12C from 13C-depleted biogenic gases (as low as −110‰) and DIC from the thermogenic pool (−30 to −50‰)11,12.
Microbial AOM may also be coupled to the reduction of Fe(III) and Mn(IV) oxides or oxyhydroxides13,14,15; from herein referred to as Fe and Mn oxides, respectively. Microbial AOM using either oxides as electron accepters has been widely reported in modern and ancient sediments13,14,16,17. Most previous studies have focused on the potential for microbial Fe-Mn oxide-dependent AOM in continental margin sediments due to metal oxide-rich sediment supply by riverine transport13,14,18. However, large amounts of Fe2+ and Mn2+ are also released by seafloor hydrothermal vents into the ocean19, where they are oxidized by oxygen during hydrothermal plume dispersal20,21. Accordingly, hydrothermal systems should support microbial metal oxide-based AOM, but to date, only Sun16 inferred that Fe(III) oxides carried by hydrothermal plumes might be coupled to AOM in the modern Okinawa Trough. Perhaps the explanation is that metalliferous sediment associated with hydrothermal vents formed at high temperatures (>90 °C)22. Like microbial AOM, there is also a paucity of information pertaining to TOM by metal oxides. To date, Kiyosu23 and Pan24 have demonstrated the process in the laboratory, while Hu25 has suggested evidence for Mn(IV) oxides dependent TOM in Triassic sandy conglomerates. For much of geological time, the source of Mn2+ was from hydrothermal activity26, while the formation of Mn carbonates with an extremely negative 13C isotope values commonly was considered as microbial Mn2+ oxidation in the water column using O2 as the terminal electron acceptor, and then subsequent reduction of Mn(III,IV) oxides through dissimilatory manganese reduction27,28.
Here, we report the occurrence of almost pure rhodochrosite, with extremely negative δ13CPDB values as low as –76.4‰, in direct association with Mn(II,III) oxides deriving from hydrothermal activity, in the Late Triassic Heqing Mn deposit in southern China (Fig. 1, Supplementary Note). However, based on petrographic and geochemical data, we interpret the formation of this rhodochrosite as resulting from the thermochemical oxidation of methane coupled to hydrothermal hausmannite, and not dissimilatory manganese reduction.
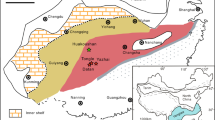
a, b Geological map of the Heqing manganese deposit. c, d Stratigraphic column of the sampling location: the third member of Triassic Songgui Formation29.
Results
Mn ores exhibit a massive black structure with white irregular blocky masses and fine spherulites distributed throughout the hand samples (Fig. 2a–d). Microscopic observation found that irregular blocky masses and fine spherulites show a microcrystalline structure (Fig. 2e–f), while the black massive structures exhibit a concentric layered microstructure resembling modern marine dendritic and botryoidal Mn nodules (Fig. 3a, b). A combination of powder and in-situ X-ray diffractometry and Raman spectroscopy analyses revealed that the nodule-like Mn oxide is hausmannite (Mn2+Mn3+2O4), the white masses are rhodochrosite (MnCO3), and the poorly crystallized mineral is pyrochroite (Mn(OH)2) (Fig. 3e, f and Supplementary Fig. S1). Scanning electron microscopy observation also revealed similar structures of manganese carbonate and oxide (Fig. 3c, d). These concentric layers of hausmannite have varying thicknesses and the thinnest layers are only micrometers in scale (Fig. 3d). Blocky mass and spherulitic rhodochrosite are distributed within hausmannite and cut through concentric layers of the latter (Fig. 3d). Furthermore, rare sphalerite debris (~2 μm) also occurs within Mn oxides masses (Supplementary Fig. S2).
a–d Hand sample of massive Mn ores (B2530-2) showing blocky mass and spherulites of manganese carbonate distributed in a Mn oxide (hausmannite) and hydroxide (pyrochroite) matrix. Areas where clumped isotope data were collected shown by arrows. e, f Microscopic images (transmitted light) of blocky mass and spherulites manganese carbonate (transmission light).
a, b Reflected light microscopy images of blocky mass and spherulitic rhodochrosite (Rds) cross-cutting a matrix of Mn oxide. c, d Backscattered SEM images showing similar features as in a and b. d locations indicated with square in c (yellow dotted line). e Characteristic XRD patterns of the Mn ore with peaks indicating hausmannite, pyrochroite, and rhodochrosite. f Representative Raman spectra of hausmannite (orange), pyrochroite (blue), and rhodochrosite (purple). Sampling locations indicated with crosses in b.
The PAAS-normalized in-situ rare earth element (REE) patterns of hausmannite show obvious negative Ce anomalies (0.57–0.83) and positive Eu anomalies (0.98–1.3) (Fig. 4a and Supplementary Table S1). The total REE content of hausmannite is extremely low (15–30 ppm). Ce and Eu anomalies of rhodochrosite are similar to those of hausmannite (Fig. 4b), but the rhodochrosite exhibits slight enrichment in light REE (the concentration of total light REE ranges from 27 to 60 ppm, with an average 40 ppm) (Fig. 4b and Supplementary Table S2). An Electron Probe Microanalyzer revealed that the rhodochrosites are almost pure, with the CaO content ranging from only 0.5–2 wt.% (Supplementary Table S3). The rhodochrosite in our samples are extremely rich in 12C and have (micro-drill) δ13C-PDB values range from –76.4 to –46.65‰, with several samples showing values within the –75 to –50‰ range (Fig. 5a, b and Supplementary Table S4). Rhodochrosite δ18O- PDB values range from –3.69 to –2.17‰ (Fig. 5a, b and Supplementary Table S4). Hausmannite δ18O- SMOW values range from 10.1 to 19.7‰ (Supplementary Table S5). The temperature of precipitation of rhodochrosite is predicted to have been between 110 °C and 135 °C (Fig. 5c) according to the clumped isotope date (n = 4; Supplementary Table S6).
Discussion
The nodule-like structure and mineral composition of hausmannite are very similar to those of modern ocean Mn nodules29. Generally, REE features, including Ce and Eu anomalies, as well as the concentration of total REE, are the main geochemical proxies used to identify the genesis of different marine Fe-Mn nodules or crusts30. REE features of hydrothermal Fe-Mn nodules or crusts usually exhibit negative Ce anomalies, positive Eu anomalies, and low concentrations of total REE due to rapid precipitation rates30. Conversely, REE features of hydrogenic Fe-Mn nodules or crusts usually exhibit positive Ce anomalies, no Eu anomalies, and high concentrations of total REE due to it having slow precipitation rates. The REE features of diagenetic Fe-Mn nodules or crusts are between hydrothermal and hydrogenic Fe-Mn nodules or crusts. Furthermore, Bau30 proposed that Ce/Ce* versus Nd and Ce/Ce* versus (Y/Ho)SN can be used to indicate the origins of Fe-Mn nodules or crusts. In-situ REE patterns of hausmannite show extremely low REE concentrations, obvious negative Ce anomalies, and slight positive Eu anomalies, which are very similar to the REE patterns of hydrothermal Mn deposits31,32. On a Ce/Ce* versus Nd diagram, hausmannite values all fall within the hydrothermal range, while on a Ce/Ce* versus (Y/Ho)SN diagram, the hausmannite samples fall within the diagenetic ranges (Fig. 4c, d). The hydrothermal Fe-Mn deposits trend toward hydrogenic Fe-Mn deposits in plots of Ce/Ce* versus Nd and Ce/Ce* versus (Y/Ho)SN due to Fe-Mn oxides surface having been exposed to seawater30. The hausmannite in our samples that fall within the diagenetic ranges on the Ce/Ce* versus (Y/Ho)SN diagram may be caused by its surfaces having had prolonged exposure to bottom seawater. Furthermore, the REE patterns of hausmannite are consistent with previous studies (bulk powder)33,34.
Oxygen isotopic values of hausmannite also support a hydrothermal origin. In our samples, we subjected the Mn ore to acidification with hydrochloric (pre-treatment) to eliminate rhodochrosite and sphalerite. Although rare clay minerals were not completely extracted from the Mn ores, it is unlikely that they would significantly impact the δ18O isotope values of hausmannite (Supplementary Table S5). Such values for oxygen isotopes are slightly higher compared to those found in modern marine Mn oxides (5.5~15.8‰)35. The difference possibly resulted from the oxygen atom in hausmannite primarily originating from O2 (+23‰), with only a small fraction coming from seawater. Based on experimental results reported by Sutherland36, it was suggested that approximately 0.58 atom oxygen of Mn oxides originate from seawater in the abiotic pathway, while 0.38–0.62 atom oxygen is derived from O2 in various biotic pathways. The calculated oxygen isotope range of hausmannite derived from seawater is between 3.84 and 12.21‰ based on rough estimates. For this study, we assumed that seawater during the Later Triassic period ranged from −1 to 1‰ based on research conducted by Sun37. Our sample analysis indicated that the precipitation temperatures of hausmannite range from 4.5 to 82 °C using the Zheng’s38 oxygen isotopic geothermometer, this corroborating our hypothesis that the hausmannite in the Heqing Mn deposit has a hydrothermal origin (Supplementary Table S5).
The hydrothermal activity was possibly driven by the eruption of basic magma in the northern margin of the Heqing sub-basin during the Late Triassic Norian period33,39. Rare sphalerite debris in hausmannite imply that hausmannite precipitation occurred close to hydrothermal vents40. Based on the rock assemblages of T3sg3, we suggest that the water column of the Heqing sub-basin is oxic or suboxic in the Late Triassic Norian period. Thus, hydrothermal fluids are released into oxygenated seawater along a paleo-fault, where a large amount of dissolved Mn2+ is oxidized and precipitated by microbial or chemical pathways to form hausmannite. It is then rapidly buried in marine sediments close to the hydrothermal vents.
Rhodochrosite is traditionally considered to be a product of Mn(III/IV) oxide reduction during early diagenesis26,41. The presence of hausmannite and its cross-cutting relationship with rhodochrosite indicates that rhodochrosite formed later by the reduction of the two Mn(III) atoms in hausmannite. The δ13CPDB isotopic signal of rhodochrosite has also been used to evaluate the origin of DIC of sedimentary manganese ore deposits42. Rhodochrosite in the Heqing deposit exhibited δ13CPDB values ranging from –46 to –75‰, with most falling between –50 and –75‰ (Fig. 5b). Such extremely depleted 13CPDB values of rhodochrosite are interpreted as methane-derived carbonate (MDCs)11,14,16,17,43. The cross-cutting relationships between the hausmannite and rhodochrosite, coupled with the isotopic composition of the latter, suggest that the precipitation of rhodochrosite was caused by a redox reaction between methane and hausmannite (reaction 1):
Previous studies proposed that the δ13CPDB values of ancient MDCs can be as proxy for methane sources11. Thermogenic methane has light C isotopic values as low as –50‰, whereas biogenic methane has even lower C isotopic values of −60 to −110‰44. Generally, the δ13CPDB isotope values of ancient carbonate are mixed with various carbon sources such as marine carbonate, decomposition of organic matter, seep methane and methanogenesis11. Thus, the δ13CPDB isotope values of MDCs usually higher than those of the seep methane11. The most δ13CPDB value in our samples is lower −60‰ (Fig. 5b), which favors biogenic methane as the main carbon source but does not preclude methane produced by the thermogenic breakdown of in situ organic matter in hydrothermal sediments. The negative covariation of C- and O-isotope and high δ18OPDB values of rhodochrosite implies that the methane may have been released through the dissociation of gas hydrates45,46,47. Indeed, high temperatures caused by magmatic or hydrothermal activity will compromise the stability of gas hydrates, allowing deep methane to rise to the seafloor16. Therefore, we infer that methane seepages were driven by dissociation of underlying gas hydrates by frequent hydrothermal activity in Heqing sub-basin.
Microbial AOM by indirect or direct metal oxides (Fe-Mn) has been suggested in some modern lake and marine sediments13,48,49. However, metalliferous sediments close to hydrothermal vents are usually heated by underlying magmatic or hydrothermal activities3,7, so thermochemical oxidation methane (TOM) by Mn oxides at high temperature is more likely than AOM, although this mechanism has only recently been documented in geological systems25. The carbonate clumped isotope thermometer (TΔ47) can effectively constrain the temperature of carbonate samples because it is independent of the oxygen isotope of seawater or porewater during carbonate growth50. Low Δ47 values of rhodochrosite suggest a relatively high temperature (110–135 °C) for the origin of the Heqing Mn deposit (Fig. 5c and Supplementary Table S5). In addition to high temperature, other processes can produce low Δ47 values in rhodochrosite. For the MDCs, low Δ47 (high TΔ47) may also be produced by kinetic isotopic effects during methane oxidation17,51,52. The extremely high oxidation rate of methane is the main factor which causing kinetic isotopic effects51,52. Extremely high oxidation of methane rates leads to high MDCs growth rates51,52. In this case, various sources of DIC (seawater DIC and methane-derived DIC) would not have sufficient time to reach equilibrium, and thus produce a low Δ47 value51,52. By contrast, various DIC species have sufficient time to re-equilibrate at low oxidation of methane rates. Methane oxidation by metal-oxides occurs at much slower rates than sulfate-dependent AOM, due to metal oxides being solids which are less accessible than dissolved sulfate anions13. Correspondingly, the precipitation rate of rhodochrosite is also slow. Thus, we tentatively suggest that the high TΔ47 values are unlikely due to kinetic isotopic effects. Furthermore, the impact of fluids during diagenesis and solid-state reordering can also lead to low TΔ47 values53,54. But there is no trace of recrystallization in our rhodochrosite, and C- and O-isotope of rhodochrosite show a negative covariation (Fig. 5a). Thus, effects of fluid-buffered during diagenesis (e.g., meteoric fluid buffered and recrystallization) was excluded. The T3Sg3 strata were buried at a maximum depth of 354 m and the Heqing Mn deposit were not buried more than 100 m during the Later Triassic33. Based on the geothermal gradient as 30 °C/Km and the surface temperature as 30 °C during Late Triassic55, the calculated peaking burial temperature ranges from 40 to 46 °C. Solid-state recording is unlikely to proceed at such burial temperature53.
In addition, we must consider the potential impact of hydrothermal fluids on solid-state reordering. The nearest alkaline porphyry from the Himalayan Period is located ten kilometers away from the Heqing Mn ore deposit56,57. Using the Raman geothermometer, the spectra of organic matter in Mn ores agree with it being amorphous carbon (low-grade organic matter), and the organic matter’s peak temperature was 128 °C58 (Fig. S3 and Supplementary Table S6). Clay minerals associated with the Mn ore sample are predominantly composed of illite and kaolinite, with a minor presence of chlorite and mixed-layer (illite-vermiculite) minerals (Supplementary Fig. S4). The crystallinity of illite, known as Kubleri-Index, is temperature-dependent59. Illite’s Kubleri-Index obtained from Mn ores using the Gaussian–Lorentzian function is roughly 1.18 Δ°2θ (Supplementary Fig. S5), corresponding to an estimated peak temperature of 100 °C60 (Supplementary Table S6). The calcite veins found in the host rock of the Heqing Mn ore deposit exhibit δ18O isotope values ranging from −12.3 to −7.6‰, which also corresponds to a temperature range of ~89–109 °C61. Collectively, the available data indicates that the maximum temperatures reached by the ores was at or below 130 °C. This calculated temperature agrees with the temperature range determined from clumped isotope measurements, which falls between 110 and 135 °C. Although further investigations are required to verify the accuracy of Raman spectra and Kubleri-Index geothermometer in low-temperature zones (<150 °C), there is a lack of clear high-temperature signal, indicating that magmatic activity has minimal impact on the Heqing area. Instead, the most parsimonious explanation is that the temperature range was influenced by hydrothermal events62. According to the model proposed by Stolper63 (for calcite), clumped isotope temperatures can be altered by a maximum of ~40 °C when the environmental temperature is held at 125 °C for a duration of over 10 million years, and by a maximum of ~25 °C when held at the same temperature for less than 5 million years. The range of maximum burial temperature is between 40 °C and 46 °C, and it is observed that the effect of solid-state reordering is overprinted by the maximum temperature of diagenetic burial, which ranges from 80 to 86 °C. This range is considerably lower than the measured clumped isotope temperature. Additionally, clumped isotope temperatures may be modified by less than 5 °C when held at 125 °C below 1 million years63. Although precise constraints on the timescale of hydrothermal events are challenging to obtain, we propose that a short timescale (between 103 and 104 years) is plausible for the duration of the heating event. This inference is supported by two lines of evidence. The first is the distance of over 10 km between the Himalayan Period alkaline porphyry and the Heqing Mn ore deposit, which suggests that magmatic activity may have had minimal influence on the stratum (i.e., <1 million years). It is possible for long-term heating (>106 years) to occur in the stratum only in proximity to magmatic activity56. The second piece of evidence is the incursion of heat flow through deep-seated crustal faults, which are typically transient in nature62. Therefore, we propose that solid-state reordering resulting from hydrothermal events accounts for the observed change in clumped isotope temperature of less than 5 °C. Based on the above discussion, we infer that the formation of highly δ13C-depleted rhodochrosite occurred at high temperatures (105–130 °C), beyond that which reasonably supports microbes driving AOM.
Notably, if rhodochrosite undergoes equilibrium crystallization at temperatures ranging from 105 to 130 °C, the isotopic fractionation between oxygen isotopes in the mineral and its parent fluid (porewater) should range from 16 to 19‰64. Thus, the precipitation of rhodochrosite is from the parent fluid with an δ18O-SMOW isotopic value of 9.6–11.8‰. Such δ18O-rich fluid is rare and far higher than normal marine water (−3 to 3‰). Consequently, there are two potential explanations for the observed circumstances: either rhodochrosite was formed through processes that generated isotopic disequilibrium with its parent fluid, or alternatively, it was produced from a porewater source with notably high δ18O values. First, the disequilibrium crystallization of rhodochrosite should be excluded due to rhodochrosite’s slow precipitation rate. Previous studies have demonstrated that δ18O-rich fluids could possibly serve as sources for authigenic methane-derived rhodochrosite, arising from various origins such as gas hydrate dissociation65, clay mineral dehydration (smectite to illite)66, and Mn oxide reduction67. Although illite and kaolinite were found in Mn ores, mixed layer clays (e.g., smectite, illite) were undetected, suggesting that clay mineral dehydration may not be responsible for enriching the δ18O isotope of porewater. While gas hydrate dissociation is a possible contributor, it is not considered a primary source since it can result in fluid enriched with δ18O isotopic values ranging only between 2.4 and 3.4‰68. This assertion is also supported by the negative covariation of C-O isotope values of rhodochrosite. Furthermore, the process by which initial oxygen isotopic values are modified through Mn oxides reduction has been documented in the authigenic Ca-rhodochrosite of the Baltic Sea67. Based on their findings, those authors speculated that the δ18O isotope may increase by up to 1‰ due to a mixture of 0.03 mole fraction of MnO2 with δ18O values of +8.9‰ and 0.97 mole fraction of porewater with δ18O values of −4.6‰67. In our samples, rhodochrosite is likely entirely derived from the reduction of hausmannite in closed porewater system. Based on the hausmannite’s δ18O-rich signal (10.1–19.7‰), we propose that the enrichment of oxygen isotopes in porewater is due to the reduction of hausmannite.
In-situ REE patterns of rhodochrosite have slightly lower Ce/Ce* (0.49–0.60) and Eu/Eu* (0.91–1.23) values than the hausmannite (Ce/Ce*: 0.57–0.83; Eu/Eu*: 0.98–1.30). The Ce anomalies and slight positive Eu anomalies also are very similar to the REE patterns of hydrothermal fluids, volcaniclastic sediments, as well as those of the Hollard Mound MDCs69. These anomalies (Ce and Eu) have main two origins; (1) REE inherited from hausmannite, or (2) hydrothermal fluid influence. Compared with hausmannite, the rhodochrosite exhibits slight enrichment in LREEs. The slight LREE-rich rhodochrosite is possibly influenced by high temperature hydrothermal fluids70. Thus, we infer that the subsurface high hydrothermal fluid possibly was involved in the formation process of rhodochrosite (or reduction of hausmannite) during the early diagenesis. This also implies that rhodochrosite may have formed in a high temperature porewater environment. In summary, both the REE and stable isotopic geochemistry of these Mn minerals are consistent with frequent hydrothermal activities in Heqing sub-basin during the Norian period.
The microbial role in AOM is limited in high temperatures (>90 °C) environments due to the metabolism of anaerobic methanotrophic microbes, which occur at temperatures below 90 °C7. Thus, methane oxidation is likely dominated by thermochemical reactions in our samples. The absence of Fe-Mn sulfides (pyrite or alabandite) in our samples implies that rhodochrosite formed in an environment where sulfate was depleted16,17. Rapid sediment accumulation of hydrothermal hausmannite may also indicate that it was buried below the sulfate-methane transition zone14—poor sulfate zone. Thus, we infer that highly δ13C-depeted rhodochrosite results from TOM by Mn oxides at high temperature. Although the laboratory experiments show TOM by metal-oxides does not proceed at temperatures below 250 °C23,24, this process can occur at lower temperatures at slower rates of reaction during a longer geological interval25.
Overall, we provide the evidence for the occurrence of TOM by Mn oxides in hydrothermal metalliferous sediments. Hydrothermal activity developed at the Heqing sub-basin during the Late Triassic Norian period where large amounts of Mn2+ were partially oxidized, precipitated as hausmannite, and then buried into the subsurface sediments closed to hydrothermal vents during the dispersal of hydrothermal plumes. In addition, hydrothermal activity compromised the stability of underlying gas hydrates, driving methane seepage. The TOM by hausmannite to form rhodochrosite occurred as upward-migrating methane encountered the subsurface high temperature metalliferous sediments.
Metalliferous sediments composed of poorly crystalline Fe-Mn oxides or hydroxides are widely distributed in modern hydrothermal fields including mid-ocean ridges, back-arc basins and intraplate volcanoes71,72. Seafloor hydrothermal fluids not only release large amounts of metal ions (Fe2+ and Mn2+) but also large amount of reducing gas (CH4 and H2S) into the ocean73. In addition, most hydrothermal fields coincide with the predicted distribution of natural gas hydrates16 so that hydrothermal activity may disrupt the stability of natural gas hydrates, and thus generates methane seepages. The potential role of metalliferous sediments as a methane sink in the hydrothermal systems have largely been neglected so far. In this study, we provide the evidence for the occurrence of thermochemical anaerobic oxidation methane by hydrothermal Mn oxides (hausmannite) in metalliferous sediments. It is worth noting that the use of Fe-Mn oxides by microbes as an electron acceptor is not an exclusive pathway for methane cycling in hydrothermal fields17. Instead, TOM can also play a vital role in this process. Rhodochrosite and kutnohorite, two forms of Mn carbonate known to exhibit extremely negative 13C isotopic values (<–25‰), are commonly found in the geological record26,27,43. Additionally, manganese carbonate deposits often have a documented hydrothermal origin26. These findings suggest that hydrothermal Fe-Mn oxides may have served as widespread methane sinks in Phanerozoic oceans. Moreover, it is possible that hydrothermal Fe-Mn oxides could have played an even more crucial role in methane consumption during the Archean Era since this period had higher inputs of hydrothermal metals (Fe2+ and Mn2+) and CH4 and lower concentrations of sulfate than modern oceans56.
Methods
Mineral analyses
The powders and thin-sections of Mn ore from the Heqing Mn ore deposit were analyzed using a Bruker D8 ADVANCE X-ray diffractometer, with data collected over the 2θ range of 5°–75° during counting times of 1° min−1 at the State Key Laboratory of GPMR, China University of Geosciences (CUG) in Wuhan, China. To identify the various clay minerals, oriented aggregates were prepared from the <2 um fraction. This particular fraction was extracted via decantation from a suspension of 10 g of dried bulk Mn ores previously sieved at 63 um, utilizing distilled water. Subsequently, each sample underwent solvation with ethylene glycol for a period of 3 h. Jade 6.0 software was employed for the purpose of mineral identification and processing of the data. High-resolution Micro-Raman was conducted at the State Key Laboratory of Biogeology and Environmental Geology, CUG in Wuhan, China, using a WITec300 Confocal Raman Imaging system. All Raman spectra were processed with the WITec Project Five Plus software. The Raman spectra of organic matter were subjected to peak deconvolution using the WITec Project 5 computer program. A Gaussian–Lorentzian function with a high degree of fitting (R2 > 0.99) was employed in this study. The Raman spectrum of organic matter exhibits two regions of interest, namely the first-order region (1000–1800 cm−1) and second-order region (2500–3100 cm−1), with specific emphasis on the former. This region is characterized by up to five distinctive bands for organic matter, namely G, D1, D2, D3, and D4. Utilizing the peak fitting flowchart proposed by Kouketsu58, we found that the Raman spectra of organic matter in our samples corresponded to low-grade (amorphous carbon). In this study, the peak heating temperature (TD1°C) was computed by means of the full width at half-maximum (FWHM) of a series of disordered bands, which include D1 and D2-band, via the following equation TD1°C = −2.15 × (FWHM-D1) + 478.
Elemental analyses
Microscopic analysis was performed on thin sections of the Mn ores via optical microscope and field-emission scanning electron microscopy at the State Key Laboratory of GPMR, CUG in Wuhan, China. Prior to the EF-SEM observations, the thin sections underwent a coating with a few nanometers of platinum gold (Au). The analyses were carried out on a Zeiss Sigma 300 instrument under working conditions that involved a distance from the sample of 8 mm, voltages of 20 kV, and a beam current of 10 nA. In preparation for analysis, samples were initially coated with a thin conductive carbon film, with measures taken to minimize variability in carbon film thickness between specimens and to achieve an approximately uniform coating thickness of 20 nm; this follows the protocols outlined by Zhang and Yang74. Operation conditions for quantitative WDS analyses included an accelerating voltage of 15 kV, beam current of 10 nA, and a 10 µm spot size. The content of C was calculated based on the mole ratio of C:O = 1:3, and then involved into the ZAF correction procedure. The peak counting time was 10 s for Fe, Ca, Mg, Mn. The background counting time was 1/2 of the peak counting time on the high- and low-energy background positions. Diopside (Ca, Mg), Pyrope (Fe), Rhodonite (Mn) were used in this analysis as standards.
LA-ICP-MS analyses
Chemical analyses of the Mn ores were conducted by in-situ methods directly on polished thin sections at the State Key Laboratory of GPMR, CUG in Wuhan, China. A 193 nm RESOlution S-155 laser-ablation system coupled with a Thermo iCAP RQ inductively coupled plasma mass spectrometer was used. Samples were ablated using a 50 μm spot size, 10 Hz repetition rate, and 4 J/cm2 corresponding energy density. In this study, we adopted multiple reference materials including NIST SRM 610 and 612, BIR-1G, BCR-2G, and BHVO-2G75,76 as external standards without the use of an internal standard for concentration determination77. The analytical uncertainty is better than 5% for REEs and 10% for the remaining elements78. Raw data reduction was performed offline using the ICPMSDataCal software77. In calculating total major element oxides for quantification, it was assumed that all Mn in the Mn oxide samples were in the 3+ oxidation states.
Rhodochrosite C-O isotope analyses of micro-drill samples
Isotopic analysis of carbon and oxygen was performed on the Mn ores utilizing a Finnigan MAT-253 isotope ratio mass spectrometer (IRMS) at the State Key Laboratory of GPMR, CUG in Wuhan, China. An automated carbonate preparation system known as Kiel IV was used, where 100% H3PO4 was introduced to the samples for 220 s at a temperature of 70 °C. Every nine samples including a random repeated sample were followed by a standard. All isotopic ratios are expressed as per mil relative to the Vienna Peedee Belemnite (V-PDB) standard. The internal precision (1 SD) was less than ±0.1 for 13C and 18O values, and the external precision was better than ±0.5 for δ13C and δ18O values.
Hausmannite O isotope analyses
All the samples underwent acidification with HCl as a pre-treatment process. Acidified subsamples were leached for 3 h in a 10% HCl solution, rinsed seven times using deionized water, and air-dried overnight in an oven set at 100 °C to ensure complete dehydration prior to analysis. To liberate oxygen from hausmannite, pure BrF5 was quantitatively reacted with it and heated with a CO2 laser, following Clayton and Mayeda79 at analytical laboratory of Beijing Research Institute of Uranium Geology, China. The δ18O isotope data were reported relative to Vienna-Standard Mean Ocean Water (V-SMOW), with internal precisions of better than 0.2‰ for δ18O in a single analysis.
Rhodochrosite clumped isotope analyses of micro-drill samples
Clumped isotope were analyzed at the State Key Laboratory of GPMR, CUG in Wuhan, China. Sample pre-treatments involved adding 200 mg of dried bulk powder samples and 2 ml of analytical grade H2O2 into a centrifuge tube for duration of 1–2 h, followed by subsequent washing and drying of the samples. Clumped isotope (Δ47) analyses were performed using the sample preparation line and dual inlet-equipped MAT253 IRMS. An aliquot of 18–25 mg rhodochrosite powder was first digested in phosphoric acid at concentrations of 102–105% and temperatures of 90 °C, followed by CO2 gas purification through multiple cryogenic traps. The ensuing CO2 gas underwent purification through multiple cryogenic traps, including a Porapak-Q gas chromatograph column held at −20 °C. To eliminate water and low vapor pressure gases, U-shaped cryogenic glass traps were employed. The process entailed utilizing liquid nitrogen for freezing and CO2 collection at −196 °C, as well as a combination of liquid nitrogen and alcohol for unfreezing and releasing CO2 at −90 °C. Furthermore, a U-shaped cryogenic glass trap, measuring 30 cm in length and packed with Porapak Q between 80 and 100 Mesh, was utilized along with a mixture of liquid nitrogen and ethylene glycol (−20 °C) to further purify CO2 by removing organic contaminants and sulfides. Glass traps were ultimately used to transfer the purified gas to a finger tube for confinement before measurement on the IRMS80.
Ten acquisitions with eight cycles were performed for each sample or standard analysis, and each sample was subjected to three measurements. Nonlinearity corrections were carried out using standard protocols, which incorporated equilibrated gas at temperatures of 10, 25, and 50 °C, as well as heated CO2 gas at 1000 °C81. In addition, the purified CO2 gas’s δ18O isotopic values were analyzed utilizing this specially modified IRMS (MAT253). The δ18O values were calibrated relative to the international reference standard NBS-19 (δ18O = −2.20‰) and Chinese national standard GBW04416 (δ18O = −11.59‰) and expressed in standard δ-notation as permille deviations from the Vienna Pee Dee Belemnite (VPDB) international standard. Precision levels for duplicate analyses of GBW04416 and selected study samples exceeded ±0.1‰. Based on clumped isotope temperature, the oxygen isotope geothermometer proposed by Kim64 was used to calculate the oxygen isotope values of parent fluid of precipitation rhodochrosite.
The raw clumped isotope data were calibrated to the absolute reference frame (ARF), by applying +0.082‰ to account for acid digestion at 90 °C-related fractionation, and subsequently by further calibration utilizing newly determined clumped isotope values of ETH standards (i.e., ETH-1: +0.258‰, ETH-2: +0.256‰, ETH-3: +0.691‰, and ETH-4: +0.507‰)80. In previous several studies, an acid fractionation factor of +0.082‰ is widely accepted, and thus we used it to calibrate our data82. In order to reduce inter-laboratory bias, we chose the ETH standards reported in Bernasconi82 to correct our data62. We chose the calibration from Petersen83 to calculate clumped isotope-based temperatures (using IUPAC parameters, new Δ47-TE values, and new Δ*25-X), although other temperature calibrations would result in only small differences in TΔ47 values and would not affect our interpretations84,85. The different mineralogies can use a same calibration equation, due to full acid fractionation factor vary can cancel out equilibrium clumping varies between carbonate mineralogies83.
Data availability
The data underlying the research presented in our paper is available in the Supplementary Information and have been deposited in the Mendeley Data (Huang Qin (2023), “Huang et al_2023_COMMSENV”, Mendeley Data. V1, https://doi.org/10.17632/44fj25xmy6.1).
References
Berbesi, L. A., di Primio, R., Anka, Z., Horsfield, B. & Wilkes, H. Methane leakage from evolving petroleum systems: masses, rates and inferences for climate feedback. Earth Planet. Sci. Lett. 387, 219–228 (2014).
D’Hondt, S., Rutherford, S. & Spivack, A. J. Metabolic activity of subsurface life in deep-sea sediments. Science 295, 2067–2070 (2002).
Reeburgh, W. S. Oceanic methane biogeochemistry. Chem. Rev. 107, 486–513 (2007).
Knittel, K. & Boetius, A. Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63, 311–334 (2009).
Hinrichs, K. U., Hayes, J. M., Sylva, S. P., Brewer, P. G. & DeLong, E. F. Methane-consuming archaebacteria in marine sediments. Nature 398, 802–805 (1999).
Boetius, A. et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626 (2000).
Kallmeyer, J. & Boetius, A. Effects of temperature and pressure on sulfate reduction and anaerobic oxidation of methane in hydrothermal sediments of Guaymas Basin. Appl. Environ. Microb. 70, 1231–1233 (2004).
Biddle, J. F. et al. Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J. 6, 1018–1031 (2012).
Wankel, S. D. et al. Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction. Environ. Microbiol. 14, 2726–2740 (2012).
Cai, C. F., Zhang, C. M., He, H. & Tang, Y. J. Carbon isotope fractionation during methane-dominated TSR in East Sichuan Basin gasfields, China: a review. Mar. Petrol. Geol. 48, 100–110 (2013).
Peckmann, J. & Thiel, V. Carbon cycling at ancient methane-seeps. Chem. Geol. 205, 443–467 (2004).
Drake, H. et al. Extreme C-13 depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat. Commun. 6, 7020 (2015).
Beal, E. J., House, C. H. & Orphan, V. J. Manganese- and iron-dependent marine methane oxidation. Science 325, 184–187 (2009).
Egger, M. et al. Iron oxide reduction in methane-rich deep Baltic Sea sediments. Geochim. Cosmochim. Acta 207, 256–276 (2017).
Ettwig, K. F. et al. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl Acad. Sci. USA 113, 12792–12796 (2016).
Sun, Z. L. et al. Hydrothermal metal supplies enhance the benthic methane filter in oceans: an example from the Okinawa Trough. Chem. Geol. 525, 190–209 (2019).
Cai, C. F. et al. Anaerobic oxidation of methane by Mn oxides in sulfate-poor environments. Geology 49, 761–766 (2021).
Li, J. W., Peng, X. T., Bai, S. J., Chen, Z. Y. & Van Nostrand, J. D. Biogeochemical processes controlling authigenic carbonate formation within the sediment column from the Okinawa Trough. Geochim. Cosmochim. Acta 222, 363–382 (2018).
Gartman, A. & Findlay, A. J. Impacts of hydrothermal plume processes on oceanic metal cycles and transport. Nat. Geosci. 13, 396–402 (2020).
Dekov, V. M. et al. Metalliferous sediments from Eolo Seamount (Tyrrhenian Sea): hydrothermal deposition and re-deposition in a zone of oxygen depletion. Chem. Geol. 264, 347–363 (2009).
Mills, R., Elderfield, H. & Thomson, J. A dual origin for the hydrothermal component in a metalliferous sediment core from the mid-atlantic ridge. J. Geophys. Res. Sol Earth 98, 9671–9681 (1993).
Schouten, S., Wakeham, S. G., Hopmans, E. C. & Damste, J. S. S. Biogeochemical evidence that thermophilic archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microb. 69, 1680–1686 (2003).
Kiyosu, Y. & Imaizumi, S. Carbon and hydrogen isotope fractionation during oxidation of methane by metal oxides at temperatures from 400 degrees to 530 degrees C. Chem. Geol. 133, 279–287 (1996).
Pan, C. C., Yu, L. P., Liu, J. Z. & Fu, J. M. Chemical and carbon isotopic fractionations of gaseous hydrocarbons during abiogenic oxidation. Earth Planet. Sci. Lett. 246, 70–89 (2006).
Hu, W. X. et al. Thermochemical oxidation of methane induced by high-valence metal oxides in a sedimentary basin. Nat. Commun. 9, 5131 (2018).
Maynard, J. B. The chemistry of manganese ores through time: a signal of increasing diversity of earth-surface environments. Econ. Geol. 105, 535–552 (2010).
Polgári, M. et al. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 47, 87–109 (2012).
Ossa, F. et al. Aerobic iron and manganese cycling in a redox-stratified Mesoarchean epicontinental sea. Earth Planet. Sci. Lett. 500, 28–40 (2018).
Sousa, I. M. C. et al. Mineralogy and chemical composition of ferromanganese crusts from the Cruzeiro do Sul Lineament-Rio Grande Rise, South Atlantic. J. S. Am. Earth Sci. 108 https://doi.org/10.1016/j.jsames.2021.103207 (2021).
Bau, M. et al. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 381, 1–9 (2014).
Chetty, D. & Gutzmer, J. REE redistribution during hydrothermal alteration of ores of the Kalahari Manganese Deposit. Ore Geol. Rev. 47, 126–135 (2012).
Sasmaz, A., Turkyilmaz, B., Ozturk, N., Yavuz, F. & Kumral, M. Geology and geochemistry of Middle Eocene Maden complex ferromanganese deposits from the Elazig-Malatya region, eastern Turkey. Ore Geol. Rev. 56, 352–372 (2014).
Chang, H. L. Study on geochemical characteristics and tracer of sedimentary environment in Heqing manganese deposit (Yunnan Province: Kunming Univesity of Science and Technology, 2013).
Yu, S. J. Analysis of ore-controlling factors of Heqin manganese deposit. J. Mineral. Petrol. 20, 30–37 (2000).
Sutherland, K. M. et al. Ferromanganese crusts as recorders of marine dissolved oxygen. Earth Planet Sc Lett 533, https://doi.org/10.1016/j.epsl.2019.116057 (2020).
Sutherland, K. M., Wankel, S. D. & Hansel, C. M. Oxygen isotope analysis of bacterial and fungal manganese oxidation. Geobiology 16, 399–411 (2018).
Sun, Y. D. et al. Lethally hot temperatures during the early triassic greenhouse. Science 338, 366–370 (2012).
Zheng, Y. F. Calculation of oxygen isotope fractionation in metal-oxides. Geochim. Cosmochim. Ac 55, 2299–2307 (1991).
Yu, S. J. Genesis of Heqin manganese deposit. J. China Univ. Min. Technol. 33, 109−114 (2004).
Zhu, Q. K & Zhou, H. Y. Reviews and prospects of submarine metalliferous sediments of hydrothermal origin. Mar. Sci., 45, 8, https://doi.org/10.11759/hykx20191221001 (2021).
Johnson, J. E., Webb, S. M., Ma, C. & Fischer, W. W. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim. Cosmochim. Acta 173, 210–231 (2016).
Okita, P. M., Maynard, J. B., Spiker, E. C. & Force, E. R. Isotopic evidence for organic-matter oxidation by manganese reduction in the formation of stratiform manganese carbonate ore. Geochim. Cosmochim. Acta 52, 2679–2685 (1988).
Hein, J. R. & Koski, R. A. Bacterially mediated diagenetic origin for chert-hosted manganese deposits in the Franciscan Complex, California Coast Ranges. Geology 15, 722–726 (1987).
Whiticar, M. J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314 (1999).
Aloisi, G. et al. Methane-related authigenic carbonates of eastern Mediterranean Sea mud volcanoes and their possible relation to gas hydrate destabilisation. Earth Planet. Sci. Lett. 184, 321–338 (2000).
Lin, Q., Wang, J. S., Algeo, T. J., Su, P. B. & Hu, G. W. Formation mechanism of authigenic gypsum in marine methane hydrate settings: evidence from the northern South China Sea. Deep Sea Res. Part I 115, 210–220 (2016).
Luff, R., Wallmann, K. & Aloisi, G. Numerical modeling of carbonate crust formation at cold vent sites: significance for fluid and methane budgets and chemosynthetic biological communities. Earth Planet Sci. Lett. 221, 337–353 (2004).
Krylov, A. A. et al. Authigenic rhodochrosite from a gas hydrate-bearing structure in Lake Baikal. Int. J. Earth Sci. 107, 2011–2022 (2018).
Michaelis, W. et al. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 297, 1013–1015 (2002).
Ghosh, P. et al. C-13-O-18 bonds in carbonate minerals: a new kind of paleothermometer. Geochim. Cosmochim. Acta 70, 1439–1456 (2006).
Loyd, S. J. et al. Methane seep carbonates yield clumped isotope signatures out of equilibrium with formation temperatures. Nat. Commun. 7, 12274 (2016).
Zhang, N. Z. et al. Clumped isotope signatures of methane-derived authigenic carbonate presenting equilibrium values of their formation temperatures. Earth Planet. Sci. Lett. 512, 207–213 (2019).
Henkes, G. A. et al. Temperature limits for preservation of primary calcite clumped isotope paleotemperatures. Geochim. Cosmochim. Acta 139, 362–382 (2014).
Passey, B. H. & Henkes, G. A. Carbonate clumped isotope bond reordering and geospeedometry. Earth Planet. Sci. Lett. 351, 223–236 (2012).
McElwain, J. C., Beerling, D. J. & Woodward, F. I. Fossil plants and global warming at the Triassic-Jurassic boundary. Science 285, 1386–1390 (1999).
Fu, Y. et al. Geochronology of the giant Beiya gold-polymetallic deposit in Yunnan Province, Southwest China and its relationship with the petrogenesis of alkaline porphyry. Ore Geol. Rev. 71, 138–149 (2015).
Deng, J. et al. Delineation and explanation of geochemical anomalies using fractal models in the Heqing area, Yunnan Province, China. J. Geochem. Explor. 105, 95–105 (2010).
Kouketsu, Y. et al. A new approach to develop the Raman carbonaceous material geothermometer for low-grade metamorphism using peak width. Island Arc 23, 33–50 (2014).
Guggenheim, S. et al. Report of the Association Internationale pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2001: Order, disorder and crystallinity in phyllosilicates and the use of the “crystallinity index”. Clay Miner. 50, 406–409 (2002).
Baludikay, B. K. et al. Raman microspectroscopy, bitumen reflectance and illite crystallinity scale: comparison of different geothermometry methods on fossiliferous Proterozoic sedimentary basins (DR Congo, Mauritania and Australia). Int. J. Coal Geol. 191, 80–94 (2018).
Shackleton, N. J. et al. Paleotempreature history of the Cenozoic and the initiation of Antarctic glaciation: Oxygen and carbon isotope analysis in DSDP site 277, 279, and 281. In Initial Reports of the Deep Sea Drilling Project, 29. (U. S. Government Printing Office, 1975), https://doi.org/10.2973/dsdp.proc.29.117.1975.
Chang, B. et al. Massive formation of early diagenetic dolomite in the Ediacaran ocean: constraints on the “dolomite problem”. Proc. Natl Acad. Sci. USA 117, 14005–14014 (2020).
Stolper, D. A. & Eiler, J. M. The kinetics of solid-state isotope-exchange reactions for clumped isotopes: a study of inorganic calcites and apatites from natural and experimental samples. Am. J. Sci. 315, 363–411 (2015).
Kim, S. T., Kang, J. O., Yun, S. T., O’Neil, J. R. & Mucci, A. Experimental studies of oxygen isotope fractionation between rhodochrosite (MnCO3) and water at low temperatures. Geochim. Cosmochim. Acta 73, 4400–4408 (2009).
Han, X. Q., Yang, K. H. & Huang, Y. Y. Origin and nature of cold seep in northeastern Dongsha area, South China Sea: Evidence from chimney-like seep carbonates. Chin. Sci. Bull. 58, 3689–3697 (2013).
Hesse, R. Pore water anomalies of submarine gas-hydrate zones as tool to assess hydrate abundance and distribution in the subsurface - What have we learned in the past decade? Earth Sci. Rev. 61, 149–179 (2003).
Neumann, T., Heiser, U., Leosson, M. A. & Kersten, M. Early diagenetic processes during Mn-carbonate formation: Evidence from the isotopic composition of authigenic Ca-rhodochrosites of the Baltic Sea. Geochim. Cosmochim. Acta 66, 867–879 (2002).
Maekawa, T. Experimental study on isotopic fractionation in water during gas hydrate formation. Geochem. J. 38, 129–138 (2004).
Jakubowicz, M., Dopieralska, J. & Belka, Z. Tracing the composition and origin of fluids at an ancient hydrocarbon seep (Hollard Mound, Middle Devonian, Morocco): a Nd, REE and stable isotope study. Geochim. Cosmochim. Acta 156, 50–74 (2015).
Bau, M. & Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 155, 77–90 (1999).
Bodei, S., Buatier, M., Steim-Nann, M., Adatte, T. & Wheat, C. G. Characterization of metalliferous sediment from a low-temperature hydrothermal environment on the Eastern Flank of the East Pacific Rise. Mar. Geol. 250, 128–141 (2008).
Hrischeva, E. & Scott, S. D. Geochemistry and morphology of metalliferous sediments and oxyhydroxides from the Endeavour segment, Juan de Fuca Ridge. Geochim. Cosmochim. Acta 71, 3476–3497 (2007).
Boetius, A. & Wenzhofer, F. Seafloor oxygen consumption fuelled by methane from cold seeps. Nat. Geosci. 6, 725–734 (2013).
Zhang, R. X. & Yang, S. Y. A mathematical model for determining carbon coating thickness and its application in electron probe microanalysis. Microsc. Microanal. 22, 1374–1380 (2016).
Jochum, K. P. et al. Determination of reference values for NIST SRM 610-617 glasses following ISO guidelines. Geostand. Geoanal. Res. 35, 397–429 (2011).
Jochum, K. P., Willbold, M., Raczek, I., Stoll, B. & Herwig, K. Chemical characterisation of the USGS reference glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G using EPMA, ID-TIMS, ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 29, 285–302 (2005).
Liu, Y. S. et al. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 257, 34–43 (2008).
Chen, L. et al. Accurate determinations of fifty-four major and trace elements in carbonate by LA-ICP-MS using normalization strategy of bulk components as 100%. Chem. Geol. 284, 283–295 (2011).
Clayton, W. M. & Mayeda, T. K. The use of bromine pent a fluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 27, 43–52 (1963).
Chang, B. A. et al. Effects of different constants and standards on the reproducibility of carbonate clumped isotope (Delta(47)) measurements: insights from a long-term dataset. Rapid Commun. Mass. Spectrom 34, e8678 (2020).
Dennis, K. J. et al. Defining an absolute reference frame for “clumped” isotope studies of CO2. Geochim. Cosmochim. Acta 75, 7117–7131 (2011).
Bernasconi, S. M. et al. Reducing uncertainties in carbonate clumped isotope analysis through consistent carbonate-based standardization. Geochem. Geophy. Geosyst. 19, 2895–2914 (2018).
Petersen, S. V. et al. Effects of improved O-17 correction on interlaboratory agreement in clumped isotope calibrations, estimates of mineral-specific offsets, and temperature dependence of acid digestion fractionation. Geochem. Geophy. Geosyst. 20, 3495–3519 (2019).
Bonifacie, M. et al. Calibration of the dolomite clumped isotope thermometer from 25 to 350 degrees C, and implications for a universal calibration for all (Ca, Mg, Fe) CO3 carbonates. Geochim. Cosmochim. Acta 200, 255–279 (2017).
Muller, I. A. et al. Calibration of the oxygen and clumped isotope thermometers for (proto-)dolomite based on synthetic and natural carbonates. Chem. Geol. 525, 1–17 (2019).
Acknowledgements
We are grateful to Chad Wittkop, Leslie James Robbins, Kaipeng Ji and Yunrong Huang for their assistance with the discussion. This work was financially supported by the National Natural Science Foundation of China (Nos. 41972091). There is no permissions required for the sampling in this study.
Author information
Authors and Affiliations
Contributions
Q.H. and S.-Y.J. designed this study; Q.H. wrote the manuscript with contributions from S.-Y.J. and D.-H.P.; Q.H. and X.-P.W. collected the samples and performed the experiments; L.-Y.L. and H.Y. participated in the interpretation of the data; K.K. participated in the discussion and edit of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Kyoko Yamaoka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Joe Aslin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Q., Jiang, SY., Pi, DH. et al. Thermochemical oxidation of methane by manganese oxides in hydrothermal sediments. Commun Earth Environ 4, 224 (2023). https://doi.org/10.1038/s43247-023-00891-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-00891-6
- Springer Nature Limited